Abstract
Human cytomegalovirus (hCMV) is a ubiquitous pathogen that causes congenital infection and severe infections in immunocompromised patients. Chronic hCMV infection may also play an important role in immunosenescence and adverse health outcomes in older adults. THP-1, a human monocytic cell line and its derived macrophages serve as a useful cell culture model for mechanistic studies of hCMV infection and its underlying biology. A major methodological challenge is the lack of a quick and reliable tool to accurately determine the efficiency of hCMV infection in THP-1 derived macrophages. In this study, we developed a flow cytometry based method using commercially available monoclonal antibody (MAb) against hCMV immediate early (IE) antigen that can accurately determine infection efficiency. We used 0.5% formaldehyde for fixation, 90% methanol for permeabilization, and incubation with FITC conjugated MAb at 37 °C. The method was tested by hCMV infection with laboratory Towne strain in the presence or absence of hydrocortisone. It was also compared with the routine flow cytometry protocol using Cytofix/Cytoperm solution and with immunofluorescence. The results indicate that this new method is reliable and time saving for accurate determination of infection efficiency. It may facilitate further investigations into the underlying biological mechanisms of hCMV infection.
Keywords: hCMV, Infection efficiency, THP-1, Flow cytometry
Human cytomegalovirus (hCMV) is a ubiquitous pathogen that causes congenital infection (Azam et al., 2001; Lazzarotto et al., 2000) as well as severe and disseminated infections in immuno-compromised patients(Deeks et al., 2012; Rowshani et al., 2005). In immunocompetent individuals, hCMV can establish a latent or persistent infection with subsequent reactivations, leading to clinically important consequences (Smith et al., 2004; Wreghitt et al., 2003; Osawa and Singh, 2009). For example, chronic hCMV infection has been implicated in T-cell clonal expansion, immunosenescence, and adverse health outcomes in older adults (Pawelec et al., 2005; Koch et al., 2007; Leng, 2011; Schmaltz et al., 2005; Wang et al., 2010; Aiello et al., 2008). Reservoir for hCMV in persistent infection appears to be cells of the myeloid lineage, particularly peripheral blood monocytes which are easily accessible for evaluation (Taylor-Wiedeman et al., 1991; Reeves and Sinclair, 2008; Soderberg-Naucler et al., 1997). In fact, our recent studies in older adults have shown that presence of hCMV viral DNA in peripheral blood monocytes as detected by nested PCR is a better diagnostic marker of chronic/persistent CMV infection than positive anti-CMV IgG serology in terms of expansion of CMV-specific CD8+ T cells detected via Class I tetramer analysis (Leng et al., 2011a), immune activation as marked by elevated neopterin levels (Leng et al., 2011b), and chronic inflammation by elevated IL-6 levels (Li et al., 2014).
As isolated human peripheral blood monocytes are available only in limited quantity and short-lived in culture, THP-1, a human monocytic cell line and its derived macrophages have become a useful cell culture model for mechanistic studies of hCMV infection and its underlying biology (Weinshenker et al., 1988; Lee et al., 1999; Ioudinkova et al., 2006; Sanchez et al., 2012). For example, a number of studies have used THP-1 derived macrophages to investigate not only hCMV viral entry, replication, reactivation, and gene expression, but also the impact of hCMV on function and regulation of host cells such as lipid metabolism, apoptosis and cytokine production (Ioudinkova et al., 2006; Yew et al., 2010; Sanchez and Spector, 2006; Sanchez and Dong, 2010; Moon et al., 2003; Murayama et al., 1997). A major methodological challenge, however, is the lack of a quick and reliable tool for accurate determination of infection efficiency. This is particularly important in THP-1 derived macrophages as CMV infection efficiency is typically low and highly variable in these cells, often requiring additional agents such as hydrocortisone to improve infection efficiency (Lee et al., 1999; Sanchez et al., 2012). Many previous studies have employed immunofluorescence (IF) for this purpose (Lee et al., 1999; Ioudinkova et al., 2006; Sanchez et al., 2012; Fu et al., 2014; Van et al., 2015). However, IF is technically cumbersome and time consuming. The objective of this study was to develop a novel flow cytometry-based tool for accurate determination of the efficiency of hCMV infection in THP-1 derived macrophages.
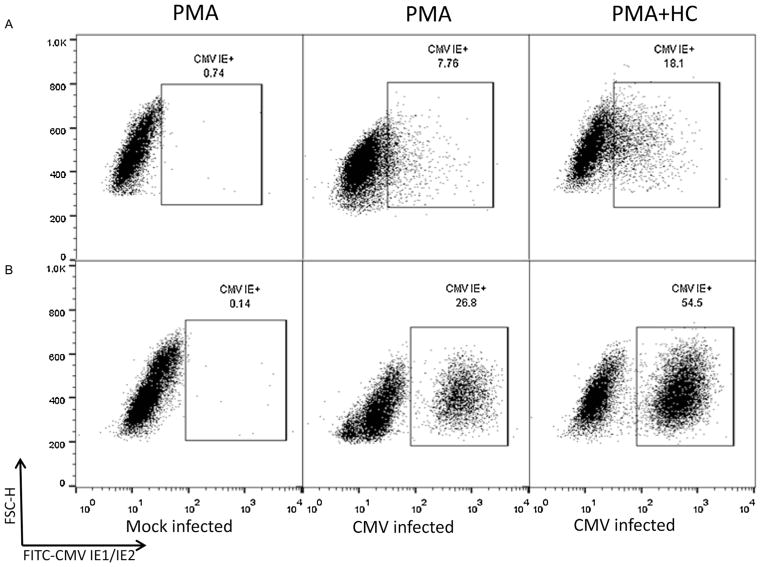
Laboratory hCMV strain Towne (ATCC; VR 977) was routinely propagated in MRC-5 cells. To prepare THP-1 derived macrophages, THP-1 cells (ATCC) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, glutamine, penicillin and streptomycin (completed medium). For flow cytometry analyses, cells were counted, seeded into 48-well tissue culture plates at 2 × 105, and differentiated by incubation with phorbolmyristate acetate (PMA; EMD/Millipore, St. Charles, MO) at a concentration of 50 ng/mL for 24 h before infection. To assess the effect of hydrocortisone (HC) on CMV infection efficiency in these cells, a separate set of culture was prepared in the presence of PMA (50 ng/mL) and HC (5 mM; Sigma, St Louis, MO) as previously reported (Sanchez et al., 2012). Adherent cells in both culture sets were infected with hCMV Towne at a multiplicity of infection (MOI) of 2 in the presence of PMA or PMA plus HC for 24 h, after which virus inoculum was removed and cultures were fed with either complete medium alone or complete medium containing PMA and HC for additional 24 h, respectively. Cells were detached by accutase (Sigma) for 10 min, washed with Dulbecco’s phosphate buffered saline (DPBS), and then processed under either of the following two conditions: (1) cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA) at 4 °C for 20 min, washed twice with Perm/Wash buffer (BD Biosciences), and incubated at 4 °C for 30 min with a fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibody (MAb) against hCMV immediately early (IE) antigens (FITC-CMV IE1/IE2 MAb, clone 8B1.2, EMD/Millipore, Billerica, MA). After staining, cells were washed twice with Perm/Wash buffer, resuspended in 1% paraformaldehyde in DPBS, and stored at 4 °C until analysis; or (2) cells were fixed in 0.5% formaldehyde in DPBS at 37 °C for 10 min, kept at 4 °C for another 10 min. Cells were pelleted at 500 × g for 10 min, resuspended and permeabilized in DPBS with 90% methanol on ice for 10 min; washed with FACS buffer (0.5% BAS, 0.01% sodium azide in DPBS) twice, and incubated with the same FITC-CMV IE1/IE2 MAb as above at 37 °C for 90 min. After staining, cells were washed twice with FACS buffer, resuspended in 1% paraformaldehyde in DPBS, and stored at 4 °C until analysis. Matched mouse MAb isotype control (clone GC270, EMD/Millipore) and cells with no hCMV infection (mock control) were used to exclude nonspecific binding. Samples were analyzed on a Calibur flow Cytometer and CellQuest software (BD Biosciences). Analyses were gated on live cells using forward and side scatter. The data obtained were analyzed using FlowJo software (Tree-Star Inc., Ashland, OR). Fig. 1 shows the results comparing these two methods. As shown in Fig. 1A, the routine protocol with Cytofix/Cytoperm solution and Perm/Wash buffer from BD Biosciences did not distinguish hCMV-infected cells expressing IE antigen from uninfected cells in the presence of PMA (“PMA”) or PMA plus HC (“PMA + HC”). The new method using 0.5% formaldehyde for fixation, 90% methanol for permeabilization, and incubation with FITC conjugated MAb at 37 °C, however, clearly distinguished the two cell populations under both “PMA” and “PMA + HC” conditions (Fig. 1B). Mock-infected control showed minimal background staining with both methods. Consistent with previously reported data, hCMV infection efficiency in the presence of PMA plus HC (“PMA + HC”) was close to 60% and was higher than that (20–40%) obtained without HC (“PMA”).
Fig. 1.
THP-1 cells were treated with PMA (“PMA”) or PMA plus hydrocortisone (“PMA + HC”) and mock infected or hCMV (Towne strain) infected as labeled. Cell were then fixed, permeabilized and stained for the expression of hCMV IE antigen using either the routine method with Cytofix/Cytoperm solution (panels in row (A)) or the new method using 0.5% formaldehyde and 90% methanol (panels in row (B)).
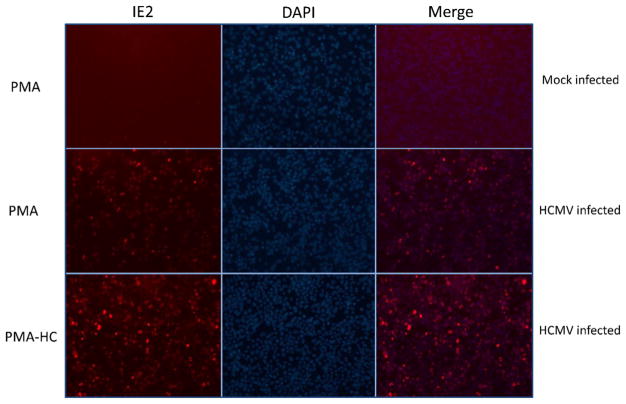
To compare the new method with IF, THP-1 cells were seeded at the density of 2 × 105 onto 8-well chamber slide (Lab-Tek II, Nalge Nunc, Thermo Fisher Scientific Inc., Waltham, MA). Induction of differentiation by PMA with and without HC as well as infection of hCMV Towne strain was carried out in the same way as described above. Twenty-four hours post-infection, cells were washed with DPBS and fixed in 4% paraformaldehyde in DPBS for 20 min at room temperature, permeabilized with cold methanol for 5 min, washed with DPBS twice, and blocked with diluted normal horse serum. Cells were incubated with mouse anti-hCMV IE2 MAb (clone 5A8.2, EMD/Millipore) for 1 h containing 0.1% immunohistochemical grade BSA. Cells were washed with DPBS twice and incubated with secondary antibody Alexa Flor 350 Donkey anti-mouse IgG (Life Technologies, Grand Island, NY) for 1 h, then cells were washed with DPBS twice, mounted with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), and visualized using a Nickon Elipse Ni-U microscope with Intensilight C-HGFIE. Images were captured by epifluorescentor imaging technique. For measurement of the number of cells expressing IE antigen, 8–10 images were recorded per cover slip and approximately 100 cells were counted per experimental condition. Fig. 2 demonstrates results from a representative of three experiments. hCMV infection efficiency was obtained from the Merge images (double staining with DAPI and anti-hCMV IE2 Alexa Flor 350) of live cells expressing hCMV IE antigen and estimated to be approximately 20% under the experimental condition of “PMA” and estimated 50% under that of “PMA + HC”. Mock-infected control showed minimal background staining under both “PMA” and “PMA + HC” conditions.
Fig. 2.
THP-1 cells were treated with PMA (“PMA”) or PMA plus hydrocortisone (“PMA + HC”) and then mock infected or hCMV (Towne strain) infected as labeled in rows. In columns, “IE2” indicates staining with primary mouse anti-hCMV IE antigen (IE2) MAb followed by secondary antibody with Alexa Flor 350; “DAPI” indicates live cells; “Merge” indicates live cells expressing hCMV IE antigen that were positive for both DAPI and IE2 staining.
To the best of our knowledge, this is the first time that a flow cytometry-based method was utilized for accurate determination of hCMV infection efficiency in THP-1 derived macrophages. While a FITC conjugated MAb against hCMV IE antigen is commercially available, the conventional protocol with Cytofix/Cytoperm solution and Perm/Wash buffer from BD Biosciences, which is routinely employed in most flow cytometry laboratories, is unable to distinguish hCMV-infected and uninfected cell populations (Fig. 1A, personal communication with Sanchez). The new method using 0.5% formaldehyde for fixation, 90% methanol for permeabilization, and incubation with FITC conjugated MAb at 37 °C likely improves permeabilization and intracellular staining of hCMV-infected cells and, thus, can clearly distinguish between hCMV-infected and uninfected cell populations. Lee et al. (2005) reported using flow cytometry analysis with the same MAb for the determination of resistance of hCMV to ganciclovir. However, human foreskin fibroblasts were used in their analysis and hCMV-infected and uninfected cell populations were not clearly separated. While similar results were obtained using IF, the new method is less time consuming and gives more accurate determination of infection efficiency. Building on the versatility of multi-channel flow cytometry, this new tool can also be used for further investigations into potential impact of hCMV infection on phenotype, function and regulation of THP-1 derived macrophages.
In addition to IF, immunoperoxidase staining assay was employed in earlier studies (Weinshenker et al., 1988; Turtinen et al., 1989). However, this assay yielded inconsistent results in our hands due to high level of background staining (data not shown). Another technical difficulty for both IF and immunoperoxidase staining assay is that CMV-infected cells were disproportionately removed from glass coverslips during wash steps. Detection of hCMV viral DNA either in cellular DNA or culture supernatants by polymerase chain reaction (PCR) was also utilized in a number of studies for assessing hCMV infection in THP-1 derived macrophages (Sanchez et al., 2012; Fu et al., 2014; Turtinen and Seufzer, 1994; Jayarama et al., 2006). However, PCR-based assay cannot determine infection efficiency and positive result in cellular DNA sample could be due to hCMV virus deposited to the surface of THP-1 cells rather than true infection (Beisser et al., 2001). More recently, various hCMV strains have been cloned as bacterial artificial chromosomes (BAC) and engineered to express the green fluorescent protein (GFP) so that GFP can be used as a reporter and cells infected by these engineered CMV mutants can be identified by flow cytometry (Van et al., 2015; Murphy et al., 2003; Chan et al., 2009). BAC derived hCMV strains represent another method that is broadly used to study the biology of these viruses. We used hCMV laboratory strain Towne in this study for assay development. It is hoped that our new method can be used to determine infection efficiency of other hCMV strains without requiring BAC clones.
In conclusion, this time saving flow cytometry-based tool offers significant advantages over IF and other existing methods for the determination of hCMV infection efficiency in THP-1 derived macrophages. It employs commercially available FITC conjugated MAb against hCMV IE antigen and clearly separates hCMV-infected and uninfected cell populations. Since virtually every cell infected by hCMV expresses IE antigen, this method offers high sensitivity and accuracy. It is hoped that it will serve as an invaluable tool for rapid determination of hCMV infection efficiency and facilitate further investigations into the underlying biological mechanisms of hCMV infection.
Acknowledgments
This work is supported in part by NIH grants R21-AG-043874 (PI: Dr. Sean X. Leng), R01-AI-108907 (PI: Dr. Sean X. Leng), and funding from the Irma and Paul Milstein Program for Senior Health of the Milstein Medical Asian American Partnership (MMAAP) Foundation (www.mmaapf.org) (to Dr. Sean X. Leng).
Footnotes
Conflict of interest
No pertinent conflict of interest.
References
- Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A: Biol Sci Med Sci. 2008;63:610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam AZ, Vial Y, Fawer CL, Zufferey J, Hohlfeld P. Prenatal diagnosis of congenital cytomegalovirus infection. Obstet Gynecol. 2001;97:443–448. doi: 10.1016/s0029-7844(00)01140-6. [DOI] [PubMed] [Google Scholar]
- Beisser PS, Laurent L, Virelizier JL, Michelson S. Human cytomegalovirus chemokine receptor gene US28 is transcribed in latently infected THP-1 monocytes. J Virol. 2001;75:5949–5957. doi: 10.1128/JVI.75.13.5949-5957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Yurochko AD. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A. 2009;106:22369–22374. doi: 10.1073/pnas.0908787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24:501–506. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Fu M, Gao Y, Zhou Q, et al. Human cytomegalovirus latent infection alters the expression of cellular and viral microRNA. Gene. 2014;536:272–278. doi: 10.1016/j.gene.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Ioudinkova E, Arcangeletti MC, Rynditch A, et al. Control of human cytomegalovirus gene expression by differential histone modifications during lytic and latent infection of a monocytic cell line. Gene. 2006;384:120–128. doi: 10.1016/j.gene.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Jayarama V, Marcello J, Ohagen A, et al. Development of models and detection methods for different forms of cytomegalovirus for the evaluation of viral inactivation agents. Transfusion. 2006;46:1580–1588. doi: 10.1111/j.1537-2995.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Ozcelik D, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini MP. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr. 2000;137:90–95. doi: 10.1067/mpd.2000.107110. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lee GC, Chan YJ, Chiou CJ, Ahn JH, Hayward GS. Factors affecting human cytomegalovirus gene expression in human monocyte cell lines. Mol Cells. 1999;9:37–44. [PubMed] [Google Scholar]
- Lee GC, Lee DG, Choi SM, et al. Use of time-saving flow cytometry for rapid determination of resistance of human cytomegalovirus to ganciclovir. J Clin Microbiol. 2005;43:5003–5008. doi: 10.1128/JCM.43.10.5003-5008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX. Role of chronic cytomegalovirus infection in T-cell immunosenescence and frailty: more questions than answers. J Am Geriatr Soc. 2011;59:2363–2365. doi: 10.1111/j.1532-5415.2011.03815.x. [DOI] [PubMed] [Google Scholar]
- Leng SX, Qu T, Semba RD, et al. Relationship between cytomegalovirus (CMV) IgG serology, detectable CMV DNA in peripheral monocytes, and CMV pp65(495-503)-specific CD8+ T cells in older adults. AGE (Dordr) 2011a;33:607–614. doi: 10.1007/s11357-011-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Li H, Xue QL, et al. Association of detectable cytomegalovirus (CMV) DNA in monocytes rather than positive CMV IgG serology with elevated neopterin levels in community-dwelling older adults. Exp Gerontol. 2011b;46:679–684. doi: 10.1016/j.exger.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Weng P, Najarro K, et al. Chronic CMV infection in older women: longitudinal comparisons of CMV DNA in peripheral monocytes, anti-CMV IgG titers, serum IL-6 levels, and CMV pp65 (NLV)-specific CD8+ T-cell frequencies with twelve year follow-up. Exp Gerontol. 2014;54:84–89. doi: 10.1016/j.exger.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon MS, Lee GC, Kim JH, Yi HA, Bae YS, Lee CH. Human cytomegalovirus induces apoptosis in promonocyte THP-1 cells but not in promyeloid HL-60 cells. Virus Res. 2003;94:67–77. doi: 10.1016/s0168-1702(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Murayama T, Ohara Y, Obuchi M, et al. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line. THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J Virol. 1997;71:5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Yu D, Grimwood J, et al. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa R, Singh N. Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care. 2009;13:R68. doi: 10.1186/cc7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79:381–386. doi: 10.1097/01.tp.0000148239.00384.f0. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Dong JJ. Alteration of lipid metabolism in cells infected with human cytomegalovirus. Virology. 2010;404:71–77. doi: 10.1016/j.virol.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Spector DH. Cyclin-dependent kinase activity is required for efficient expression and posttranslational modification of human cytomegalovirus proteins and for production of extracellular particles. J Virol. 2006;80:5886–5896. doi: 10.1128/JVI.02656-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Dong JJ, Battley J, Jackson KN, Dykes BC. Human cytomegalovirus infection of THP-1 derived macrophages reveals strain-specific regulation of actin dynamics. Virology. 2012;433:64–72. doi: 10.1016/j.virol.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Smith MS, Bentz GL, Alexander JS, Yurochko AD. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol. 2004;78:4444–4453. doi: 10.1128/JVI.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Turtinen LW, Seufzer BJ. Selective permissiveness of TPA differentiated THP-1 myelomonocytic cells for human cytomegalovirus strains AD169 and Towne. Microb Pathog. 1994;16:373–378. doi: 10.1006/mpat.1994.1037. [DOI] [PubMed] [Google Scholar]
- Turtinen LW, Assimacopoulos A, Haase AT. Increased monokines in cytomegalovirus infected myelomonocytic cell cultures. Microb Pathog. 1989;7:135–145. doi: 10.1016/0882-4010(89)90032-6. [DOI] [PubMed] [Google Scholar]
- Van DE, Sauviller S, Lau B, et al. Glucocorticosteroids trigger reactivation of human cytomegalovirus from latently infected myeloid cells and increase the risk for HCMV infection in D+R+ liver transplant patients. J Gen Virol. 2015;96:131–143. doi: 10.1099/vir.0.069872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GC, Kao WH, Murakami P, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker BG, Wilton S, Rice GP. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988;140:1625–1631. [PubMed] [Google Scholar]
- Wreghitt TG, Teare EL, Sule O, Devi R, Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603–1606. doi: 10.1086/379711. [DOI] [PubMed] [Google Scholar]
- Yew KH, Carsten B, Harrison C. Scavenger receptor A1 is required for sensing HCMV by endosomal TLR-3/-9 in monocytic THP-1 cells. Mol Immunol. 2010;47:883–893. doi: 10.1016/j.molimm.2009.10.009. [DOI] [PubMed] [Google Scholar]