Abstract
Traditionally, gas chromatography – mass spectrometry (GC/MS) analysis has used a targeted approach called selected ion monitoring (SIM) to quantify specific compounds that may have adverse health effects. Due to method limitations and the constraints of preparing duplicate samples, the information that could be obtained from separately collecting the full scan chromatogram of the sample has often been sacrificed. However, a hybrid technique called synchronous SIM/scan mode that switches back and forth between the two acquisition methods has become available from equipment manufacturers that maintains the accuracy and sensitivity of SIM for targeted analysis while also providing the full scan chromatogram for discovery of non-target compounds. We have explored the value and performance of this new technology using calibration data and real-world breath samples from a joint EPA/NIOSH collaboration that assessed the safety of firefighters’ protective gear during controlled structural burns. Collecting field samples is costly and must be performed strategically to ensure that time points and replicates are accurate and representative of the intended population. This is especially difficult, if not impossible, to accomplish with firefighters who are working under volatile conditions. The synchronous SIM/scan method decreases the number of field samples that need to be collected by half and reduces error in trying to recreate time points since a breath sample from a single sorbent tube can be used to collect both the SIM and scan data simultaneously. This work demonstrates the performance of the technology using calibration data. As a practical demonstration of the method, we investigate thirty-six firefighter breath samples, document organic compounds of interest, and identify additional non-target compounds.
Keywords: Selected ion monitoring/scan (SIM/scan), Gas chromatography-mass spectrometry (GC/MS), Automatic thermal desorption (ATD), Volatile organic compound (VOC), Polyaromatic hydrocarbon (PAH), breath research
1. Introduction
Many environmental and health studies begin with broad investigations of potentially harmful sources, which are followed by more specific methods that focus only on constituents of interest. For example, target screening revealed that of the thousands of compounds in liquid JP-8 jet fuel, four n-alkanes (C9 to C12) constituted the fingerprint of the fuel and that a series of single-ring aromatic compounds (benzene, toluene, naphthalene, etc.) could be used as markers of adverse health outcomes. Subsequently, only a handful of target compounds were analyzed from the breath of Air Force base personnel to efficiently assess exposures [1]. The inverse of this approach has also been implemented. For example, if certain halogenated compounds from water disinfection are suspected to be harmful, then only those compounds are targeted in exhaled breath to document potential exposures [2]. However, what if there are unanticipated sources of contamination in the water, such as methyl tertiary butyl ether (MTBE) or trichloroethylene and vinyl chloride from surface infiltration from spills (e.g., [3,4])? What if an unknown infectious state of the human subject introduced bacterial off-gas products into the sample [5,6])? In these cases, the targeted methods would fail to detect these compounds and products, which could interfere with sample integrity or lead to a false assessment of health state. Therefore, a combination of targeted and non-targeted work could be important both for protecting health and in other applications of clinical practice, such as pulmonary testing and hypoxia, forensic science, and national security [7,8,9]. This has traditionally required separate instrumental methods as described below, and depending upon sampling schemes, could even require additional sample collections.
Targeted and non-targeted analysis
For targeted compound quantification in gas chromatography-mass spectrometry (GC/MS) investigations, the MS acquisition mode called selected ion monitoring (SIM) is used where only a handful of specific analytes are detected at a time. This is the preferred approach because it provides improved sensitivity and selectivity over the less specific option of analyzing all of the compounds using a non-targeted approach. SIM mode is convenient when only a select number of analytes are important to the study, and all other compounds present in a sample can be ignored. This is not a novel concept; in fact, it is fully integrated into the environmental protection agency’s (EPA)’s methods for assessing ambient volatile organic compounds (VOCs) programs described and optimized originally for EPA Compendium Methods TO-14 and TO-15 [10–12]. There are times, however, when we have no preconception as to what may be present in the environment or in biological fluids, and thus a screening approach is appropriate. In GC/MS, this is referred to as scan mode, where the instrument acquires a continuous range of ion fragmentation data to detect all possible compounds within the sample. However, scan mode has traditionally had less sensitivity and specificity compared with SIM mode, making SIM mode the preferred method for targeted analysis of regulated compounds or those
Synchronous SIM/scan analysis
Synchronous SIM/scan mode is a hybrid technique that gathers broad-based data simultaneously into two separate data files with the improved sensitivity and specificity of targeted analysis [13,14]. For discovery analyses, when both targeted and non-targeted components are applicable, synchronous SIM/scan mode can provide a useful way to meet both data quality objectives. While sacrificing a small amount of sensitivity, a full total ion current (TIC) of the sample can be simultaneously obtained along with the SIM chromatogram. This allows for accurate quantification of target ions in the SIM chromatogram while still providing access to the full TIC of the sample to search for and quantify unknown compounds [13,14]. A few previous demonstrations of Agilent’s synchronous SIM/scan mode have been reported. GC/MS with SIM/scan mode has been utilized to identify target and non-target organic contaminants in water samples [15] and tobacco smoke [16]. Thermal desorption-GC/MS (TD-GC/MS) with flame photometric detection in SIM/scan mode has also been used to identify and quantify volatile substances in fumigants [17]. PerkinElmer developed a similar technology known as selected ion full ion (SIFI), which also collects full scan and SIM spectra simultaneously [18]. SIFI has been used in 24 h diffusive air sampling to monitor co-eluting compounds while quantifying select VOCs during TD-GC/MS analysis [19,20].
Firefighters’ exposure study
In this work, synchronous SIM/scan mode was applied to analyze firefighter breath samples collected by the National Institute for Occupational Safety and Health (NIOSH) and analyzed at EPA. The present collaborative study was conducted as a follow-on of previous work [21–23]. Here, we assessed exposure levels of firefighters to VOCs and polyaromatic hydrocarbons (PAHs) from controlled structure burns. Although firefighters wear turnout gear and self-contained breathing apparatus (SCBA) throughout the course of these standard exercises, they are still exposed to low levels of these harmful compounds, presumably through their skin in the neck region where their hoods provide insufficient protection due to the porous nature and movement of the hoods during firefighting activities [21,23–25]. In this study, firefighters were assigned to different firefighting positions to see if the exposure levels varied based upon firefighting duties during the exercise. The focus of the present work is to assess the real-world application of synchronous SIM/scan mode using various calibration standards and breath samples from firefighters; a detailed article interpreting the exposures of firefighters will be prepared in a separate publication.
2. Experimental
2.1 Materials and Chemicals
Carbograph 2TD/1TD dual bed thermal desorption (TD) tubes (catalog no. C2-AXXX-5126) and BIO-VOC samplers were purchased from Markes International (Cincinnati, OH, USA). PAH standards (catalog no. 31011) and the Rxi-5Sil 30 m MS Capillary Column with a 0.25 mm ID, 0.25 μm film thickness and a 5 m Integra Guard column with 0.25 mm ID (part no. 13623-124) were purchased from Restek Corporation (Bellefonte, PA, USA). HPLC grade methanol was purchased from Fisher Scientific (Hampton, NH, USA). Research grade helium gas (99.9999%) and Ultra zero air were supplied by Airgas (Morrisville, NC, USA). The TO-14A 43 Component Mix at 1 ppm in nitrogen was supplied by Linde Electronics & Specialty Gases (Stewartsville, NJ, USA).
2.2 Sample Collection
Sample collection was performed at the University of Illinois Fire Service Institute using a protocol approved by the University of Illinois and NIOSH Institutional Review Boards (IRB) with informed consent of all participants. Additional details about this study, which had a number of specific aims beyond the scope of this paper, are provided in [26]. Exhaled breath samples were collected from firefighters who participated in controlled structure burns conducted by NIOSH using a previously established breath sampling regimen [21]. The study was performed at the Fire Service Institute at the University of Illinois. Three groups of 12 firefighters participated in four controlled burns on different days. The controlled burns mimicked residential settings with modern furniture. Firefighters were assigned to different positions, including command/pump, attack, search, outside vent, overhaul/backup, and overhaul/rapid intervention team (RIT). Firefighters who went inside the structures (e.g., attack and search) were required to wear self-contained breathing apparatus (SCBA), while firefighters participating outside the structure were not required to wear SCBA (e.g., command/pump and outside vent). Firefighters performing overhaul did not always wear SCBA while outside the structure, but did don SCBA before entering the structure after suppression to perform overhaul (i.e., search for smoldering items or residual flames). Breath samples were taken before, directly after, and 1 h post-exposure. Participants exhaled into BIO-VOC samplers, and 129 mL of end-tidal breath was then loaded onto Carbograph 2TD/1TD dual bed TD tubes. The TD tubes were shipped to U.S. EPA on ice packs and stored at 4°C until analysis.
2.3 Standard Preparation and Calibration
Upon receipt from the vendor, the Carbograph 2TD/1TD dual bed TD tubes that were used for calibration were conditioned at 350 °C for 2 h followed by 10 h at 380 °C while purging with 75 ml/min of research grade helium using a TC-20 tube conditioner (Markes International, Cincinnati, OH). The conditioned tubes were then blank checked on the ATD-GC/MS to determine the background levels of VOCs on the tubes. After laboratory analysis, the tubes were conditioned for 2 h at 380 °C using the TC-20 tube conditioner.
PAH standards were prepared using a 16 component Restek Corporation calibration mix. The 2,000 ng/μL PAH standard was diluted in HPLC grade methanol to prepare a set of calibration standards of concentration 0.02, 0.05, 0.10, 0.20, 0.50, 1.0, and 2.0 ng/μL. PAH standards were loaded onto Carbograph 2TD/1TD dual bed tubes using a direct injection method. One μL of methanol was drawn into a 10 μL syringe using an air gap and a solvent plug. Next, 1 μL of the liquid PAH calibration standard was drawn into the syringe. A sorbent tube was placed into the injection port of a flash loading system, which was heated to 127 °C. The PAH solution was injected onto the tube in a steady stream of helium gas at a flow rate of 50 cc/min for 4 min.
After the PAHs were loaded onto the sorbent tubes, the VOCs were then loaded onto the same tubes at matched concentration levels from low to high using a TO-14A 43 Component Mixture at 1 ppm in nitrogen. An Easy VOC syringe sampler was used to deliver a constant volume of 200 mL of gas onto each tube. The range of VOC calibration points was achieved by varying the flow rates of the calibration gas (TO-14A) and the dilution gas (humidified air). Tylan mass flow controllers (Coastal Instruments, Burgaw, NC, USA) were utilized to control the flow rates, and higher flow rates of the TO-14A calibration gas were utilized as the required VOC concentration increased. For each calibration level, the conditions were 50% relative humidity and 20 °C. VOC standards were loaded in concentrations of 0.5, 1.0, 2.0, 5.0, 10.0, 25.0, and 50.0 parts per billion by volume (ppbv). Pure methanol and humidified air without VOCs was loaded onto three sorbent tubes per calibration to serve as a “zero” calibration reference point. Three sorbent tubes were loaded for each calibration point, and seven tubes were loaded with the 0.5 ppbv VOC, 0.02 ng PAH standard in the first calibration curve preparation to determine the method detection limit (MDL). Since the PAHs are less volatile than the VOCs and are well retained by the chosen sorbents, the PAHs were not displaced from the Carbograph 2TD/1TD tubes during loading of the VOCs.
To calibrate the system, two empty glass tube helium blanks were analyzed followed by a lab blank, which consisted of a conditioned Carbograph 2TD/1TD dual bed tube. The calibration standards were then analyzed from low concentration to high, with three replicates per concentration. At the end of the calibration sequence, two daily calibration check standards (10 ppbv VOC, 0.5 ng PAH) were included to assess instrument drift. During sample analysis, lab blanks and daily calibration check standards were included in the sequence every 13 samples to assess instrument drift and performance. When the area counts for the compounds in the daily calibration check standard fell out of the +/− 30% range of the calibration value, the system was recalibrated. In total, three calibrations were performed during the analysis of this sample set.
2.4 ATD-GC/MS Analysis
Breath samples were desorbed using a PerkinElmer (PE) 650 TurboMatrix automated thermal desorption (ATD) system (PerkinElmer LAS, Shelton, CT, USA), and compounds were analyzed in an Agilent 6890N gas chromatograph coupled to an Agilent 5975 inert XL mass spectrometer (GC/MS) (Agilent Technologies, Santa Clara, CA, USA) instrument. Thermal desorption and GC/MS conditions reported in [27] were utilized as a guide to select initial parameters and were optimized for this particular application. A proprietary ion focusing trap was provided by PerkinElmer. Thermal desorption was achieved using a purge time of 5 min, desorption flow rate of 20 mL/min, and desorption time of 15 min. The trap temperature was increased linearly from 10-385 °C with a trap hold of 10 min. The tube temperature was set to 375 °C, and the valve temperature was 270 °C. The column flow was 2 mL/min, the outlet split was set to 6 mL/min, and the inlet split option was not utilized.
A Rxi-5Sil 30 m MS Capillary Column with a 0.25 mm ID, 0.25 μm film thickness and a 5 m Integra Guard column with 0.25 mm ID was utilized for chromatographic separation with helium as the carrier gas. The initial oven temperature was 35 °C for 2 min, 6 °C/min to 190 °C, 28 °C/min to 310 °C with an 8 min hold. The quadrupole, ion source, and transfer line temperatures were held at 176, 290, and 290 °C, respectively. A solvent delay of 1.50 min was used. Ions were monitored from 35–300 m/z. In SIM/scan mode, scan spectra were collected at a rate of 2^2. The PAH and VOC compounds and their target and qualifier ion m/z are listed in Table 1.
Table 1.
Target and Qualifier Ions and Selected Dwell Times for VOCs and PAHs Analyzed in SIM/scan Mode
| Compound | Retention Time (min) | Target Ion (m/z) | Qualifier Ion (m/z) | Target and Qualifier Ion Dwell Times (ms) |
|---|---|---|---|---|
| Benzene | 2.68 | 78.1 | 77.1 | 25 |
| Toluene | 4.28 | 92.1 | 91.1 | 25 |
| Ethylbenzene | 6.39 | 106.1 | 105.1 | 25 |
| m,p-Xylene | 6.65 | 106.1 | 105.1 | 25 |
| Styrene | 7.15 | 104.1 | 103.1 | 25 |
| o-Xylene | 7.19 | 106.1 | 105.1 | 25 |
| 4-Ethyltoluene | 9.08 | 120.1 | 105.1 | 25 |
| 1,3,5-Trimethylbenzene | 9.25 | 120.1 | 105.1 | 25 |
| Naphthalene | 14.77 | 128.0 | 127.0 | 100 |
| Acenaphthylene | 20.75 | 151.9 | 151.0 | 100 |
| Acenaphthene | 21.44 | 154.0 | 153.0 | 100 |
| Fluorene | 23.47 | 166.0 | 165.0 | 100 |
| Phenanthrene | 27.12 | 178.0 | 176.0 | 100 |
| Anthracene | 27.29 | 178.0 | 176.0 | 100 |
| Fluoranthene | 30.17 | 202.1 | 101.0 | 50 |
| Pyrene | 30.49 | 202.1 | 101.0 | 50 |
2.5 Data Analysis
Chromatographic peak integrations were performed using ChemStation software version D.02.00. For all data files pertaining to the three calibrations, target compounds were integrated from both the scan and the SIM chromatograms to obtain raw area counts. Target compounds were integrated from both the scan and SIM chromatograms for a selected subset of 36 samples out of a total of 446 firefighter samples analyzed in this study. The target compounds in the remainder of the firefighter samples were only integrated from the SIM chromatograms for use in subsequent exposure investigations but remain available for further evaluations of the MS techniques. Detailed analytical results of all of the firefighter samples will be published elsewhere. Relevant sample information and parameters were exported from ChemStation into Microsoft Excel for external data processing. The calibration curves were background corrected by subtracting the area counts obtained for the 0 ppbv VOC, 0 ng PAH sample for each compound from each subsequent calibration level. The calibration curves were fit to second-order polynomial regression equations. The regression equations were utilized to calculate the concentration of each VOC based upon the area counts reported by the instrument. These concentration values were then corrected for instrument drift using the weighted average of the calibration check standards analyzed before and after each set of 13 samples analyzed (see Section 2.3 Standard Preparation and Calibration). The concentration values for each compound were then background corrected by subtracting out the average concentration value obtained for the lab blanks (conditioned, analyzed tubes) for that calibration sequence.
3. Results and Discussion
3.1 PAH and VOC Method Development
3.1.1 PAH and VOC Standard Loading onto Carbograph Adsorbent Tubes
While the analysis of VOCs was the main objective of the NIOSH study, we utilized these samples to perform some method development at EPA to see if we could also detect PAHs in the firefighter breath samples. PAHs are products of incomplete combustion, several of which are known to be potentially carcinogenic to humans, and therefore of interest to firefighter exposure [28–29]. As part of this work, a novel procedure for VOC and PAH dual standard preparation and loading was investigated. In this method, PAHs and VOCs were loaded onto the same set of Carbograph 2TD/1TD dual bed tubes to avoid having to use separate tubes for each set of compounds, which decreased the number of tubes required to calibrate the ATD-GC/MS system by half. This calibration method also more closely represents the condition of the samples on the tubes. Since both the VOCs and PAHs are present simultaneously, any issues caused by this potential interference will be present in both the calibration and sample tubes.
This procedure for loading both the PAHs and VOCs onto the same set of Carbograph 2TD/1TD tubes significantly reduced instrument analysis time during calibration. Three TD tubes were loaded per calibration point. For this method, eight calibration points were selected to define the sample range. With three replicates per calibration level, 24 TD tubes were needed for standard loading, which also required approximately 24 h of instrument analysis time. If the PAH and VOC standards had to be loaded separately, then the required number of TD tubes as well as the instrument analysis time would be doubled. This significantly increases the cost of the study, as TD tubes are expensive, and also decreases efficiency because of the significant increase in time. Due to instrument drift, the accuracy of the calibration points analyzed at the beginning of a calibration sequence lasting for more than 48 h is likely to be lower compared with the calibration samples analyzed toward the end of the sequence, which are likely to have much lower area counts, depending on the stability of the individual instrument. For the purposes of our study and the large number of samples, the dual PAH/VOC calibration was an efficient approach.
3.1.2 ATD-GC/MS SIM/scan Method Development
The firefighter samples were analyzed in synchronous SIM/scan mode to allow for discovery analysis of non-target compounds in the samples while maintaining high sensitivity and accuracy for analysis of known compounds of interest to the study. The SIM parameters were optimized using strategies outlined in [12] and [30], as described here. The most abundant fragment ion for each compound was selected as the target ion, and typically the second most abundant ion was chosen as the qualifier ion. The accurate m/z of the target and qualifier ions utilized in the final method was determined by analyzing the standard with target ions ±0.1, 0.2 around the mass centroid of the chosen target ion, and the most abundant accurate ion m/z was utilized in the final method. Each ion was tested with dwell times of 25, 50, 75, and 100 ms, and the dwell time that yielded between 10–20 scans across each chromatographic peak was used for that ion in the final method. Table 1 lists the VOCs and PAHs targeted in the SIM portion of the method along with the retention times, target ion m/z, qualifier ion m/z, and dwell times for each ion.
Shown in Fig. 1 is a screenshot of a TIC of the VOC portion of a 50 ppbv VOC, 2.0 ng PAH standard from the 4/29/16 calibration. Due to the significant differences in the VOC and PAH concentrations, the chromatograms are shown separately at different scales. The top portion of the figure shows the full scan chromatogram while the bottom portion shows the SIM chromatogram. The scan chromatogram contains additional compounds from the Linde 43-component calibration mix that were not included in the target compound list. All of the target VOC compounds were baseline separated during chromatography except for m- and p-xylene, which co-elute at 6.65 min, and styrene and o-xylene, which co-elute at 7.15–7.19 min. Since m- and p-xylene are constitutional isomers, these compounds were analyzed together in this method. Styrene and o-xylene, however, have unique target and qualifier ions, and were therefore analyzed separately using the data collected in SIM mode (See Table 1).
Figure 1.
VOC chromatogram. Close up of the VOC portion of the 50 ppbv VOC, 2.0 ng PAH standard chromatogram from the 4/29/16 calibration. From left to right (SIM): benzene (2.68 min), toluene (4.28 min), ethylbenzene (6.39 min), m,p-xylene (6.65 min), styrene/o-xylene (7.15/7.19 min), 4-ethyltoluene (9.08 min), 1,3,5-trimethylbenzene (9.25 min), and 1,2,4-trimethylbenzene (9.89 min) (non-target compound also isolated in SIM mode).
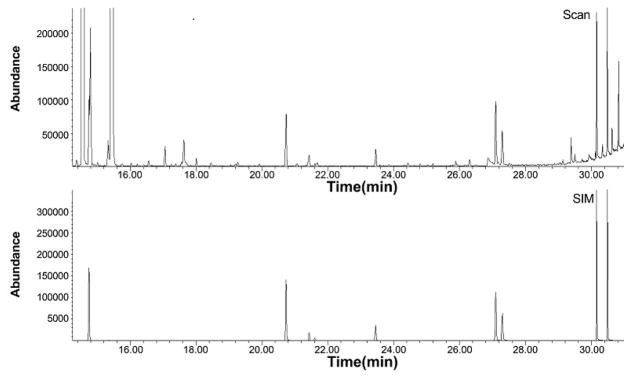
Shown in Fig. 2 is the PAH portion of the 50 ppbv VOC, 2.0 ng PAH standard from the 4/29/16 calibration shown above. The scan chromatogram is shown in the top panel and the SIM chromatogram containing the target compounds is shown in the bottom panel. Some of the latest eluting VOCs from the Linde 43-component calibration mix can be seen in the scan chromatogram around the time that naphthalene elutes at 14.7 min (top portion of figure). The PAHs were all well separated during chromatography. The GC/MS conditions outlined in [31] were used as a starting point to determine optimal temperatures for PAH analysis. Poor desorption of acenaphthene, fluorene, and anthracene from the Carbograph 2TD/1TD tubes was observed in this method.
Figure 2.
PAH chromatogram. Close up of the PAH portion of the 50 ppbv VOC, 2.0 ng PAH standard chromatogram from the 4/29/16 calibration. From left to right (SIM): naphthalene (14.77 min), acenaphthylene (20.75 min), acenaphthene (21.44 min), fluorene (23.47 min), phenanthrene (27.12 min), anthracene (27.29 min), fluoranthene (30.17 min), and pyrene (30.49 min).
The MDL of each compound was determined using the method given in the Code of Federal Regulations (40CFR136 Appendix B). Briefly, seven replicates of the 0.5 ppbv VOC, 0.02 ng PAH standard from the 4/29/16 calibration were analyzed, and the standard deviation of the seven replicates was multiplied by 3.14 (the Student’s t-value for a single-tailed t-test with 99 percent confidence and six degrees of freedom).
3.2 Calibration Curves
The calibration data were fit to second-order polynomial regression equations for the three calibrations performed. The ion signals for the target compounds were integrated from both the SIM and scan chromatograms, and calibration curves from each analysis mode were plotted and compared to one another. For all three calibrations, the VOCs had curves with R2 values greater than or equal to 0.9997 for the calibration curves plotted using both the SIM and scan data (see Table S1).
The PAH calibration curves showed wider variability in the obtained R2 values, which ranged from 0.7177–0.9998 for the SIM curves and from 0.4884–0.9997 for the scan curves. The lower R2 values obtained for the PAH calibration curves were due to poor desorption of the PAHs from the Carbograph 2TD/1TD tubes, which could not be corrected through optimization of the ATD method parameters. This method appears to be better suited to PAHs using a different type of TD tube, such as Tenax (data to be published elsewhere). The expected concentrations of VOCs and PAHs in the firefighter breath samples necessitated selecting calibration ranges for the VOCs and PAHs that were approximately 100-fold different. The VOC amounts loaded onto the Carbograph tubes ranged from 1.6–250 ng, while the PAH amounts ranged from only 0.02–2.0 ng. The lower amounts of the PAHs on the tubes made detection of the low level PAHs challenging, leading to some non-detects for PAHs that do not have a high signal response in GC/MS, such as anthracene. Due to the higher sensitivity and accuracy of SIM mode for quantifying low abundance ions in complex samples, the equations generated using the SIM data are assumed to be the most accurate and representative of the true system response, and these SIM calibration curve equations were utilized to analyze the bulk of the firefighter sample data.
3.3 Difference Plots
Next, the data were evaluated to ensure that they were evenly and tightly distributed around the average calculated values for each calibration point in the calibration curves. Difference plots are a useful technique for visualizing the level of agreement within a data set and to identify potential outliers. Difference plots were created to assess the spread of the data for each target compound across the calibration concentration levels. The percent error was calculated by subtracting the average area counts for each compound from each individual measurement (termed the “Real Value”), dividing by the average value, and multiplying by 100%:
| [Eqn. 1] |
These percentages for each compound were then plotted against the calibration level to which they pertain. The difference plots for the 4/29/16 calibration as calculated from the SIM and scan data for the VOCs can be seen in Figures 3a and 3b. The PAHs were not included in the same difference plots as the VOCs because the compounds had different degrees of spread. Smaller percentages indicate that the replicate measurements at that calibration level were more precise, while larger percentages indicate wider variability in the three replicate measurements during calibration. Unsurprisingly, the most significant spread in the plots can be seen at the lowest calibration level, 0 ppbv VOC. The spread at the lower calibration levels is inflated due to dividing by the lower number of average area counts measured on these tubes in Eqn. 1. Overall, the spread in the percentages does not significantly change across the calibration levels as the concentration increases, indicating that the variability in the method precision is consistent. This implies that the same statistical parameters can be applied to the data at each calibration level without incurring any additional error. While the difference plots constructed using the SIM and scan data show similar spread for the higher calibration levels, some slight differences can be seen in the 0 ppbv level. The scan plots show slightly more visible spread than the SIM plots at the lowest calibration levels, which is likely exacerbated by the low levels of the compounds on the tubes, since the 0 ppbv calibration standard is basically just measuring the sampling tube and system background of the VOCs. While some compounds such as benzene are typically identified with sample peaks, other compounds like 4-ethyltoluene often do not show any signal at the 0 ppbv calibration level and therefore show up as zeroes or with extremely small area counts, which can also increase variability. Difference plots for the SIM and scan results from the 4/29/16 and 5/17/16 calibrations showing similar results can be seen in the Supplemental Information as Supplemental Figs. S1–S2. Difference plots for the PAH data, which showed significant spread compared to the VOC data, can also be found in the Supplemental Information. The most significant PAH outliers were acenaphthene, fluorene, and anthracene, which were often poorly detected due to the desorption issues experienced from the Carbograph 2TD/1TD tubes mentioned previously. Therefore, these three compounds were omitted from the difference plots. Naphthalene, phenanthrene, and fluoranthrene showed much tighter distributions in the SIM and scan plots for all three calibrations while acenaphtyhlene and pyrene generally showed more variability (see Supplemental Figs. S3–S5).
Fig. 3(a) and (b).
Difference plots for the 6/15/16 VOC SIM and Scan Data
The plots show the degree of spread and reproducibility of the three replicate calibration measurements for the VOC SIM and scan calibration data. The percent differences of the three calibration measurements for each compound are plotted versus the calibration level to which they pertain. Lower calibration levels show more variability than higher levels due to the lower amounts of compounds on the tubes. In the scan plot, the 0 ppb calibration level shows more variability than the corresponding SIM level likely due to the lower sensitivity of the analysis method. The calibration levels (1–8) correspond to the following VOC concentrations: 0, 0.5, 1, 2, 5, 10, 25, and 50 ppbv.
3.4 Percent Error between SIM and Scan Calibrations
The percent error between the average area counts measured in SIM and scan at each calibration level was then calculated to determine how much the raw area counts acquired from the peak integrations in scan mode differed from those obtained in SIM mode. Since SIM is considered the more accurate of the two quantitation methods, it was used as the theoretical or known value in the percent error calculation, shown in Eqn. 2:
| [Eqn. 2] |
Where SIMavg is the average of the three replicate measurements of the calibration level quantified in SIM mode and scanavg is the average of the three replicate measurements of the calibration level quantified in scan mode. The percent errors for the 4/29/16, 5/17/16, and 6/15/16 calibrations are included in Supplemental Table S2. The percent errors for the VOCs were typically less than 1% for all compounds, especially at the calibration levels of 2 ppbv and higher. 4-Ethyltoluene showed a higher percent error of 14.5% at the 0.5 ppbv calibration level, indicating that there may have been some interference that led to a higher area count measurement in scan. For the lower concentration levels, SIM is clearly more accurate at quantification. At the higher concentration levels, however, when higher levels of VOCs are present, the area count measurements from the scan peak integrations are almost identical to those from SIM mode.
In general, the PAHs showed higher percent errors than the VOCs, indicating that the accuracy of the area count measurements were significantly affected by quantifying in scan instead of SIM mode. The higher variability in the PAH data is likely explained by the much lower concentrations of these compounds in the calibration standards compared with the VOCs. At each calibration level, there is 100-times more VOCs than PAHs on the calibration tube due to the large difference in abundance of these compounds expected in the firefighter breath samples. Therefore, the PAHs are at trace levels compared to the VOCs and require the extra sensitivity of SIM mode in order to be accurately quantified. For many of the PAHs, especially at the low calibration levels, the area counts acquired in scan are consistently lower than those obtained in SIM mode, indicating that scan mode underestimated the amount of PAHs in these samples.
3.5 Scan versus SIM Plots
Next, to evaluate the linearity of the response in scan versus SIM, the area counts measured in scan for each calibration level were plotted versus the area counts obtained in SIM. The LINEST formula in Excel was utilized to determine the standard deviation of the slope, the standard deviation of the intercept, and the standard error of the regression. These statistical parameters can be found in Table S3. Most VOCs from the three calibrations appeared to have linear responses over the calibration range with R2 values close to 1.0 (0.9999) and low slope standard deviations (10−4 to 10−5, see Table S3). The important thing to note from Table S3 is that most of the slopes are approximately 0.99–1.01. This indicates that analyzing the samples in scan mode did not introduce a systematic error causing the area counts to be significantly over- or underestimated in scan mode compared to SIM mode. Slopes of 1.0 indicate that the area count responses are equivalent in SIM and scan for most compounds in most of the calibrations.
m,p-Xylene and styrene from the 6/15/16 calibration deviated from this observation. Styrene had a slope of only 0.782 and m,p-xylene had a slope of 1.28, indicating that styrene was underestimated in scan while m,p-xylene was over-estimated (see Table S3). These errors in area count measurements can be exacerbated by ion interference in the scan chromatogram. While the PAHs showed linear responses, some of their R2 values were less than 1 (0.85–0.999), and they had higher slope standard deviations than the VOCs (10−3 to 10−1), indicating a higher degree of variability. The higher variability in the PAHs can likely be attributed to their lower concentrations, making them more difficult to detect accurately in scan mode. Some of the PAHs also had slopes less than 1 (anthracene in the 5/17/16 calibration, and acenaphthene and phenanthrene in the 6/15/16 calibration, see Table S3), showing some underestimation of area counts in scan mode. Both the VOCs and PAHs showed high standard errors of regression, indicating low precision and a high degree of scatter in the data. This shows that some accuracy and sensitivity was lost by integrating in scan mode, which was expected. Despite the difference in sensitivity, the lack of curvature in the scan versus SIM plots indicates that the scan data were well correlated with the SIM data without showing concentration-dependent signal dampening.
3.6 Investigation of Real Firefighter Samples
3.6.1 Evaluation of Firefighter Data in SIM and Scan Modes
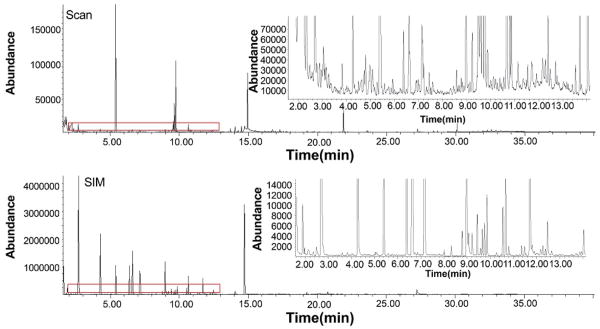
Thirty-six real firefighter samples were analyzed using data collected from both the SIM and scan analyses. These data were collected from three firefighters who were assigned to attack, overhaul/backup, and outside ventilation positions on different days and contributed pre-exposure, post-exposure and 1-hour post-exposure breath samples during each exercise. Subsets of these samples were analyzed under each of the three calibrations previously discussed. An example post-exposure SIM/Scan chromatogram from a firefighter who participated in overhaul/backup is shown in Fig. 4. As seen in Fig. 4, the scan chromatogram contains signal for additional ion peaks that were not included in the SIM target list. A section of the chromatogram was enlarged from 2.0 to 12.0 min to show the difference in signal between scan and SIM mode. While SIM clearly has less noise due to the nature of targeted analysis, the presence of the additional ion peaks in the scan chromatogram also provides additional compounds for discovery analysis that were not included in the target list.
Fig. 4.
SIM and Scan Chromatograms for a Firefighter Sample
Post-exposure 2nd/overhaul firefighter chromatogram from one of the 36 samples analyzed in this study. The scan chromatogram is shown in the top panel and the SIM chromatogram is shown in the bottom panel. Compounds were identified in the scan chromatogram that were not included in the SIM target list. A section of the chromatogram was enlarged from 2.0 to 12.0 min to show the difference in the number peaks between scan and SIM mode. Scan includes additional compounds for discovery analysis but also has noise than SIM due to the lack of product ion mass filters.
The area counts obtained for each target compound in SIM were compared to those calculated in scan. The percent errors for these areas were calculated along with the differences in retention times for the compounds integrated in SIM and scan. The average percent errors for each compound were then calculated using the absolute values of the percent errors for the compounds that were detected in both SIM and scan mode for a given firefighter sample. These values along with the number of non-detects for each compound in SIM and scan mode are listed in Table 2. Most of the VOCs had consistent area counts measured in SIM and scan with percent errors less than 10%, indicating that the analyses of these compounds were not significantly affected by the lower sensitivity of scan mode (see Table 2). However, this was not true for 4-ethyltoluene. In approximately 20% of the 36 samples, the percent error for 4-ethyltoluene between the SIM and scan measurements was 20% or greater. In most of these cases, the area counts for 4-ethyltoluene were higher in scan than in SIM. Unlike most of the other VOCs targeted in this study, 4-ethyltoluene (retention time (RT)=9.08 min) was found to suffer from interference from other non-target compounds, such as benzaldehyde (RT=8.96 min) in the majority of the firefighter sample chromatograms. This interference is most likely leading to high area count measurements for 4-ethyltoluene in many of the scan chromatograms. 4-Ethyltoluene was also not consistently detected in all 36 of the firefighter samples, with 15 non-detects in SIM mode and 19 in scan mode (see Table 2). In cases such as this where compounds co-elute and interference is an issue, evaluating the target ion in SIM is more accurate for quantification.
Table 2.
Average Percent Errors of SIM and Scan Area Counts for Firefighter Samples
| Compound | Average Percent Error (%) | Standard Deviation | Number of Non-Detects (SIM Mode) | Number of Non-Detects (Scan Mode) |
|---|---|---|---|---|
| Benzene | 0.42 | 0.60 | 0 | 0 |
| Toluene | 3.24 | 4.88 | 0 | 0 |
| Ethylbenzene | 2.24 | 6.14 | 1 | 2 |
| m,p-Xylene | 3.74 | 11.80 | 1 | 2 |
| Styrene | 2.25 | 5.94 | 3 | 4 |
| o-Xylene | 2.27 | 6.28 | 1 | 1 |
| 4-Ethyltoluene | 24.14 | 24.27 | 15 | 19 |
| 1,3,5-Trimethylbenzene | 3.88 | 5.38 | 10 | 11 |
| Naphthalene | 6.50 | 13.10 | 4 | 3 |
| Acenaphthylene | 0.01 | 0.02 | 23 | 23 |
| Acenaphthene | 0.00 | - | 35 | 35 |
| Fluorene | 0.57 | 1.35 | 28 | 29 |
| Phenanthrene | 10.94 | 15.86 | 20 | 26 |
| Anthracene | 0.00 | - | 35 | 34 |
| Fluoranthene | 5.37 | 8.29 | 28 | 30 |
| Pyrene | 3.49 | 8.54 | 27 | 30 |
The data for the PAHs are difficult to interpret due to the high number of compound peaks that were not detected in either SIM or scan in the 36 firefighter samples selected (see Table 2). Phenanthrene and naphthalene have higher percent errors, which may also suggest that these compounds suffer from interference. Since the PAHs were present in much lower amounts compared to the VOCs (approximately 100-fold lower), the PAH ion signals were more difficult to distinguish from the background, and therefore the scan measurements showed higher deviation from the SIM values for these compounds. Phenanthrene showed low errors for five firefighter samples but high disagreement for five others, several of which showed no signal in SIM mode. This most likely indicates a false identification of phenanthrene from the scan analysis in the samples that did not have any ion signal in SIM mode. The remaining target PAHs showed no consistency between area count measurements in SIM and scan due to the low levels of these compounds in the firefighter samples and the noise of the scan chromatogram interfering with accurate compound detection.
Despite the low percent errors between the area count measurements for many of the VOCs and PAHs, SIM is more sensitive to the target ion signal than scan mode. Among the 36 real samples analyzed, 23 of the VOCs and PAH compounds were identified only in SIM mode but not in scan, while only eight compounds were identified in scan but not in SIM. Most of the compounds exclusively identified in scan mode had low area counts and therefore may have been false positive identifications. Thus SIM has the advantage of being both more sensitive and accurate than scan. SIM mode also produces peaks with cleaner chromatography than scan. An example of this can be seen in Fig. 5, which depicts the extracted ion chromatograms for 1,3,5-trimethylbenzene (m/z 105.1) collected in SIM and scan. In this figure, the peak for 1,3,5-trimethylbenzene collected in scan is poor quality and appears jagged, while the peak collected in SIM is smooth and symmetrical. This demonstrates that SIM can be advantageous for accurate peak integration and quantification. These data were obtained from a firefighter sample analyzed during the 6/15/16 calibration that did not show any significant differences in percent errors between area counts measured in SIM and scan mode (i.e., the area count measurements for both peaks were about the same).
Fig. 5.
Comparison of SIM and Scan Chromatogram Signal
SIM (left) and scan (right) extracted ion chromatograms for 1,3,5-trimethylbenzene (m/z 105.1) at 9.20 min. 1,3,5-Trimethylbenzene has cleaner peak signal in SIM than in scan despite both peaks having similar area counts. This demonstrates the advantage of still using SIM for quantitative analyses even though scan sometimes yields similar area counts.
3.6.2 Preliminary Investigation of Non-targeted Compounds
The simultaneous acquisition of scan data allowed us to inspect breath samples for compounds in addition to those targeted in SIM mode. Although not the focus of this specific NIOSH investigation of firefighter safety, this capability is useful to determine unsuspected exposures that would be missed by targeted analysis. The 36 selected real-world breath samples were investigated for non-target compounds, and ions of interest from the scan chromatograms that appeared in multiple samples were searched using the NIST library in the ChemStation software. A subset of twelve features that were consistently observed in the scan chromatograms were selected as examples and are shown in Table 3. The probable molecular formula, compound identity, most abundant ion mass to charge ratios, and the retention time of each feature based on the fragment ion pattern of the product ion mass spectra are included in Table 3.
Table 3.
Non-target compounds detected in the scan chromatograms of the firefighter samples.
| Formula | Probable Identity | Ions (m/z) | RT (min) |
|---|---|---|---|
| C5H12O | Methyl tert-butyl ether | 73, 57 | 2.00 |
| C2H6S2 | Dimethyl disulfide | 94, 79 | 3.83 |
| C5H6S | 3-Methyl-thiophene | 97, 98 | 4.52 |
| C8H16 | 3-Methylene heptane | 70, 55 | 4.75 |
| C9H16 | 3,5,5-Trimethylcyclohexene | 68, 109 | 5.76 |
| C7H6O | Benzaldehyde | 105, 77 | 9.00 |
| C6H6O | Phenol | 94, 66 | 9.48 |
| C8H24O4Si4 | Octamethyl-cyclotetrasiloxane | 281, 282 | 9.76 |
| C12H10 | Biphenyl | 153, 154 | 19.25 |
| C12H24O | Dodecanal | 57, 43 | 19.91 |
| S | Sulfur | 192, 64 | 22.01 |
| C19H38 | 1-Nonadecene | 97,83 | 27.36 |
Herein we see additional candidates for future study, both as exogenous markers, and potential endogenous metabolites that may prove important for assessing changing health state or fatigue. Some of the compounds in this table have been previously detected in smoke/fire and combustion sources. For example, benzaldehyde has been found in motor vehicle emissions [32], phenol is a common component of cigarette smoke [33,34]), and dimethyl disulfide has been detected as the major gas emitted during wildfires [35]. Several of these compounds, including sulfur and biphenyl, are also known to originate from fuel sources such as diesel fuel and crude oil [36]. Methyl tert-butyl ether has also commonly been used as an additive to gasoline [37]. Therefore, it is expected that many of these compounds known to exist in smoke and fuel sources would also be detected in firefighter breath samples. These candidate compounds listed in Table 3 could provide valuable information about the exposure levels of individual firefighters that goes beyond the VOC and PAH compounds targeted in this study. While the qu antitation of target VOCs and PAHs may indicate that a significant exposure has occurred, elevation in a non-target compound may show that the individual has been exposed to a different harmful chemical during firefighting. In some cases, the identity of the biomarker is not necessarily as important as its ability to provide consistent exposure information patterns. Discovery analysis, especially with exhaled breath samples, has recently been further developed beyond exogenous compound investigations to capture more constituents of the human exposome and extended to high resolution mass spectrometric methods [38,39].
4. Conclusions
In general, the synchronous SIM/scan procedure is a valuable tool for assessing the character of real-world samples. The comparisons between both modes demonstrate excellent agreement for calibrations and real-world samples. This approach provides robust results for specific compounds of interest as well as an overview of other compounds that might become probative for future study designs. For example, the potential appearance of benzaldehyde, phenol, dimethyl disulfide, sulfur, and biphenyl observed as a function of firefighter activities indicates that these compounds may also be used to document exposures. This additional information comes at no risk to the primary goal of targeted analysis and reduces the effort and cost of field work in that one does not need to collect separate samples to perform discovery analysis. The dual PAH/VOC standard loading technique provides a convenient method to simultaneously load standards onto a single thermal desorption tube. This method can be used to decrease the number of tubes needed for calibration by half, saving both time and decreasing the risk of instrument drift. This method was shown to work well for VOCs and select PAHs.
Supplementary Material
Highlights.
A SIM/Scan method was developed to evaluate target and non-target compounds.
Calibrated the SIM/scan method for eight VOC and eight PAH standards.
Utilized a new dual PAH/VOC standard loading method onto thermal desorption tubes.
Thirty-six firefighter samples were analyzed in SIM/scan mode.
Non-target compounds of interest to exposure were discovered in the scan chromatograms.
Acknowledgments
The authors are appreciative for the participation of the volunteer subjects who provided breath samples for this method development and evaluation. Participants were compensated up to $599 to participate in this study. This study was approved by the NIOSH and University of Illinois Institutional Review Boards. This study was funded by the U.S. Department of Homeland Security, Assistance to Firefighters Grant (EMW-2013-FP-00766) and also made possible through a partnership with the CDC Foundation. The authors also acknowledge Lee Marotta from Perkin Elmer for technical assistance with the PAH method development and John-Michael Marquardt from Perkin Elmer for instrument support. This research has been subjected to EPA review and approved for publication. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of NIOSH. Mention of trade names and commercial products does not constitute endorsement or recommendation for use.
References
- 1.Pleil JD, Smith LB, Zelnick SD. Personal exposure to JP-8 jet fuel and exhaust at Air Force bases. Environ Health Perspect. 2000;108(3):183–192. doi: 10.1289/ehp.00108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleil JD, Lindstrom AB. Exhaled human breath measurement for assessing exposure to halogenated volatile organic compounds. Clin Chem. 1997;43(5):723–730. [PubMed] [Google Scholar]
- 3.Lindstrom AB, Pleil JD. A Methodological Approach for Exposure Assessment Studies in Residences using Volatile Organic Compound Contaminated Water. J Air Waste Manag Assoc. 1996a;46:1058–1066. [PubMed] [Google Scholar]
- 4.Lindstrom AB, Pleil JD. Alveolar Breath Sampling and Analysis to Assess Exposures to Methyl Tertiary Butyl Ether (MTBE) During Motor Vehicle Refueling. J Air Waste Manag Assoc. 1996b;46:676–682. doi: 10.1080/10473289.1996.10467502. [DOI] [PubMed] [Google Scholar]
- 5.Pleil JD, Miekisch W, Beauchamp JD, Funk WE. Adapting biomarker technologies to adverse outcome pathways (AOPs) research: Current thoughts on using in vivo discovery for developing in vitro target methods. J Breath Res. 2015;9:039001. doi: 10.1088/1752-7155/9/3/039001. [DOI] [PubMed] [Google Scholar]
- 6.Leja M, Amal H, Lasina I, Skapars R, Sivins A, Ancans G, Tolmanis I, Vanags A, Kupcinskas J, Ramonaite R, Khatib S. Analysis of the effects of microbiome-related confounding factors on the reproducibility of the volatolomic test. J Breath Res. 2016;10(3):037101. doi: 10.1088/1752-7155/10/3/037101. [DOI] [PubMed] [Google Scholar]
- 7.Brodrick E, Davies A, Neill P, Hanna L, Williams EM. Breath analysis: Translation into clinical practice. J Breath Res. 2015;9(2):027109. doi: 10.1088/1752-7155/9/2/027109. [DOI] [PubMed] [Google Scholar]
- 8.Harshman SW, Geier BA, Fan M, Rinehardt S, Watts BS, Drummond LA, Preti G, Phillips JB, Ott DK, Grigsby CC. The identification of hypoxia biomarkers from exhaled breath under normobaric conditions. Journal of Breath Research. 2015;9(4):047103. doi: 10.1088/1752-7155/9/4/047103. [DOI] [PubMed] [Google Scholar]
- 9.Pleil JD, Risby T, Herbig J. Breath biomonitoring in national security assessment, forensic THC testing, biomedical technology and quality assurance applications: report from PittCon 2016. J Breath Res. 2016;10(2):029001. doi: 10.1088/1752-7155/10/2/029001. [DOI] [PubMed] [Google Scholar]
- 10.US Environmental Protection Agency. US EPA Compendium Method TO-14A, Determination of Volatile Organic Compounds (VOCs) in Ambient Air using Specially Prepared Canisters with Subsequent Analysis by Gas Chromatography, EPA/625/R-96/010b. Center for Environmental Research Information, Office of Research and Development; Cincinnati, OH: Jan, 1999. [Google Scholar]
- 11.US Environmental Protection Agency. US EPA Compendium Method TO-15, Determination of Volatile Organic Compounds (VOCs) in Air Collected In Specially-Prepared Canisters And Analyzed By Gas Chromatography/Mass Spectrometry (GC/MS), EPA/625/R-96/010b. Center for Environmental Research Information, Office of Research and Development; Cincinnati, OH: Jan, 1999. [Google Scholar]
- 12.Pleil JD, Vossler TL, McClenny WA, Oliver KD. Optimizing sensitivity of SIM mode of GC/MS analysis for EPA’s TO-14 air toxics method. J Air & Waste Manag Assoc. 1991;41(3):287–93. [Google Scholar]
- 13.Meng C-K. [accessed Mar. 2017];Improving Productivity with Synchronous SIM/Scan, Agilent Technical Note, 5989-3108EN. 2005 May; http://www.chem.agilent.com/temp/radCD7E8/00052484.pdf.
- 14. [accessed Sept. 2016];Strategies for Developing Optimal Synchronous SIM-Scan Acquisition Methods-AutoSIM/Scan Setup and Rapid SIM, Agilent Technical Note, 5989-5669EN. 2006 Sep; https://www.agilent.com/cs/library/technicaloverviews/Public/5989-5669EN.pdf.
- 15.Gómez MJ, Gómez-Ramos MM, Agüera A, Mezcua M, Herrera S, Fernández-Alba AR. A new gas chromatography/mass spectrometry method for the simultaneous analysis of target and non-target organic contaminants in waters. J Chromatogr A. 2009;1216(18):4071–82. doi: 10.1016/j.chroma.2009.02.085. [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Yang S, Chen G, Wu Y, Hou Y, Xu G. Simultaneous determination of non-volatile, semi-volatile and volatile organic acids in tobacco by SIM–Scan mode GC–MS. J Sep Sci. 2008;31(4):721–6. doi: 10.1002/jssc.200700318. [DOI] [PubMed] [Google Scholar]
- 17.Fahrenholtz S, Hühnerfuss H, Baur X, Budnik LT. Determination of phosphine and other fumigants in air samples by thermal desorption and 2D heart-cutting gas chromatography with synchronous SIM/Scan mass spectrometry and flame photometric detection. J Chromatogr A. 2010;1217:8298–8307. doi: 10.1016/j.chroma.2010.10.085. [DOI] [PubMed] [Google Scholar]
- 18.PerkinElmer. [Date accessed: March 2017];SIFI – Simultaneous Collection of Full Scan and Selected Ion Recording Mass Spectral Data. [ https://www.perkinelmer.com/Content/TechnicalInfo/TCH_SIFIGCMS.pdf]
- 19.McClenny WA, Oliver KD, Jacumin HH, Daughtrey EH, Whitaker DA. 24 h diffusive sampling of toxic VOCs in air onto Carbopack X solid adsorbent followed by thermal desorption/GC/MS analysis– laboratory studies. J Environ Monit. 2005;7:248–256. doi: 10.1039/b412213e. [DOI] [PubMed] [Google Scholar]
- 20.McClenny WA, Jacumin HH, Oliver KD, Daughtrey EH, Whitaker DA. Comparison of 24 h averaged VOC monitoring results for residential indoor and outdoor air using Carbopack X-filled diffusive samplers and active sampling – a pilot study. J Environ Monit. 2006;8:263–269. doi: 10.1039/b507850d. [DOI] [PubMed] [Google Scholar]
- 21.Fent KW, Eisenberg J, Evans D, Sammons D, Robertson S, Striley C, Snawder J, Mueller C, Kochenderfer V, Pleil J, Stiegel M, Horn GP, editors. NIOSH. Health hazard evaluation report: evaluation of dermal exposure to polycyclic aromatic hydrocarbons in fire fighters. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2013. NIOSH HETA Report No. 2010-0156-3196. [Google Scholar]
- 22.Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, Mueller C, Horn GP, Dalton J. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. Ann Occup Hyg. 2014;58(7):830–45. doi: 10.1093/annhyg/meu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fent KW, Evans DE, Booher D, Pleil JD, Stiegel MA, Horn GP, Dalton J. Volatile Organic Comp\ounds Off-gassing from Firefighters’ Personal Protective Equipment Ensembles after Use. J Occup Environ Hyg. 2015;12(6):404–414. doi: 10.1080/15459624.2015.1025135. [DOI] [PubMed] [Google Scholar]
- 24.Baxter CS, Hoffman JD, Knipp MJ, Reponen T, Haynes EN. Exposure of Firefighters to Particulates and Polycyclic Aromatic Hydrocarbons. J Occup Environ Hyg. 2014;11:D85–D91. doi: 10.1080/15459624.2014.890286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pleil JD, Stiegel MA, Fent KW. Exploratory breath analyses for assessing toxic dermal exposures of firefighters during suppression of structural burns. J Breath Res. 2014;8:037107. doi: 10.1088/1752-7155/8/3/037107. [DOI] [PubMed] [Google Scholar]
- 26.Fent KW, Alexander B, Roberts J, Robertson S, Toennis C, Sammons D, Bertke S, Kerber S, Smith S, Horn G. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. 2017 doi: 10.1080/15459624.2017.1334904. Submitted. [DOI] [PubMed] [Google Scholar]
- 27.Provost R, Marotta L, Thomas R. A Single-Method Approach for the Analysis of Volatile and Semivolatile Organic Compounds in Air Using Thermal Desorption Coupled with GC-MS. LCGC N Am. 2014;27(17):624–631. [Google Scholar]
- 28.IARC. Monographs on the evaluation of the carcinogenic risks to humans: naphthalene. Vol. 82. Lyon, France: World Health Organization, International Agency for Research on Cancer; 2002. [Date accessed: March 2017]. [ http://monographs.iarc.fr/ENG/Monographs/vol82/mono82-8.pdf] [Google Scholar]
- 29.IARC. Monographs on the evaluation of the carcinogenic risks to humans: some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. Vol. 92. Lyon, France: World Health Organization, International Agency for Research on Cancer; 2010. [Date accessed: March 2017]. [ http://monographs.iarc.fr/ENG/Monographs/vol92/mono92.pdf] [Google Scholar]
- 30. [accessed Mar. 2017];Setting up a SIM Acquisition Method MS ChemStation, Agilent Technical Note. :a05042. http://www.agilent.com/cs/library/support/documents/a05042.pdf.
- 31.Szelewski M. [accessed Mar. 2017];Synchronous SIM/Scan Low-Level PAH Analysis Using the Agilent Technologies 6890/5975 inert GC/MSD, Agilent Technical Note, 5989-4184EN. 2005 Nov; http://www.cqiie.com/uploads/soft/2009/091013/20091013_034225_00.pdf.
- 32.Grosjean D, Grosjean E. Airborne carbonyls from motor vehicle emissions in two highway tunnels. Res Rep Health Eff Inst. 2002;107:57–78. [PubMed] [Google Scholar]
- 33.Smith CJ, Perfetti TA, Morton MJ, Rodgman A, Garg R, Selassie CD, Hansch C. The Relative Toxicity of Subsitituted Phenols Reported in Cigarette Mainstream Smoke. Toxicol Sci. 2002;69(1):265–278. doi: 10.1093/toxsci/69.1.265. [DOI] [PubMed] [Google Scholar]
- 34.Spears AW. Quantitative Determination of Phenol in Cigarette Smoke. Anal Chem. 1963;35(3):320–322. [Google Scholar]
- 35.Meinardi S, Simpson IJ, Blake NJ, Blake DR, Sherwood Rowland F. Dimethyl Disulfide (DMDS) and Dimethyl sulfide (DMS) emissions from biomass burning in Australia. Geophys Res Lett. 2003;30(9):1–7. [Google Scholar]
- 36.Adams NG, Richardson DM. Isolation and Identification of Biphenyls fro West Edmond Crude Oil. Anal Chem. 1953;25(7):1073–1074. [Google Scholar]
- 37.Achten C, Püttmann W. Method for determination of methyl tert-butyl ether in gasoline by gas chromatography. J Chromatogr A. 2001;910(2):377–383. doi: 10.1016/s0021-9673(00)01220-6. [DOI] [PubMed] [Google Scholar]
- 38.Pleil JD, Stiegel MA. The evolution of environmental exposure science: Using breath-borne biomarkers for “discovery” of the human exposome. Anal Chem. 2013;85:9985–9990. doi: 10.1021/ac402306f. [DOI] [PubMed] [Google Scholar]
- 39.Pleil JD, Isaacs KK. High-resolution mass spectrometry: basic principles for using exact mass and mass defect for discovery analysis of organic molecules in blood, breath, urine and environmental media. J Breath Res. 2016;10(1):012001. doi: 10.1088/1752-7155/10/1/012001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.