Abstract
Background
The traditional definition of engagement in HIV care in terms of only clinic attendance and viral suppression provides a limited understanding of how persons living with HIV (PLWH) interact with the health care system.
Methods
We conducted a retrospective analysis of patients with ≥1 HIV clinic visits at the Duke Adult Infectious Diseases Clinic between 2008 and 2013. Health care utilization was characterized by 4 indicators: clinic attendance in each half of the year (yes/no), number of emergency department (ED) visits/year (0, 1, or 2+), inpatient admissions/year (0, 1, 2+), and viral suppression (never, intermittent, always). Health care engagement patterns were modeled using latent class/latent transition analysis.
Results.
A total of 2288 patients (median age, 46.4 years; 59% black, 71% male) were included in the analysis. Three care engagement classes were derived from the latent class model: “adherent” “nonadherent,” and “sick.” Patients age ≤40 years were more likely to be in the nonadherent class (odds ratio, 2.64; 95% confidence interval, 1.38–5.04) than other cohort members. Whites and males were more likely to transition from nonadherent to adherent the following year. Nonadherent patients were significantly more likely to disengage from care the subsequent year than adherent patients (23.6 vs 0.2%, P < .001).
Conclusions
A broader definition of health care engagement revealed distinct and dynamic patterns among PLWH that would have been hidden had only previous HIV clinic attendance had been considered. These patterns may be useful for designing engagement-targeted interventions.
Keywords: health care utilization, HIV care continuum, HIV engagement in care, latent class analysis
The benefits of linkage and retention in HIV care are unequivocal and primarily driven by effective antiretroviral therapy (ART) [1]. Regular engagement in HIV care facilitates sustained viral suppression, which is associated not only with improved health for people living with HIV (PLWH), but also with decreased HIV transmission [2, 3]. With a marked increase in the prevalence of comorbid noncommunicable chronic diseases among PLWH over the last 15 years, HIV clinics are increasingly important as de facto primary care points of access for many HIV-infected individuals [4]. Therefore, it stands to reason that missed clinic visits have been independently associated with all-cause mortality among PLWH [3, 5]. Given the benefits of receiving longitudinal HIV care for PLWH, the prompt identification of persons at highest risk for falling out of care and the development of strategies to re-engage them remain top priorities of HIV health services research. The commitment of researchers and governmental agencies to HIV care engagement is evidenced by the development of the HIV care continuum, a model first introduced as part of the US National HIV/AIDS strategy in 2010, emphasizing the importance of a well-defined process-based approach to getting PLWH from diagnosis to viral suppression [6]. Updates of the HIV care continuum consistently demonstrate a steep dropoff between PLWH who receive care and PLWH who are retained in care, necessitating innovative strategies aimed at preventing at-risk PLWH from completely disengaging [7].
The HIV care continuum is dependent on definitions of the selected reporting metrics. Although the Institute of Medicine (IOM) and the Department of Health and Human Services have both released definitions of “retention in HIV care,” these definitions are completely derived from encounter-level reporting [8, 9]. Although these metrics may be adequate for reporting purposes, they are static and give little insight into patient behaviors associated with subsequent care disengagement. Recent studies have investigated patient-level determinants associated with HIV care disengagement. In a retrospective analysis of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), Rebeiro et al. found that men, blacks, and injection drug users were more likely to disengage from care than others in the cohort [10]. However, simply looking at nonmodifiable patient factors as predictors of care disengagement may be inadequate for identifying patients at risk of care disengagement. We hypothesize that looking beyond patient demographics and examining patient behavior, particularly pertaining to health care utilization, will improve our ability to identify patients at highest risk of HIV care disengagement. Ultimately, an in-depth understanding of health care utilization patterns could provide a novel perspective on the design of interventions aimed at retaining high-risk PLWH in care.
Latent class analysis (LCA) is a statistical methodology that permits detection of groups (latent classes) that cannot be directly observed on the basis of categorical indicator variables alone [11, 12]. This methodology has been increasingly used in the behavioral sciences to detect otherwise unobservable at-risk subgroups within a population based on patterns of individual risk-associated behaviors [13, 14]. For example, LCA was used to better define substance abuse patterns among women living with HIV and to associate those patterns with likelihood of antiretroviral adherence [13] The use of computational methods like LCA to deconstruct complex patient decision-making associated with care disengagement may enrich our ability to detect patients at highest risk of falling out of HIV care and subsequently help target retention interventions to appropriate individuals. We evaluate the use of health care engagement behaviors (emergency department utilization and inpatient admission) to empirically understand patterns of HIV care engagement and to identify groups of individuals at highest risk for disengagement from HIV care. We also use latent transition analysis (LTA), a related methodology for the analysis of the movement of individual observations between latent classes over time, to examine patient-level factors associated with moving between care disengagement risk groups [11].
METHODS
Study Population and Design
We conducted a retrospective analysis of PLWH (age ≥18 years) who received HIV care from the Duke University Adult Infectious Diseases (ID) Clinic between January 2008 and December 2013. The Duke ID Clinic provides medical care to approximately 1900 PLWH. HIV care is rendered by 17 full-time ID-trained faculty, 2 physician assistants, and 7 infectious diseases fellows. In 2010, 22.7% of HIV clinic patients received primary care outside the clinic. All patients who attended ≥1 medical provider appointments at the clinic during the study period were included in the analysis. No exclusion criteria were applied to the cohort. Clinical data from cohort members were abstracted from the electronic medical record (EMR) using the Duke Enterprise Data Unified Content Explorer (DEDUCE), a data interface that allows for query of patient-level data from all clinical encounters within the Duke University Health System since 1996 [15].
Indicator and Outcome Variables
For LCA of care engagement patterns, 4 indicator variables were utilized: emergency department (ED) visits per year (0, 1, ≥2), inpatient admissions per year (0, 1, ≥2), attended HIV clinic appointments each half of the calendar year (yes/no), and achieved virologic suppression, defined as ≤400 copies/mL (never, sometimes, always). Patients who had no viral loads (missing or not obtained) in a given calendar year of observation were assigned to the “never suppressed” class for purposes of the viral suppression indicator variable. Only encounters in which patients were seen by a medical provider (physician/nurse practitioner/physician assistant) were included. To assess the association of patient demographics with transition between class membership over time, we also included sex, race (white, nonwhite), and age (<40 years, ≥40 years) in the latent transition model. The outcome of interest was disengagement from HIV care, defined as absence of any clinic encounters in a given calendar year after attending ≥1 clinic appointments in the previous year [7, 8]. All-cause mortality was a secondary outcome.
Statistical Analysis
Trends in mortality rates within the clinic cohort between calendar years were assessed using linear trend estimation. Latent classes were modeled using PROC LCA in SAS [16]. Models including 2–6 latent classes were assessed for model fit. Model identification for each solution was assessed by an expectation-maximization algorithm and set to a maximum of 10 000 iterations. We programmed the model estimation to allow for 100 repetitions of model estimation for each solution using 20 random sets of starting values to ensure that the definitive maximum log-likelihood ratio was identified. Bayesian information criterion (BIC) and the adjusted Bayesian information criterion (aBIC) were assessed to determine the best model [17]. Patients were placed in latent classes by assigning them to the class for which they had the highest posterior probability of membership. To examine the association among demographic characteristics and class membership, we used multivariable logistic regression within PROC LCA, simultaneously estimating class membership and odds ratios associated with demographic characteristics in the same model [18]. Point estimates were reported as odds ratios and 95% confidence intervals.
To examine transition of individuals between latent classes over time, we conducted a latent transition analysis using PROC LTA in SAS [16]. We used a latent transition model with a number of classes identical to the number of classes used for the best latent class model. LTA models were also set for a maximum of 10 000 iterations. Model estimation was conducted with 100 repetitions and using 20 randomly selected starting values. To determine the association between patient factors and the probability of transitioning between latent classes from year to year, we added the above demographic covariates to our LTA model. Model estimates were reported as transition probabilities between classes, and the associated transition matrices between classes by transition time point are reported below. All analysis was conducted using SAS, version 9.4 (Cary, NC), and the SAS-based add-on package PROC LCA/LTA, version 1.3.2 (University Park, PA).
RESULTS
Cohort Characteristics
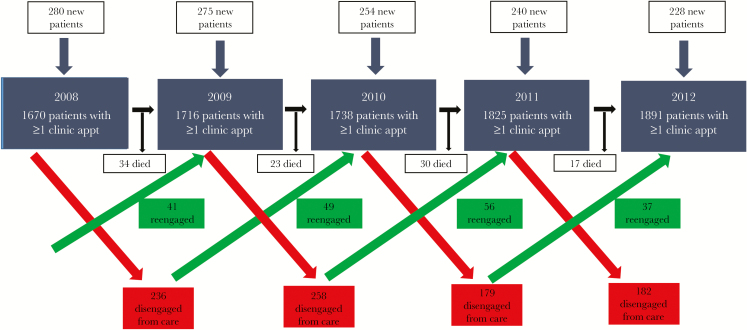
Overall, 2288 unique patients received HIV care at the Duke University ID Clinic between 2008 and 2012 and had adequate health care utilization data for inclusion in the analysis. Of the patients in the analysis cohort, 59% were black/African American, 71% were male, and 2.8% were Hispanic of any race. Mean age of cohort members (SD) was 46.4 (11.7) years (Table 1). In 2008, 280 (17%) of 1670 patients who attended ≥1 clinic visits were new patients compared with 228 (12%) of 1891 in 2012 (P < .001) (Figure 1). The mortality rate among the clinic population decreased over the study period, from 2% in 2008 to 1.1% in 2013 (P = .004).
Table 1.
Patient Characteristics Stratified by Calendar Year
| Characteristic | 2008–2009 (n = 1670) |
2009–2010 (n = 1716) |
2010–2011 (n = 1738) |
2011–2012 (n = 1825) |
2012–2013 (n = 1891) |
|---|---|---|---|---|---|
| Age, median (IQR), y | 45.2 (38.2–51.8) | 45.8 (38.4–52.4) | 46.6 (38.7–53.3) | 47.1 (39.1–53.8) | 47.5 (39.1–54.5) |
| Male sex, n (%) | 1193 (71) | 1251 (73) | 1253 (72) | 1304 (71) | 1342 (71) |
| Race, n (%) | |||||
| Black | 955 (57) | 976 (57) | 1009 (58) | 1084 (59) | 1138 (60) |
| White | 584 (35) | 588 (34) | 595 (34) | 608 (33) | 630 (33) |
| Other | 131 (8) | 152 (9) | 134 (7) | 133 (8) | 123 (7) |
| Ethnicity, n (%) | |||||
| Non-Hispanic | 1622 (97) | 1657 (97) | 1689 (97) | 1776 (97) | 1835 (97) |
| Hispanic | 48 (3) | 59 (3) | 49 (3) | 49 (3) | 56 (3) |
| New patients in previous year, n (%) | 280 (17) | 275 (16) | 254 (15) | 240 (13) | 228 (12) |
| No. of ED visits in previous calendar year (%) | |||||
| 0 | 1373 (82) | 1423 (83) | 1455 (84) | 1501 (82) | 1582 (84) |
| 1 | 182 (11) | 205 (12) | 198 (11) | 220 (12) | 211 (11) |
| 2 or more | 115 (7) | 88 (5) | 85 (5) | 104 (6) | 98 (5) |
| No. of admissions in previous calendar year (%) | |||||
| 0 | 1432 (86) | 1500 (87) | 1552 (89) | 1628 (89) | 1674 (89) |
| 1 | 147 (9) | 131 (8) | 114 (7) | 131 (7) | 139 (7) |
| 2 or more | 91 (5) | 85 (5) | 72 (4) | 66 (4) | 78 (4) |
| Attended clinic visits each half of previous calendar year | 1187 (71) | 1210 (71) | 1230 (71) | 1424 (78) | 1471 (77) |
| Viral suppression in previous year, n (%) | |||||
| Never/no viral load | 926 (55) | 701 (41) | 758 (44) | 633 (35) | 658 (35) |
| Some of the time | 187 (11) | 154 (9) | 131 (7) | 149 (8) | 83 (4) |
| All of the time | 557 (33) | 861 (50) | 849 (49) | 1043 (57) | 1150 (61) |
| Re-engaged in current year | 80 (5) | 41 (2) | 49 (3) | 56 (3) | 37 (2) |
| Disengaged in current year | 236 (14) | 258 (15) | 179 (10) | 182 (10) | 255 (13) |
| Died in current year | 34 (2) | 23 (1) | 30 (2) | 17 (1) | 21 (1) |
Abbreviations: ED, emergency department; IQR, interquartile range.
Figure 1.
Duke ID clinic patient flow diagram of HIV patients in care, 2008–2012.
Over the observation period, the proportion of patients who attended clinic visits each half of the calendar year increased steadily from 71% in 2008 to 78% in 2012 (P = .01). There was also a clear increase in persons achieving viral suppression at all times during the year over the study period (61% in 2012 vs 33.4% in 2008, P < .001) (Table 1). Although there was an overall decrease in the proportion of patients requiring 2 or more ED visits over the time of observation (Ptrend = .03), the proportion of patients requiring 2 or more ED visits over the last 4 years of observation did not change significantly. We did observe a steady decline in the proportion of patients requiring ≥2 inpatient admissions during the study period (Ptrend < .001).
Latent Class Description
Based on predetermined criteria for model selection, a 3-class model was found to be an optimal fit for the data (aggregate BIC, 216.8; aggregate aBIC, 143.7). Based on item-response probabilities, we named the 3 latent classes as follows: “adherent” (43.5% of cohort), “nonadherent” (32.9% of cohort), and “sick” (23.6% of cohort) (Table 2). The adherent class was characterized by infrequent utilization of the ED (<0.1% with 2+ visits per year), few inpatient admissions (<0.1% with 2+ inpatient admissions per year), excellent clinic attendance (89.6% attended ≥1 clinic appointments in each half of the calendar year), and reasonable virologic suppression (54.1% with suppressed viral load at all times). Persons in the nonadherent class neither utilized the ED frequently (2.4% with 2+ visits per year) nor were admitted to the hospital frequently (2% with 2+ inpatient admissions per year). Patients in the nonadherent class used the ED and required inpatient hospitalization more frequently than patients in the adherent class (P < .001). Nonadherent class members also were unlikely to attend clinic visits in each half of the calendar year (5.1%) and seldom achieved durable virologic suppression (19.8% with suppressed viral load at all times). Patients in the sick cohort utilized the ED more frequently (20.6% with 2+ visits per year) and required inpatient hospitalization more frequently than persons in the other 2 classes (23% with 2+ inpatient admissions per year). They had good clinic attendance, though (71.2% attended clinic appointments in each half of the year), and had viral suppression rates similar to the adherent class (52.2% suppressed at all times).
Table 2.
Item Response by Latent Class, 2008–2012
| Latent Class proportion of Cohort | Class I “Adherent” (0.435), % |
Class II “Nonadherent” (0.329), % |
Class III “Sick” (0.236), % |
|---|---|---|---|
| ED visits per year | |||
| 0 | 96.4 | 90.1 | 47.7 |
| 1 | 3.6 | 7.5 | 31.7 |
| 2+ | <0.1 | 2.4 | 20.6 |
| Inpatient admissions per year | |||
| 0 | 99.5 | 96.0 | 62.3 |
| 1 | 0.5 | 2.0 | 24.7 |
| 2+ | <0.1 | 2.0 | 23.0 |
| Clinic visits in each half of year | |||
| Yes | 89.6 | 5.1 | 71.2 |
| No | 10.4 | 94.9 | 18.8 |
| Virologic suppression (400 copies/mL) | |||
| Never | 37.2 | 76.7 | 40.8 |
| Sometimes | 8.7 | 3.5 | 7.0 |
| Always | 54.1 | 19.8 | 52.2 |
Abbreviation: ED, emergency department.
Factors Associated With Class Membership
Neither sex nor race was associated with membership in the nonadherent class (Table 3). However, women were significantly more likely to be in the sick class at baseline compared with men (odds ratio [OR], 1.50; 95% confidence interval [CI], 1.07–2.10). In addition, whites were significantly less likely to be members of the sick class than nonwhites (OR, 0.24; 95% CI, 0.14–0.32). PLWH age <40 years were significantly more likely to be members of the nonadherent class than patients age 40 years and older (OR, 2.64; 95% CI, 1.38–5.04). Interestingly, there was no association between age and membership in the sick class.
Table 3.
Odds Ratios of Latent Class Membership at Baseline
| Class I “Adherent” OR (95% CI) |
Class II “Nonadherent” OR (95% CI) |
Class III “Sick” OR (95% CI) |
|
|---|---|---|---|
| Female | Ref | 0.66 (0.30–1.45) | 1.50 (1.07–2.10) |
| White | Ref | 0.70 (0.42–1.14) | 0.24 (0.14–0.32) |
| Age <40 y | Ref | 2.64 (1.38–5.04) | 1.15 (0.72–1.82) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Latent Transition Analysis and Distal Outcomes
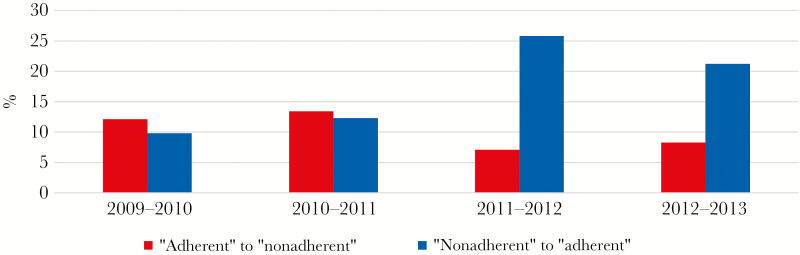
A 3-class model based on findings from the LCA was deemed the optimal fit for the LTA model. Members of the adherent class and the nonadherent class showed similar frequencies of transition away from their class in any given year (adherent, 19.8%; nonadherent, 20.9%). In 2009–2010, cohort members were more likely to transition from the adherent class to the nonadherent (12.1%) class than from the nonadherent class to the adherent class (9.8%). By the end the observation period (2012–2013), the trend completely reversed, with more patients transitioning from the nonadherent class to the adherent class (21.2%) than the opposite direction (8.3%) (Figure 2). Patients in the nonadherent class were also more likely to transition to the sick class the following year than persons in the adherent class (9.8% vs 6.0%, P < .001).
Figure 2.
Proportion of cohort transitioning between latent class I and class II by year, 2009–2013.
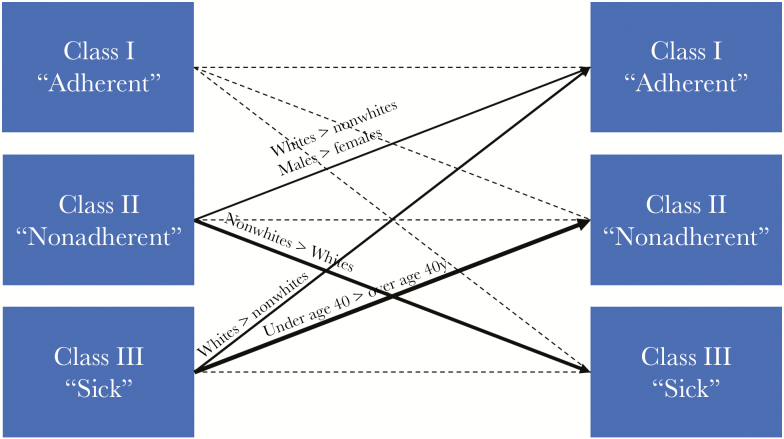
On assessment of patient-level factors associated with latent class transition, males were more likely than females to transition from the nonadherent class to the adherent class in the subsequent year. Whites were also more likely than nonwhites to transition from the nonadherent class to the adherent class the following year. Conversely, nonwhites were more likely than whites to transition from the nonadherent class in a given year to the sick class the subsequent year. Persons age ≤40 years were also more likely to transition from the sick class to the nonadherent class than older patients (Figure 3).
Figure 3.
Transition patterns in class membership by selected demographics. Weight of line represents magnitude of likelihood of transition. Only transitions with P < .05 are depicted in the figure.
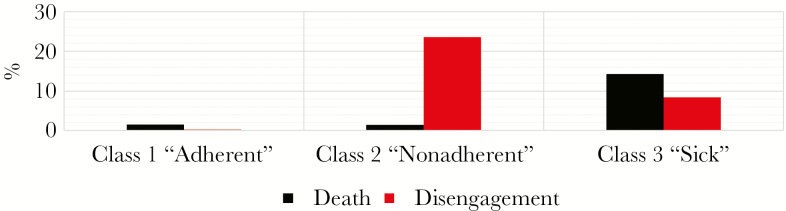
Overall, patients in the nonadherent class were significantly more likely to completely disengage from care the subsequent year than persons in the adherent class (23.6% vs 0.2%, P < .001). There were no differences in probability of death the following year between the 2 classes (adherent: 1.5%, nonadherent: 1.4%). Predictably, death rates were highest in the sick class (14.3%), and care disengagement probabilities were higher in the sick class than in the adherent class (Figure 4).
Figure 4.
Probability of death or disengagement the following year based on current year latent class, 2009–2013.
DISCUSSION
Our findings demonstrate that a broader definition of engagement in care that incorporates other aspects of health care utilization can be used to discriminate clinically important patterns of health care engagement among PLWH. Specifically, with the benefit of LCA, we were able to identify a subset of clinic patients who were at significantly higher risk for care disengagement than the rest of our HIV clinic cohort. These findings provide evidence that considering health care utilization behaviors outside the HIV clinic can enhance our ability to identify patients at risk of disengaging from HIV care. This study extends the utility of LCA as a technique to more broadly understand HIV-related health care behaviors, building on prior work examining HIV testing and health care engagement, and patterns of substance use, mental illness, and family conflict, as predictors of engagement in HIV care [19, 20].
The importance of HIV care continuity for PLWH has been well documented, and as a result, maintaining PLWH in care is a top priority for relevant governmental and diplomatic agencies [2, 5, 21, 22]. Disengaging from HIV care is directly associated with all-cause mortality among PLWH [3, 5]. In addition, an increase in the likelihood of detectable viremia in patients who disengage has undeniable public health consequences posed by the increased risk of HIV transmission [23, 24]. As a result, other groups have formulated risk stratification tools to identify at-risk patients within their clinical cohorts [25–27]. These risk predictions methods have focused on HIV-related behavioral tendencies (HIV clinic nonattendance, nonadherence with ART), static demographic risk factors (race/ethnicity and sex), or comorbidities (substance abuse). Our study takes an alternative view of a patient’s propensity to disengage from longitudinal HIV care, focusing on health care utilization behaviors that are easily retrievable from the EMR. The analysis here adds value to solely using HIV clinic attendance patterns and demographics as predictors of engagement. Although missed visits may be explained by a single barrier to care, taking into consideration a patient’s entire health care utilization behavior reports on more than just an inability to get to clinic visits. For example, a person who does attend clinic visits twice a year yet presents to the emergency department 5 times a year without a single admission likely has the ability to get to HIV clinic appointments but has not set clinic attendance as a personal priority. Alternatively, persons who do not attend clinic appointments and only attend the ED when they are ill enough to require hospitalization likely have a fixed barrier to health care access that is only overcome in cases of critical illness. These health care utilization behaviors are fundamentally different, and these hypotheses and patient groups could not be explored if only missed visits and viral suppression were taken into consideration. Additionally, 5.1% of patients in the nonadherent class (approximately 1.65% of the entire cohort) would have been misclassified as adherent if only clinic attendance were taken into consideration.
Our combined LCA/LTA also gave us the opportunity to make observations on general health care utilization patterns among our clinical cohort. For example, persons in the adherent class were more likely to have no clinic visits to the ED than nonadherent patients (96.3 vs 90.1%, P < .001). Adherent patients were also less likely to require inpatient admission than nonadherent patients. We also observed that fewer patients were transitioning from adherent class to nonadherent class in the later years of the study period. This observation could possibly be explained by attrition—nonadherent patients dropping out through the years, leaving a more adherent clinic population overall. Alternatively, the reversal in trend could be due to improvements in the tolerability of contemporary antiretroviral regimens, making patients more likely to remain adherent to medication and less likely to miss follow-up visits.
A couple of interesting observations were noted in the multivariable analysis of class membership and transition. Although whites were less likely to be members of the nonadherent class, this observation did not reach statistical significance (OR, 0.70; 95% CI, 0.42–1.14). However, whites were more likely to transition from nonadherent to adherent than nonwhites. Whites were also significantly less likely to belong to the sick class and more likely to move into another class if they initially belonged to the sick class then nonwhites, consistent with findings from other studies of both PLWH and non-HIV-infected populations [28–31]. Females were also more likely to remain in the nonadherent class from year to year than men, which is possibly attributable to the high prevalence of extenuating medical and social conditions that present barriers to optimal care engagement behaviors (substance abuse, depression, lack of social support, caregiver responsibilities) [32]. In corroboration with prior reports, persons age <40 years were more likely to be members of the nonadherent class than others in the cohort (OR, 2.64; 95% CI, 1.38–5.04) [33–35].
Our findings are consistent with prior studies. For example, Woodward and others reported on a risk prediction tool based on clinic attendance, medication adherence, substance abuse, and prior treatment failure to successfully predict clinic patients likely to miss their next clinic encounter at the Vanderbilt Comprehensive Care Clinic (VCCC) [25]. These initiatives to identify clinic patients at risk for disengagement from HIV care are critical, especially in the context of reports suggesting that up to 60% of new HIV transmissions in the United States may be from persons aware of their HIV diagnosis but not retained in care [23]. Our methodology is also easily implementable in a variety of clinic settings. In choosing our indicator variables, we purposefully included covariates that could be easily calculated as part of real-time reports on contemporary EMR platforms. In practice, these covariates would be collected and input into a programmed algorithm that would be presented as a “class II (nonadherent) flag” on the patient’s chart, available immediately to the entire multidisciplinary care team in real time at the point of care. For clinics without the ability to collect these data through the EMR, the data can be abstracted from the institution’s data warehouse, and latent class analysis can be easily performed on any statistical package at an interval of the clinic’s choice (eg, quarterly).
Our study has several limitations. Our model may misclassify patients newly entered into HIV care, as these patients generally have detectable HIV RNA levels at treatment initiation and might therefore have a “sometimes” suppressed indicator for virologic suppression. A sensitivity analysis excluding new patients from the latent class model did not substantially change the distribution of our latent class models or their prediction of distal outcomes. Also, our closed health care hospital model may exclude patient hospitalizations and ED visits outside of the health care system. Given the definitive predictive ability of our latent classes for the disengagement from care outcome, it seems unlikely that adding these encounters (in the event that data on these encounters were accessible) would significantly change the ability of latent classes to predict outcomes of interest. More broadly, our methodology does not take regional differences in barriers to care (eg, ADAP eligibility/scope, Medicaid expansion by state) into consideration. Fortunately, the modeling methodology is flexible enough that individual facilities can tailor the response to our proposed indicator variables to their particular circumstance. Finally, we acknowledge that this strategy may be harder to implement in standalone clinics not affiliated with an inpatient or emergency care facility, or medical facilities without electronic medical record systems.
In conclusion, we have demonstrated that PLWH health care utilization patterns outside of the HIV clinic are useful predictors of subsequent disengagement from care. Importantly, this LCA model utilizes data that are easily attainable from EMR platforms, making it generalizable to HIV clinics in numerous settings and possible to implement in HIV clinics with limited resources. The identification of these high-risk patients greatly enhances our ability to target clinic-based retention interventions to individuals who need them the most.
Acknowledgments
Financial support. This work was supported by the Duke Interdisciplinary Research Training Program in AIDS (T32 AI007392) and the Duke Center for AIDS Research (P30 AI064518).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thompson MA, Aberg JA, Hoy JF et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 2. Cohen MS, Chen YQ, McCauley M et al. ; HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horberg MA, Hurley LB, Silverberg MJ et al. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS 2013; 27:442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong C, Gange SJ, Moore RD et al. Multimorbidity among persons living with HIV in the U.S. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mugavero MJ, Westfall AO, Cole SR et al. ; Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis 2014; 59:1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The White House. Executive Order—HIV Care Continuum Initiative http://www.whitehouse.gov/the-press-office/2013/07/15/executive-order-hiv-care-continuum-initiative. Accessed 9 September 2017.
- 7. HIV AIDS Bureau, Health Resources Services Administration, Department of Health and Human Services. HIV Care Continuum https://hab.hrsa.gov/about-ryan-white-hivaids-program/hiv-care-continuum. Accessed 9 September 2017.
- 8. Department of Health and Human Services. HIV Core Indicators http://aids.gov/pdf/hhs-common-hiv-indicators.pdf. Accessed 15 November 2017.
- 9. HIV AIDS Bureau, Health Resources Services Administration, Department of Health and Human Services. HIV/AIDS Bureau’s Performance Measure Portfolio http://hab.hrsa.gov/deliverhivaidscare/november2013webinar.pdf. Accessed 25 June 2017.
- 10. Rebeiro PF, Abraham AG, Horberg MA et al. Sex, race, and HIV risk disparities in discontinuity of HIV care after antiretroviral therapy initiation in the United States and Canada. AIDS Patient Care STDS 2017; 31:129–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lanza ST, Collins LM, Schafer JL, Flaherty BP. Using data augmentation to obtain standard errors and conduct hypothesis tests in latent class and latent transition analysis. Psychol Methods 2005; 10:84–100. [DOI] [PubMed] [Google Scholar]
- 12. Lanza ST, Flaherty BP, Collins LM. Latent class and latent transition analysis. In: Weiner IB. Handbook of Psychology. Vol. 4. Hoboken, NJ: John Wiley and Sons; 2003:663–85. [Google Scholar]
- 13. Carter A, Roth EA, Ding E et al. Substance use, violence, and antiretroviral adherence: a latent class analysis of women living with HIV in Canada. AIDS Behav 2018; 22:971–85. [DOI] [PubMed] [Google Scholar]
- 14. Bohora S, Chaffin M, Shaboltas A et al. Latent class analysis of HIV risk behaviors among Russian women at risk for alcohol-exposed pregnancies. AIDS Behav 2017; 21(Suppl 2):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath MM, Rusincovitch SA, Brinson S et al. Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE). J Biomed Inform 2014; 52:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling 2007; 14:671–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral and Health Sciences. Hoboken, NJ: John Wiley and Sons, 2010. [Google Scholar]
- 18. Bandeen-Roche K, Miglioretti DL, Zeger SL, Rathouz PJ. Latent variable regression for multiple discrete outcomes. J Am Stat Assoc 1997; 92:1375–86. [Google Scholar]
- 19. Dangerfield DT 2nd, Craddock JB, Bruce OJ, Gilreath TD. HIV testing and health care utilization behaviors among men in the United States: a latent class analysis. J Assoc Nurses AIDS Care 2017; 28:306–15. [DOI] [PubMed] [Google Scholar]
- 20. Robinson AC, Knowlton AR, Gielen AC, Gallo JJ. Substance use, mental illness, and familial conflict non-negotiation among HIV-positive African-Americans: latent class regression and a new syndemic framework. J Behav Med 2016; 39:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulett KB, Willig JH, Lin HY et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS 2009; 23:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis 2007; 44:1500–2. [DOI] [PubMed] [Google Scholar]
- 23. Skarbinski J, Rosenberg E, Paz-Bailey G et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175:588–96. [DOI] [PubMed] [Google Scholar]
- 24. Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–50. [DOI] [PubMed] [Google Scholar]
- 25. Woodward B, Person A, Rebeiro P et al. Risk prediction tool for medical appointment attendance among HIV-infected persons with unsuppressed viremia. AIDS Patient Care STDS 2015; 29:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robbins GK, Johnson KL, Chang Y et al. Predicting virologic failure in an HIV clinic. Clin Infect Dis 2010; 50:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daigle GT, Jolly PE, Chamot EA et al. System-level factors as predictors of adherence to clinical appointment schedules in antiretroviral therapy in Cambodia. AIDS Care 2015; 27:836–43. [DOI] [PubMed] [Google Scholar]
- 28. Parker MM, Moffet HH, Schillinger D et al. Ethnic differences in appointment-keeping and implications for the patient-centered medical home–findings from the Diabetes Study of Northern California (DISTANCE). Health Serv Res 2012; 47:572–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schectman JM, Schorling JB, Voss JD. Appointment adherence and disparities in outcomes among patients with diabetes. J Gen Intern Med 2008; 23:1685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mugavero MJ, Lin HY, Allison JJ et al. Racial disparities in HIV virologic failure: do missed visits matter?J Acquir Immune Defic Syndr 2009; 50:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerver SM, Chadborn TR, Ibrahim F et al. High rate of loss to clinical follow up among African HIV-infected patients attending a London clinic: a retrospective analysis of a clinical cohort. J Int AIDS Soc 2010; 13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hader SL, Smith DK, Moore JS, Holmberg SD. HIV infection in women in the United States: status at the millennium. JAMA 2001; 285:1186–92. [DOI] [PubMed] [Google Scholar]
- 33. Hadland SE, Milloy MJ, Kerr T et al. Young age predicts poor antiretroviral adherence and viral load suppression among injection drug users. AIDS Patient Care STDS 2012; 26:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinkin CH, Hardy DJ, Mason KI et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 2004; 18(Suppl 1):S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gordillo V, del Amo J, Soriano V, González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS 1999; 13:1763–9. [DOI] [PubMed] [Google Scholar]