Abstract
Background
The goal of this study was to characterize the adoption rate and regional variation in bilateral internal mammary artery (BIMA) use during coronary artery bypass grafting (CABG) in the United States.
Methods
Observational study of 100% sample of fee-for-service Medicare beneficiaries aged 65 years or older, continuously enrolled in Parts A and B from 2009 to 2014 (n = 162,860,439). Rates of beneficiaries receiving a BIMA versus single internal mammary artery (SIMA) during CABG are expressed per 1,000 beneficiaries and aggregated by Hospital Referral Region (HRR). An HRR is a validated unit for quantifying regional variation in health care.
Results
The absolute national rate of BIMA use declined during the study period from 0.21 claims per 1,000 beneficiaries in 2009 to 0.13 in 2014 (p < 0.001). When indexed to overall CABG volume, no change was seen in the frequency of BIMA use over time (p = 0.883). SIMA use ranged from 1.3 to 8.5 claims per 1,000 Medicare beneficiaries, whereas BIMA use ranged from 0 to 1.5 (p < 0.001). A significant correlation was found between regional volume of SIMA use and likelihood of BIMA use (correlation coefficient 0.673, p < 0.001). Although both SIMA and BIMA use correlated with regional volume of diagnostic cardiac catheterization, the correlation was stronger for SIMA use (correlation coefficient 0.962 versus 0.682, p < 0.001).
Conclusions
Over the past 5 years, no growth was seen in BIMA use among Medicare beneficiaries, and the frequency of BIMA use during CABG remained low. There was significant regional variation in BIMA use, however, which demonstrates opportunity for continued growth of BIMA grafting.
Since the first large retrospective analysis of bilateral internal mammary artery (BIMA) versus single internal mammary artery (SIMA) use during coronary artery bypass grafting (CABG) by Lytle and colleagues [1] in 1983, there has been continued research interest in multi-arterial grafting. Use of a BIMA has been associated with improved long-term survival and freedom from repeat revascularization in several retrospective and meta-analyses [2–5]. Although the results of the 10-year primary end point of the Arterial Revascularization Trial (ART) are pending, the proportion of CABG procedures done with multi-arterial grating remains low, and an interim analysis of ART demonstrated no difference in mortality rate between the BIMA and SIMA groups [6].
In Europe it has been estimated that approximately 20% of CABG procedures are performed with a BIMA approach, and in the United States the rate of BIMA use is less than 5% [7]. ElBardissi and colleagues [8] examined trends in isolated CABG from The Society of Thoracic Surgeons (STS) database from 2000 to 2009. At the beginning of the study period, the rate of BIMA use was 3.5% and increased to only 4.1% in 2009 despite a disproportionate growth in the literature to support a survival benefit associated with BIMA use during that time.
The observed lack of adoption of multi-arterial grafting, despite literature demonstrating clinical benefits of this technique over single arterial grafting, has many causes. Logistically, harvesting a second arterial conduit does slightly increase operative times [9]. Second, there is no general consensus on the optimal target coronary artery when using a second arterial graft in the setting of multivessel disease [10]. Finally, there is concern over the added risk of sternal wound complications among subgroups of patients such as patients who are obese and have diabetes, that comprise a growing proportion of patients presenting for CABG [11–13]. Despite these concerns, the new STS guidelines on arterial revascularization place a class IIa recommendation based on level B evidence for use of a BIMA in patients who do not have excessive risk of sternal complications [14].
Given these new guidelines, the goal of this analysis was to describe the adoption rate and regional variation in BIMA use during CABG among a large, contemporary cohort of Medicare beneficiaries and to characterize factors associated with regional variation in the use of BIMA grafting in the United States.
Patients and Methods
Data Source
We used a 100% national sample from the physician and supplier and denominator files of the Centers for Medicare and Medicaid Services from January 1, 2009, through December 31, 2014, to identify all CABG procedures performed on Medicare-eligible beneficiaries during each of those years. The physician and supplier file contains all claims submitted by physicians for performance of procedures under the Medicare Part B program, including Current Procedural Terminology (CPT) codes, International Classification of Diseases-Ninth Revision (ICD-9) diagnosis codes, date of procedure, and age, sex, and race/ethnicity of the beneficiary undergoing the procedure [15, 16]. The denominator file contains information about eligibility by year for the Medicare Part B program and information about age, sex, and race/ethnicity of eligible beneficiaries. We excluded patients younger than 65 years or older than 99 years and patients with unknown race/ethnicity. This analysis was performed through an Institutional Review Board approval of the Dartmouth Atlas of Healthcare.
The analysis was started by using the full complement of ICD-9 codes for coronary revascularization that used venous and arterial grafts (36.10 to 36.16). This group of patients represented all CABG procedures among Medicare beneficiaries. Patients were then divided into a SIMA cohort (CPT 33533, coronary artery bypass using single arterial graft) and a BIMA cohort (CPT 33534, coronary artery bypass using two coronary arterial grafts).
Study End Points
The primary end point of the analysis was the rate of SIMA versus BIMA grafting among patients undergoing primary CABG during the study period. Secondary end points included geographic variation in rates of SIMA versus BIMA grafting and the association between arterial conduit use and density of overall CABG volume, diagnostic stress test volume, and diagnostic cardiac catheterization volume.
Statistical Analysis
After establishing our study cohort of SIMA and BIMA patients using the inclusion and exclusion criteria described above, we examined the incidence of each procedure over time between 2009 and 2014. We assessed rates separately by year. The numerator for calculating the crude rate consisted of the number of SIMA or BIMA procedures in each year selected; the denominator consisted of the number of beneficiaries in the 100% Medicare Part B program sample eligible. These rates were adjusted by means of the indirect method of standardization for changes in age, sex, and race/ethnicity occurring over time as described previously [17]. All rates in the analysis are expressed as the number of claims per 1,000 Medicare beneficiaries.
To examine geographic variation in procedure rates, we examined the rates of SIMA and BIMA within each Hospital Referral Region (HRR) in the United States. HRRs, as previously described by the Dartmouth Atlas of Healthcare, represent regional health care markets for tertiary medical care and are a validated means of studying geographic variation in health care delivery and surgical procedures [18–21]. There are currently 306 HRRs in the United States with each HRR containing a minimum population size of 120,000 and at least one hospital that performs major cardiovascular procedures. After defining crude rates of SIMA and BIMA grafting within each HRR for each of the years in our analysis, we adjusted each rate for differences in age, sex, and race across regions as described previously [17]. We used t tests to compare rates between regions, the Pearson correlation coefficient to test associations, and nonparametric tests of trend were used to test significance across years; probability values less than 0.05 were considered significant. All analysis was performed using SAS (SAS Institute, Cary, NC), and STATA 10 (StataCorp, College Station, TX).
Results
Trends in Arterial Revascularization Over Time
From 2009 to 2014 a total of 162,860,439 Medicare beneficiaries were queried for rates of arterial revascularization during CABG. The total number of SIMA and BIMA beneficiaries during the study period were 532,429 and 36,721, respectively. The absolute rates of SIMA and BIMA grafting during the study period per 1,000 Medicare beneficiaries are shown in Figure 1. There was a continued decline of both SIMA and BIMA grafting claims, consistent with overall declines in national CABG volumes. SIMA decreased from 2.7 claims per Medicare beneficiary in 2009 to 2.0 in 2014. The national rate of annual BIMA claims was, overall, 6.9% that of annual SIMA claims. BIMA claims declined during the study period from 0.21 claims per 1,000 Medicare beneficiaries in 2009 to 0.13 in 2014. When indexed to overall CABG volume, there was no change in the frequency of BIMA use over time (p = 0.883).
Fig 1.

Trends in single internal mammary artery (SIMA) and bilateral internal mammary artery (BIMA) claims among Medicare beneficiaries from 2009 to 2014.
Geographic Variation in Arterial Revascularization
Regional variation in rates of SIMA and BIMA grafting are shown in Figure 2 for the aggregate of 2009 to 2014. In this 5-year aggregate, SIMA use ranged from 1.3 to 8.5 claims per 1,000 Medicare beneficiaries, whereas BIMA use ranged from 0 to 1.5, demonstrating significant regional variation across HRR (p < 0.001). Although certain locations in the Southeast and Midwest had relatively high rates of BIMA use compared with the national average, the frequency of BIMA use demonstrated variation even within individual states and was not always consistent with areas that had high SIMA use. The distribution of SIMA and BIMA claims is shown in Figure 3. SIMA cases were relatively evenly distributed across the range of claims, whereas BIMA cases were heavily weighted toward the lower distribution of claims. Although there was a statistically significant correlation between regional volume of SIMA use and regional volume of BIMA use (correlation coefficient 0.673, p < 0.001), the correlation point estimate was moderate.
Fig 2.
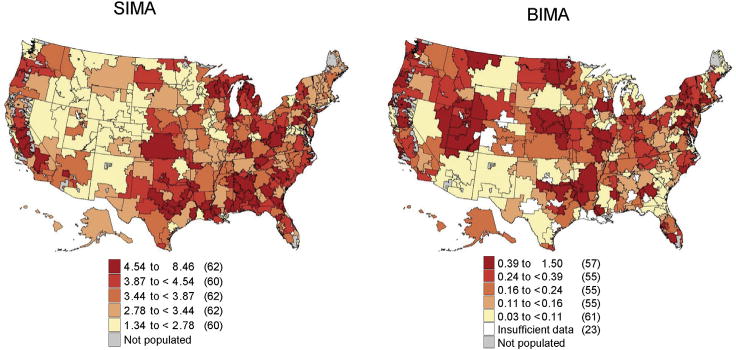
Regional rates of single internal mammary artery (SIMA) and bilateral internal mammary artery (BIMA) claims among Medicare patients aggregated from 2009 to 2014 expressed per 1,000 Medicare beneficiaries.
Fig 3.

Distribution of hospital referral region level of claims of single internal mammary artery (SIMA) and bilateral internal mammary artery (BIMA) utilization aggregated from 2009 to 2014 per 1,000 Medicare beneficiaries.
Association Between Resource Utilization and Revascularization Strategy
To assess the potential association between intensity of health care use and arterial conduit use we examined the correlation between regional variation in two diagnostic cardiovascular procedures: stress tests and cardiac catheterizations. Although both SIMA and BIMA use had a statistically significant correlation with regional volume of diagnostic stress tests, the correlation was stronger for SIMA use (coefficient 0.918 for SIMA versus 0.641 for BIMA, p < 0.001). Likewise, although both SIMA and BIMA use correlated with regional volume of diagnostic cardiac catheterization, the correlation was stronger for SIMA use (coefficient 0.962 for SIMA versus 0.682 for BIMA, p < 0.001).
Comment
In this analysis of more than 160 million Medicare recipients in the United States, we demonstrate that from 2009 to 2014 there has been no significant growth in BIMA grafting, and overall rates of BIMA use remained low.
Most of the literature on outcomes with BIMA grafting comes from retrospective studies and meta-analyses [3, 22, 23]. Overall, most of these studies have demonstrated that BIMA use is associated with improved long-term survival but an increased risk of sternal wound complications [24–26]. ART was designed as the first randomized trial to compare survival between SIMA and BIMA grafting. The primary end point of the trial was death from any cause at 10 years of follow-up [27]. A recent 5-year interim analyses of ART demonstrated no significant difference in mortality rates between groups, with a higher rate of sternal wound complications among BIMA patients [6]. Although ART provides the first randomized controlled data on multi-arterial grafting, it is important to note that the results are an interim analysis and that the survival benefit of BIMA, if present, would likely be observed beyond 5 years when saphenous vein grafts begin to fail at higher rates.
Relevant to the Medicare population studied in this analysis, retrospective analyses have demonstrated survival benefits of BIMA grafting in the elderly [28, 29]. Kurlansky and colleagues [30] recently examined the influence of age on BIMA grafting in a large series with 30 years of follow-up. The investigators observed that among patients age 65 years and older and patients age 70 years and older, there was a survival benefit of BIMA grafting with no adverse effects on rates of morbidity or mortality. Moreover, the observed survival benefit became significant approximately 5 years after operation and persisted throughout the study period. Medalion and colleagues [31] performed a similar analysis of BIMA grafting in the elderly and observed that patients receiving a BIMA who were either 65 years old or younger, between 65 and 75 years, or 75 years or older experienced a survival benefit with BIMA use compared with the age- and sex-matched general population.
Despite the observed benefits of BIMA grafting in the elderly, we demonstrate that from 2009 to 2014 there was no growth in the national frequency of BIMA use among Medicare recipients. Moreover, claims for BIMA grafting were only about 7% of total claims for SIMA grafting.
These results are consistent with a previous analysis of the STS database from 2009 that showed no growth in BIMA use with a national rate of 4.1% [8]. There are several potential explanations for the persistently low frequency of BIMA use we observed. Sternal wound complications remain a notable concern in BIMA grafting, and the recent interim ART results demonstrating twice as many sternal wound complications among BIMA patients will potentially strengthen these concerns. Moreover, surgeons may be awaiting the final, 10-year results of ART before adopting multi-arterial grafting in their practice. If there is a significant survival advantage associated with BIMA, then the potential disadvantages such as increased operative time, added complexity of operation, and increased sternal wound complication rates may be justified. Nevertheless, despite the fact that ART has not reached its primary end point, some surgeons have become early adopters of BIMA use on the basis of continued, positive retrospective data, and this varied adoption of BIMA use is reflected in our analysis.
We demonstrate that across the United States, BIMA use ranged from 0 to 1.5 claims per 1,000 Medicare beneficiaries with considerable regional variation from 2009 to 2014. So why does regional use of BIMA grafting exist? The answer to this question is both complex and multifactorial and will form the basis for ongoing investigations from our group. Nevertheless, we do present some preliminary hypothesis-generating data in this study. Previous studies on geographic variation in cardiovascular care have shown that structural characteristics of hospitals such as size, teaching status, and financial status may drive variation [32]. In our analysis we demonstrate that both regional volume of diagnostic stress testing and diagnostic cardiac catheterization were associated with BIMA use, indicating that intensity of cardiovascular care may, in part, drive BIMA use. Other studies have suggested that aside from hospital-specific factors, surgeon-specific factors such as annual volume and case mix may influence adoption of new techniques [33]. Although our analysis did not study BIMA use on an individual physician level, we do demonstrate that SIMA use correlated moderately with BIMA use. It is possible that practices with a high volume of coronary operation may adopt more complex revascularization techniques such as multi-arterial grafting at higher rates. This adoption may, in turn, have local effects among other surgeons within a practice and then regional effects among other hospitals in a given geographic area. As local experience with BIMA grafting grows and the safety and potential benefits are realized, others in the region may begin adopting the procedure. Evidence for this theory is supported by research in both vascular and orthopedic operations in which high procedure rates were explained by “enthusiasm” for a given procedure among a small number of high-volume surgeons in the region [34, 35]. In our map of geographic variation across the United States, there are states where BIMA use mirrors a large volume of coronary operations; however, there are also areas where BIMA use is low relative to high CABG volume. Therefore, the diffusion of BIMA grating throughout the United States is likely multifactorial, driven in part by CABG volume and intensity of cardiovascular care, but also by unmeasurable variables such as surgeon or group practice beliefs in the benefits of multi-arterial grafting. Further analyses will be necessary to more clearly characterize the causes of regional variation in BIMA grafting, and, if the results of ART are positive, understanding barriers to greater adoption of BIMA use will be important.
Study Limitations
Our analysis has several limitations because we used an administrative database. First, patients in this analysis are Medicare recipients; thus, the results must be understood in the context of patients being 65 years or older. Second, patients were ultimately identified in our analysis on the basis of Part B CPT codes for arterial grafting; thus, with any Medicare analysis there always exists the potential for miscoding of procedures. Finally, this analysis does not contain demographic information on the study population or data on in-hospital deaths or long-term survival. The goal of this study was to examine variation in rates of arterial grafting and not clinical outcomes which has been extensively studied by other investigators. In addition, such information would fall outside the scope of our Dartmouth Atlas data use agreement; thus, this information was not included in the analysis.
Conclusions
Over the past 5 years, there was no growth in BIMA use among Medicare beneficiaries, and the frequency of BIMA use during CABG remained low. Significant regional variation in BIMA use exits, however, which demonstrates opportunity for continued growth of BIMA grafting in the United States and the need for further research to more clearly delineate the factors that influence the decision to perform multi-arterial grafting.
Footnotes
Presented at the Poster Session of the Fifty-third Annual Meeting of The Society of Thoracic Surgeons, Houston, TX, Jan 21–25, 2017.
References
- 1.Lytle BW, Cosgrove DM, Saltus GL, Taylor PC, Loop FD. Multivessel coronary revascularization without saphenous vein: long-term results of bilateral internal mammary artery grafting. Ann Thorac Surg. 1983;36:540–7. doi: 10.1016/s0003-4975(10)60684-4. [DOI] [PubMed] [Google Scholar]
- 2.Davierwala PM, Mohr FW. Bilateral internal mammary artery grafting: rationale and evidence. Int J Surg. 2015;16(Pt B):133–9. doi: 10.1016/j.ijsu.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Locker C, Schaff HV, Dearani JA, et al. Multiple arterial grafts improve late survival of patients undergoing coronary artery bypass graft surgery: analysis of 8622 patients with multivessel disease. Circulation. 2012;126:1023–30. doi: 10.1161/CIRCULATIONAHA.111.084624. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoli G, Schiavon L, Bellini P. Does the use of bilateral internal mammary artery (IMA) grafts provide incremental benefit relative to the use of a single IMA graft? A meta-analysis approach. Eur J Cardiothorac Surg. 2002;22:781–6. doi: 10.1016/s1010-7940(02)00470-0. [DOI] [PubMed] [Google Scholar]
- 5.Puskas JD, Sadiq A, Vassiliades TA, Kilgo PD, Lattouf OM. Bilateral internal thoracic artery grafting is associated with significantly improved long-term survival, even among diabetic patients. Ann Thorac Surg. 2012;94:710–5. doi: 10.1016/j.athoracsur.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 6.Taggart DP, Altman DG, Gray AM, et al. Randomized trial of bilateral versus single internal-thoracic-artery grafts. N Engl J Med. 2016;375:2540–9. doi: 10.1056/NEJMoa1610021. [DOI] [PubMed] [Google Scholar]
- 7.Falk V. Coronary bypass grafting with bilateral internal thoracic arteries. Heart. 2013;99:821. doi: 10.1136/heartjnl-2013-303961. [DOI] [PubMed] [Google Scholar]
- 8.ElBardissi AW, Aranki SF, Sheng S, O’Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–81. doi: 10.1016/j.jtcvs.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Kieser TM. Bilateral internal mammary artery grafting in CABG surgery: an extra 20 minutes for an extra 20 years. EuroIntervention. 2013;9:899–901. doi: 10.4244/EIJV9I8A151. [DOI] [PubMed] [Google Scholar]
- 10.Calafiore AM, Di Mauro M. Bilateral internal mammary artery grafting. Expert Rev Cardiovasc Ther. 2006;4:395–403. doi: 10.1586/14779072.4.3.395. [DOI] [PubMed] [Google Scholar]
- 11.Hegazy YY, Hassanein W, Ennker J, Keshk N, Bauer S, Sodian R. The use of bilateral internal mammary artery grafting in different degrees of obesity. Thorac Cardiovasc Surg. 2017;65:278–85. doi: 10.1055/s-0037-1598028. [DOI] [PubMed] [Google Scholar]
- 12.Ruka E, Dagenais F, Mohammadi S, Chauvette V, Poirier P, Voisine P. Bilateral mammary artery grafting increases postoperative mediastinitis without survival benefit in obese patients. Eur J Cardiothorac Surg. 2016;50:1188–95. doi: 10.1093/ejcts/ezw164. [DOI] [PubMed] [Google Scholar]
- 13.Dai C, Lu Z, Zhu H, Xue S, Lian F. Bilateral internal mammary artery grafting and risk of sternal wound infection: evidence from observational studies. Ann Thorac Surg. 2013;95:1938–45. doi: 10.1016/j.athoracsur.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Aldea GS, Bakaeen FG, Pal J, et al. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann Thorac Surg. 2016;101:801–9. doi: 10.1016/j.athoracsur.2015.09.100. [DOI] [PubMed] [Google Scholar]
- 15.American Medical Association. CPT (Current Procedural Terminology) Available at https://www.ama-assn.org/practice-management/cpt. Accessed June 2, 2016.
- 16.World Health Organization. International Classification of Diseases, Ninth Revision (ICD-9) Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 17.Goodney PP, Lucas FL, Travis LL, Likosky DS, Malenka DJ, Fisher ES. Changes in the use of carotid revascularization among the medicare population. Arch Surg. 2008;143:170–3. doi: 10.1001/archsurg.2007.43. [DOI] [PubMed] [Google Scholar]
- 18.The Dartmouth Institute. Dartmouth Atlas of Healthcare. Available at www.dartmouthatlas.org. Accessed June 8, 2016.
- 19.Huber TS, Seeger JM. Dartmouth Atlas of Vascular Health Care review: impact of hospital volume, surgeon volume, and training on outcome. J Vasc Surg. 2001;34:751–6. doi: 10.1067/mva.2001.116969. [DOI] [PubMed] [Google Scholar]
- 20.Newman L. New Dartmouth Atlas: improving US cardiac care? Lancet. 2000;356:660. doi: 10.1016/S0140-6736(05)73809-5. [DOI] [PubMed] [Google Scholar]
- 21.Cooper MM. The Dartmouth Atlas of Health Care: what is it telling us? Health Syst Rev. 1996;29:44–5. 47. [PubMed] [Google Scholar]
- 22.Stevens LM, Carrier M, Perrault LP, et al. Single versus bilateral internal thoracic artery grafts with concomitant saphenous vein grafts for multivessel coronary artery bypass grafting: effects on mortality and event-free survival. J Thorac Cardiovasc Surg. 2004;127:1408–15. doi: 10.1016/j.jtcvs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Pick AW, Orszulak TA, Anderson BJ, Schaff HV. Single versus bilateral internal mammary artery grafts: 10-year outcome analysis. Ann Thorac Surg. 1997;64:599–605. doi: 10.1016/s0003-4975(97)00620-6. [DOI] [PubMed] [Google Scholar]
- 24.Yi G, Shine B, Rehman SM, Altman DG, Taggart DP. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation. 2014;130:539–45. doi: 10.1161/CIRCULATIONAHA.113.004255. [DOI] [PubMed] [Google Scholar]
- 25.Weiss AJ, Zhao S, Tian DH, Taggart DP, Yan TD. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg. 2013;2:390–400. doi: 10.3978/j.issn.2225-319X.2013.07.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi H, Goto SN, Watanabe T, Mizuno Y, Kawai N, Umemoto T. A meta-analysis of adjusted hazard ratios from 20 observational studies of bilateral versus single internal thoracic artery coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;148:1282–90. doi: 10.1016/j.jtcvs.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Taggart DP, Lees B, Gray A, et al. Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation [ISRCTN46552265] Trials. 2006;7:7. doi: 10.1186/1745-6215-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajimoto K, Yamamoto T, Amano A. Coronary artery bypass revascularization using bilateral internal thoracic arteries in diabetic patients: a systematic review and meta-analysis. Ann Thorac Surg. 2015;99:1097–104. doi: 10.1016/j.athoracsur.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Deo SV, Altarabsheh SE, Shah IK, et al. Are two really always better than one? Results, concerns and controversies in the use of bilateral internal thoracic arteries for coronary artery bypass grafting in the elderly: a systematic review and meta-analysis. Int J Surg. 2015;16(Pt B):163–70. doi: 10.1016/j.ijsu.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Kurlansky PA, Traad EA, Dorman MJ, Galbut DL, Ebra G. Bilateral versus single internal mammary artery grafting in the elderly: long-term survival benefit. Ann Thorac Surg. 2015;100:1374–81. doi: 10.1016/j.athoracsur.2015.04.019. discussion 1381–2. [DOI] [PubMed] [Google Scholar]
- 31.Medalion B, Mohr R, Frid O, et al. Should bilateral internal thoracic artery grafting be used in elderly patients undergoing coronary artery bypass grafting? Circulation. 2013;127:2186–93. doi: 10.1161/CIRCULATIONAHA.112.001330. [DOI] [PubMed] [Google Scholar]
- 32.Nallamothu BK, Rogers MA, Chernew ME, Krumholz HM, Eagle KA, Birkmeyer JD. Opening of specialty cardiac hospitals and use of coronary revascularization in Medicare beneficiaries. JAMA. 2007;297:962–8. doi: 10.1001/jama.297.9.962. [DOI] [PubMed] [Google Scholar]
- 33.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382:1121–9. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chassin MR. Explaining geographic variations. The enthusiasm hypothesis. Med Care. 1993;31(5 Suppl):YS37–44. doi: 10.1097/00005650-199305001-00006. [DOI] [PubMed] [Google Scholar]
- 35.Bederman SS, Coyte PC, Kreder HJ, Mahomed NN, McIsaac WJ, Wright JG. Who’s in the driver’s seat? The influence of patient and physician enthusiasm on regional variation in degenerative lumbar spinal surgery: a population-based study. Spine (Phila Pa 1976) 2011;36:481–9. doi: 10.1097/brs.0b013e3181d25e6f. [DOI] [PubMed] [Google Scholar]


