Abstract
Background
Neurofibromatosis 1 (NF1) leads to the development of benign and malignant peripheral nerve sheath tumors (MPNST). MPNST have been described to develop in preexisting benign plexiform neurofibromas (PN) and have a poor prognosis. Atypical neurofibromas (ANF) were recently described as precursor lesions for MPNST, making early detection and management of ANF a possible strategy to prevent MPNST. We aimed to clinically characterize ANF and identify management approaches.
Methods
We analyzed clinical, imaging, and pathology findings of all patients with NF1 and ANF at 3 institutions.
Results
Sixty-three patients had 76 ANF (32M/31F; median age 27.1 y). On MRI, most ANF appeared as distinct nodular lesions and were 18F-fluorodeoxyglucose (FDG) avid. Forty-six ANF were associated with pain, 19 with motor weakness, 45 were palpable or visible, and 13 had no clinical signs. Completely resected ANF (N = 57) have not recurred (median follow-up, 4.1 y; range, 0–14 y). Four ANF transformed into MPNST and 17 patients had a history of MPNST in a different location than was their ANF.
Conclusions
Growth of distinct nodular lesions, pain, and FDG-PET avidity should raise concern for ANF in NF1. Patients with ANF are at greater risk for development of MPNST. Complete resection of ANF may prevent development of MPNST.
Keywords: atypical neurofibroma, neurofibromatosis 1, malignant peripheral nerve sheath tumor
Importance of the study
Since ANF in NF1 were identified as precursor lesions to MPNST and outcomes for high-grade MPNST are poor, early detection and surgical removal of ANF is important to prevent development of MPNST. The clinical, imaging, and pathological features of ANF have not been fully documented previously. This study analyzed the largest cohort of 76 pathologically confirmed ANF in 63 patients using standardized data collection. Pain was the most common symptom; lesions were distinctly nodular on MRI and most were FDG-PET avid. Fully resected lesions have not shown regrowth but 4 ANF had documented transformation to high-grade MPNST and several patients had a history of MPNST prior to diagnosis of the ANF. Our data confirm the critical importance of ANF as precursor lesions to MPNST and suggest that resection of ANF, when feasible, may prevent MPNST.
Neurofibromatosis 1 (NF1) is an autosomal dominant tumor predisposition syndrome that affects 1 in 2500–3300 births. It is caused by heterozygous inactivating mutations in the NF1 tumor suppressor gene, which encodes neurofibromin, a negative regulator of RAS.1–3 NF1 is characterized by distinct clinical features, including café au lait macules, Lisch nodules, skin fold freckling, and neurofibromas.4 Patients with NF1 are at increased risk of developing benign and malignant tumors of the nervous system.5
Neurofibromas, the hallmark feature of NF1, are peripheral nerve sheath tumors with several clinicopathologic and anatomic variants, namely localized cutaneous neurofibroma, diffuse cutaneous neurofibroma, localized intraneural neurofibroma, soft tissue neurofibroma, visceral neurofibroma, and plexiform neurofibromas (PN). PN involve multiple nerve fascicles, have complex shapes, grow more rapidly in young children, and can cause substantial morbidity including pain and functional impairment.6–9 PN can undergo transformation to malignant peripheral nerve sheath tumors (MPNST), aggressive sarcomas associated with poor prognosis.
Patients with NF1 have a lifetime incidence of 8%–15.8% of developing MPNST compared with an incidence of 0.001% in the general population.10–12 In NF1, MPNST occur at a younger age and the majority arise in preexisting PN.13,14 They can present with enlarging mass, pain, and neurological deficit, but these symptoms often overlap and are difficult to distinguish from benign PN. The prognosis for MPNST in individuals with NF1 is poor, with a 5-year overall survival of 35%–50%, and to date, complete surgical resection with wide negative margins is the only curative treatment, making early detection important.15
Atypical neurofibromas (ANF) are pathologically defined lesions that have increased variable cellularity, cytological atypia, and more pronounced fascicular growth patterns but lack the widespread atypia and fascicular growth mitotic activity and necrosis seen in MPNST.16,17 In 2011 ANF were reported as precursor lesions for MPNST. A deletion at 9p21.3, which includes genes CDKN2A/2B, was identified in 15/16 (94%) ANF and in 16/23 (70%) high-grade MPNST but not in PN.18 This makes early detection and management of ANF a possible strategy to prevent MPNST. However, little is known about the clinical presentation and natural history of ANF.17,18 Our aim was to characterize ANF and identify approaches for management of these lesions.
Methods
Our study included patients with NF1 who are followed at the Catholic University in Leuven, Belgium, Guy’s and St Thomas’ NHS Foundation Trust (GSTT) in London, England, and the National Cancer Institute (NCI) in Bethesda, Maryland. The diagnosis of all cases of ANF was confirmed by one pathologist at each site using agreed upon reviewed standardized criteria, including presence of enlarged nuclei, pleomorphism, hyperchromasia, increased cellularity, absence of mitoses or minimal mitotic activity, and absence of necrosis. Using standardized data collection designed jointly by all 3 sites for this project, all patients with diagnosis of ANF were analyzed retrospectively. Data were collected on clinical presentation, including NF1 diagnostic criteria, ages at first detection, diagnosis and follow-up, ANF location, and symptoms. Imaging data of MRI and 18F-fluorodeoxygluose (FDG)-PET-CT scans, including early and delayed maximal standardized uptake values (SUVmax), were included when available. Measurements of the lesions on MRI were performed at each site with 3 diameters measured: anterior-posterior, cranio-caudal, and transverse and 3D volume based on these measurements. At the NCI, whole-body MRIs were performed on patients with NF1 and PN enrolled on the NCI NF1 natural history study (NCT00924196). Volumetric analysis was performed on the lesions, and using the same volumetric analysis method, total body PN tumor burden was calculated.19,20
Treatment interventions of biopsies and resection were analyzed with the reasons for intervention determined for each lesion, and multiple reasons were documented when applicable. Kaplan–Meier curves were generated for the age of diagnosis of ANF, the age of diagnosis of MPNST, and overall survival. All MPNST were included: MPNST prior to diagnosis of ANF or after diagnosis of ANF and whether transformed or developed in a different location. Each site had approval by its institutional ethics committee. As this was a retrospective review, informed consent was not obtained from patients.
Results
Patient Characteristics
The cohort included 63 patients—17 at the NCI, 16 at Leuven, and 30 at GSTT. Table 1 summarizes the NF1 characteristics. The 63 patients had a total of 76 pathologically confirmed ANF for which the necessary clinical data were available. Nine additional ANF were excluded from the analysis, as necessary clinical data were not available. Table 2 includes the ages and time frames of diagnosis and management of the ANF per study site. The median age at the time of pathologic diagnosis of the ANF was 27.1 years. Eighteen patients (29%) had additional lesions (1–10) that were determined to be suspicious for ANF by their clinician on clinical exams or imaging but had not been biopsied or resected at the time of this study.
Table 1.
NF1 characteristics of 63 patients with pathologically confirmed atypical neurofibroma
| Characteristic | Number | Percent | |
|---|---|---|---|
| Inheritance | De novo | 29 | 46 |
| Familial | 21 | 33 | |
| Mother/father | 10/11 | 48/52 | |
| Mosaic/segmental* | 5/1 | 8 / 2 | |
| Unknown | 7 | 11 | |
| Number of NF1 diagnostic criteria: median (range) | 4 (1–6)# | — | |
| Neurofibromas | Cutaneous | 33 | 52 |
| Subcutaneous | 5 | 8 | |
| Both | 20 | 32 | |
| None | 5 | 8 | |
| Spinal nerve root neurofibromas | 1 spinal region | 30 | 46 |
| 2 of 3 regions | 7 | 11 | |
| Cervical, thoracic, and lumbar | 14 | 22 | |
| None | 8 | 13 | |
| No spine imaging | 4 | 8 | |
* All patients with mosaic and segmental NF1 were treated at GSST.
# Patients with mosaic and segmental NF1 had only one NF1 criterion.
Table 2.
Patient characteristics by participating site: 63 patients with 76 atypical neurofibromas
| Patients with ANF | NCI | Leuven | GSTT | All Sites |
|---|---|---|---|---|
| Male/female | 10/7 | 6/10 | 16/14 | 32/31 |
| ANF (N) | 24 | 19 | 33 | 76 |
| Patients with >1 ANF | 5 | 4^ | 6^ | 15 |
| *Age, y, at diagnosis of first ANF | 19.7 (7.6–51.2) |
31.9 (15.1–58.8) |
28.5 (13.6–60) |
27.1 (7.6–60) |
| *Age, y, ANF lesion first noted (clinical or imaging) | 15.4 (5.5–40.2) |
31.4 (0.3–58.7) |
27.5 (12–58) |
25.7 (0.3–58.7) |
| *Years to ANF pathologic diagnosis | 3.8 (0.3–11) |
0.2 (0–15.1) |
0.6 (0–9) |
1.0 (0–15.1) |
| *Follow-up, y, after ANF diagnosis | 1.9 (0.1–9.9) |
4.2 (0.1–10.7) |
5.8 (0.2–14) |
4.2 (0–14) |
| Patients with additional suspicious lesions, number of lesions (range) | 9 (1–10) |
2 (2–7) |
7 (1–2) |
18 (1–10) |
*Median (range).
^Nine additional ANF from these patients were excluded from the analysis, as necessary clinical data were not available.
Clinical Characteristics of ANF
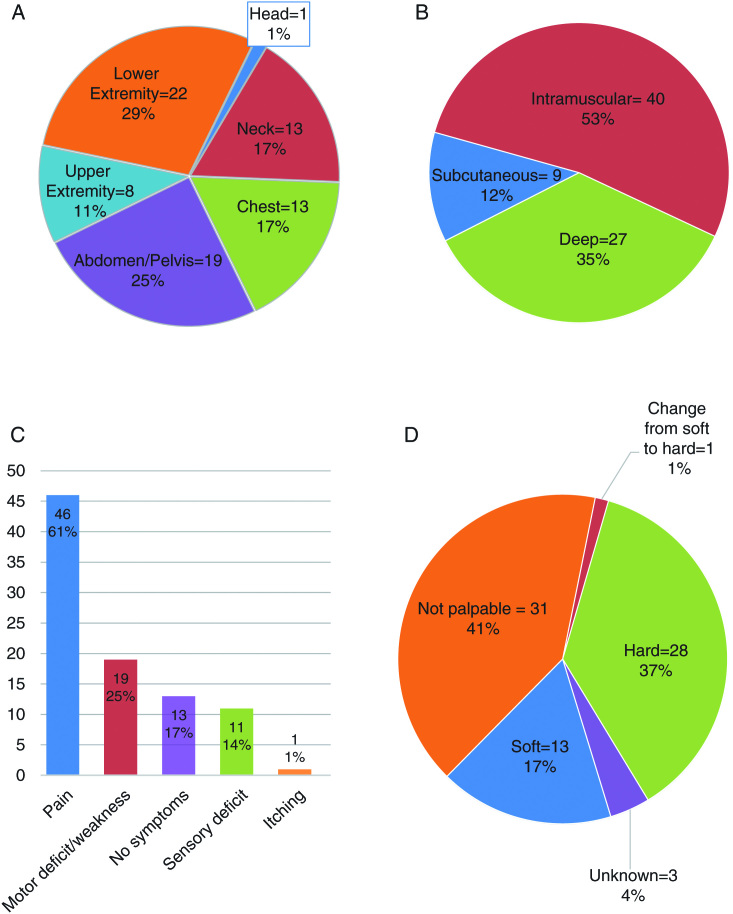
The ANF were distributed throughout the body, with 45 of the 76 lesions located in the central body, 30 lesions in the extremities, and 1 located in the head arising from the occipital nerve (Fig. 1A). The majority (n = 40) of ANF were intramuscular (Fig. 1B). (For individual patient data, see the Supplementary table.)
Fig. 1.
(A) Location of atypical neurofibromas. (B) Location of atypical neurofibromas in fascial plane. (C) Symptoms related to atypical neurofibromas. (D) Texture on palpation of atypical neurofibromas.
Fifty-one ANF caused clinical symptoms and 45 of the lesions were palpable (Fig. 1C and D). Seventy percent of ANF in the Belgian cohort (13 of 19) and 79% in the UK cohort (26 of 33) caused symptoms in comparison to 54% at the NCI (13 of 24). The most frequent symptom was pain (n = 46, 61%) and the median age of patients with and without pain was similar (28.3 y, range 13.6–60 and 25.9 y, range 7.6–57, respectively). Of the lesions associated with pain, 35 were growing. The 13 soft ANF were located predominantly in the neck and extremities, with 1 being a subcutaneous lesion in the back and another lesion being palpable in the abdomen. Thirteen ANF (8 NCI, 3 Leuven, 2 GSTT) were not associated with clinical signs or symptoms.
Imaging of ANF
Available imaging for the ANF was reviewed independently at each site (see Table 3). Only 58 of the 76 ANF had MRI of the lesion performed at a median of 15 months (range 0–11 mo) prior to diagnosis. Of these 58 ANF, 22 had a prior MRI at a median of 15.3 months earlier. Only 4 lesions decreased in size (range −4.7% to −50.6%), and 1 ANF did not change. For patients at the NCI, volumetric analysis was performed on whole-body MRIs, and the median total PN tumor volume was 1541 mL (range 186 to 3723 mL).
Table 3.
Imaging characteristics of atypical neurofibromas
| MRI (N = 58) | Median | Range |
|---|---|---|
| Longest diameter (cm) | 5.5 | 1.7–18.2 |
| Calculated 3D volume (cm3) | 65.1 | 1.6–1647 |
| Prior MRI (N = 22) | ||
| 1D growth rate (%/y) | 5.8 | −42 to 43.9 |
| 3D growth rate (%/y) | 27.4 | −51 to 379 |
| FDG-PET (N = 56) | ||
| PET avid lesions per patient (N) | 2 | 0–12 |
| Median SUVmax: 60–90 min (N = 50) | 5.6 | 0–22.3 |
| Median SUVmax: 180–240 min (N = 26) | 6.6 | 3.2–21.6 |
| Prior FDG-PET (N = 18) | ||
| Increase in SUVmax | 13 | |
| Decrease in SUVmax | 3 | |
| No change | 2 | |
Fifty-six of the 76 ANF were imaged with FDG-PET-CT scans available for review for this study, as well as 18 ANF that had prior scans available. At the NCI, FDG-PET images were obtained 60 minutes post injection, at Leuven 60 minutes or 180 minutes post injection, and at GSTT 90 and/or 240 minutes post injection. Only one ANF was not FDG avid. The majority of ANF had SUVmax >3.5, with only 4 lesions having SUVmax between 2.5 and 3.5 (3 NCI, 1 Leuven), and 2 lesions with SUVmax <2.5 (GSTT).
Interventions for ANF
Indications for intervention were lesion growth, elevated SUVmax, and clinical symptoms. Fifty-six of the 76 ANF had multiple indications for intervention and 20 had only one indication. The indications for intervention were equal, with approximately 70% per reason (growth n = 51, elevated SUVmaxn = 55, clinical symptoms n = 52). Twenty-four ANF had a biopsy as the primary intervention, while the other 52 lesions had upfront resections (2 partial resections).
Of the 13 asymptomatic lesions, 8 were biopsied or resected for only an elevated SUVmax (median SUVmax 7.0, range 2.9–16.7). Two lesions were resected because of growth on MRI, while the remaining 3 were resected for both findings on MRI and FDG-PET.
Of the 24 ANF biopsied, 3 had a repeat biopsy at a later point and 7 were resected. Of the 52 ANF with upfront resection, one partially resected lesion had a follow-up biopsy, while 7 of the lesions had secondary complete resections. Fifty-seven ANF were resected without wide margins and have not recurred on follow-up, while 2 ANF that were only partially resected have shown regrowth, one requiring a second resection. Of the patients who underwent resection, 7 (12%) had postoperative complications, which included foot drop, vocal cord dysfunction, transient paresthesia, and motor weakness that was not present preoperatively. The median follow-up time from diagnosis of the ANF to last visit or death was 4.2 years (range 0.1–14 y).
Pathology of ANF
Consistent with the pathology definition of ANF, all the lesions had enlarged nuclei, with most cases containing pleomorphic cells (n = 56) and/or hyperchromasia (n = 67). The majority of the lesions had intermediate cellularity (n = 42) with no mitotic figures. Twenty-five lesions had low cellularity and 6 had high cellularity. Eight lesions had mitotic figures ranging from 3 to 5 per 50 high-power field. No lesions showed necrosis, and hemorrhage was rare (n = 3), which could be surgical trauma related. For 3 of the 76 ANF, tissue was not available for pathology confirmation review for this study, but these had been confirmed previously by one of the 3 pathologists.
Diagnosis of MPNST in Patients with ANF
Four of the ANF in this cohort transformed into high-grade MPNST with a median time after ANF diagnosis of 1.8 years (range 0.2–3.1 y). Two of these ANF were palpable and visible, located in the calf and neck, while the other 2 lesions were in the abdomen and were not palpable or visible. All 4 lesions were associated with symptoms, including pain. On the FDG-PET closest to time of diagnosis of the ANF, the SUVmax ranged from 5.5 to 22.3. The lesions were initially only biopsied and none were fully resected.
In addition, 17 patients had a diagnosis of MPNST in a location distinct from the ANF in this cohort. Ten patients had diagnoses of their first MPNST prior to and 3 after the ANF diagnosis. The remaining 4 individuals had diagnoses of MPNST in a distinct location on the same day of the procedure that diagnosed ANF. Six of the 63 patients had a diagnosis of multiple MPNST that were not metastatic or recurrent. Three MPNST were low grade (2 patients with 1 MPNST, 1 patient who also had high-grade MPNST). Seven patients had other malignancies diagnosed (1 cholangiocarcinoma, 3 gastrointestinal stromal tumors, 1 pheochromocytoma, 1 somatostatinoma, 1 seminoma).
Ten patients in this cohort are deceased. Three of the patients died from the MPNST that had transformed from ANF and 6 patients died from MPNST in a different location. The tenth patient passed away from cholangiocarcinoma.
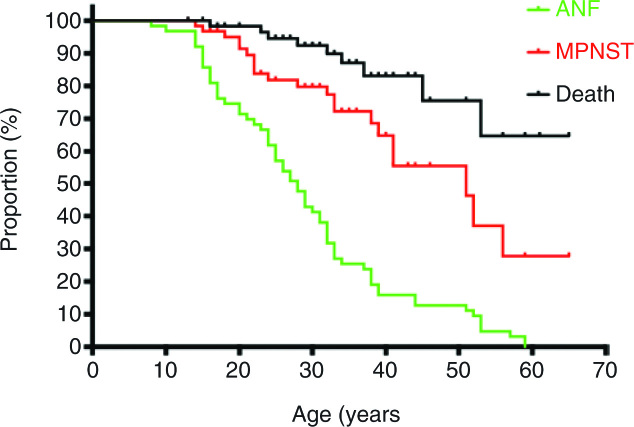
Kaplan–Meier analysis showed a median age of patients at time of ANF diagnosis of 27 years, and a median age at MPNST diagnosis of 51 years. The median for overall survival was not reached (Fig. 2).
Fig. 2.
Kaplan–Meier curve: proportion of patients with disease manifestations: age in years at first ANF, first MPNST, and death. All MPNST were included: MPNST prior to diagnosis of ANF or after diagnosis of ANF, whether transformed or developed in a different location.
Discussion
Since ANF were identified as precursor lesions to MPNST, learning more about their clinical presentation and natural history is important, as early detection and surgical removal of ANF may be important to prevent development of MPNST. This study analyzed the largest cohort to date of 76 pathologically confirmed ANF in 63 patients using standardized data collection. The median age of patients at time of diagnosis was 27.1 years, which shows that ANF are diagnosed in older patients in comparison to PN.19 This later age highlights the importance of screening NF1 patients for these lesions in early adulthood.
Clinical evaluations are of paramount importance in detecting ANF, and pain and hard palpable lesions are particularly prominent symptoms. Equally as important may be imaging with MRI, as 17% of the ANF in this cohort were not clinically detectable or symptomatic. The ANF in this cohort with MRI and FDG-PET imaging were all distinct nodular lesions, and almost all were FDG avid. It is important to note that the exact SUVmax threshold is harder to interpret due to the scans being obtained at multiple, different sites. Based on our analysis, we recommend that if nodular lesions are detected, they be followed closely at a center with NF1 expertise with history, exam, and imaging, including MRI and FDG-PET. Symptomatic, FDG-avid, or growing lesions (volume increase >20% per year in adults) warrant biopsy or resection. Given the radiation exposure, FDG-PET should be used judiciously.
It is likely that having one ANF increases the risk of having an additional ANF, as 15 patients (24%) in this cohort had a diagnosis of more than 1 ANF. Whole-body MRI data from the NCI show that most of the patients with ANF have extensive PN tumor body burden as well as multiple nodular lesions, which may be an indicator for higher risk of ANF and MPNST. Of 6 patients with whole-body MRI and MPNST at the NCI, 4 had additional distinct nodular lesions that were not biopsied (2, 2, 2, 10 per patient). Patients with severe phenotype with multiple or large burden PN or patients with an already known ANF might benefit from whole-body MRI to monitor for new lesions and change in existing lesions.21,22 Further longitudinal evaluation of current patients and a potential prospective study may help further determine this answer.
Thirty-three percent of the patients in this cohort had a diagnosis of MPNST, with 4 of those patients having documented transformation from the target ANF included in this report. This supports the hypothesis that a subset of ANF are precursors for MPNST. However, we cannot exclude the possibility that these were already MPNST when the diagnosis of ANF was established, as there may be sampling errors with core needle biopsies. The 33% incidence of MPNST in our cohort is substantially higher than the reported lifetime incidence of MPNST in NF1 ranging from 8% to 15.8%.10–12 Our Kaplan–Meier analysis suggests that as many as 50% of patients with ANF will be diagnosed with MPNST by age 51 years. Prospective studies of patients with NF1 and ANF will be required to more accurately quantitate MPNST risk and to develop surveillance strategies.
Complete removal of ANF may reduce the risk for malignant transformation. Bernthal et al showed that ANF removal with positive margins were unlikely to recur, further supporting the argument to remove ANF before they become malignant.23 Lesions not concerning for high-grade MPNST can be removed without prior biopsy, as such is the practice in Leuven. This is also the recommendation from a recent MPNST State of the Science consensus conference.24 Standardized pathologic criteria for the evaluation of ANF may allow identifying lesions, which are at greater risk for malignant transformation. A recent consensus pathology review provides guidance for the classification of ANF and proposes the term “atypical neurofibromatous neoplasms of uncertain biologic potential” (ANNUBP) for ANF considered at greater risk for transformation to MPNST.25 While multiple or deep lesions may be difficult to remove, surgery should be considered even in these cases. ANF that are not resected require close monitoring throughout life and all patients also warrant monitoring for development of additional ANF.
There are some key differences between the sites in their management of ANF (Table 4). ANF patients at the NCI were younger compared with those at the European sites, which is likely the result of the predominant patient population at NCI being children and young adults with PN. At the NCI, all patients included in this cohort were enrolled on the NF1 natural history study and therefore underwent routine whole-body MRI. They also were more likely to have an FDG-PET compared with those at the European sites. This allowed for earlier detection of these lesions, which were then followed clinically prior to biopsy or resection; this explains the longer time to diagnosis after first detection at the NCI. The higher rate of imaging at the NCI also likely explains why 8 of the 13 patients without clinical signs or symptoms were at the NCI. At the European sites, imaging was performed for a clinical indication and lesions were often removed shortly thereafter. They also were more likely to have an upfront resection and not a biopsy.
Table 4.
Differences of ANF characteristics and management between study sites
| NCI (n = 24) | Leuven (n = 19) | GSTT (n = 33) | |
|---|---|---|---|
| MRI | Whole-body MRI all patients | MRI only for concerning sites | MRI only for concerning sites |
| ANF with FDG-PET imaging | 22 (92%) | 9 (47%) | 25 (75%) |
| Asymptomatic ANF | 8 | 2 | 3 |
| Primary intervention | Biopsy: 12 Resection: 12 |
Biopsy: 1 Resection: 18 |
Biopsy: 10 Resection: 23 |
| Median age at ANF diagnosis (y) | 19.7 | 31.9 | 28.5 |
| Time to pathologic diagnosis (y) | 3.8 | 0.2 | 0.6 |
| Follow-up after ANF diagnosis (y) | 1.9 | 4.2 | 5.8 |
| Follow-up after initial lesion detection (y) | 6.3 | 5.2 | 6.9 |
While we have identified the criteria that are concerning for potential transformation (FDG avidity, lesion growth, and pain), we cannot predict which lesions will transform or when transformation will occur. More longitudinal data are needed to estimate the incidence and time frame of malignant transformation of ANF, which would help develop evidence-based guidelines for diagnosis and management of these lesions, including a risk stratification system. Currently it is not known what percentage of MPNST in NF1 arise from ANF compared with those that arise from PN without atypia. The hope would be that as we learn about ANF and their transformation to MPNST, we will have a better understanding of this. As mentioned previously, prospective assessment of the role of whole-body MRI in the identification of ANF is also needed, as this may assist in answering this question and allow for earlier detection, better monitoring, and optimized intervention of ANF. Additionally, complete characterization of genomic events that lead to the transformation from a neurofibroma to ANF and then to MPNST is needed not only to improve understanding of the transformation but also to allow for development of diagnostic biomarkers. Studies analyzing the genomic events are currently ongoing at multiple sites.
There are limitations to this study. This was a retrospective chart review; therefore, data were extracted from clinical records and there was no standardized clinical questionnaire. The approaches of diagnostic evaluation and management differed among sites, as previously noted. There was no central imaging or pathology review; however, criteria for imaging analysis and pathology review were discussed and agreed upon prior to analysis in an attempt to decrease the differences between sites.
In summary, ANF are precursor lesions to MPNST in NF1 and warrant close attention. Pain, elevated SUVmax, and growth are all concerning signs for ANF but are not unique to ANF. Future studies could provide the information that will allow for removal of those ANF that are at greatest risk for transformation with the ultimate goal of improving outcomes for patients with NF1.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was in part supported by the National Institutes of Health, the NCI, and the Center for Cancer Research Intramural Research Program.
Acknowledgment
Hilde Brems is a postdoctoral researcher of the Research Foundation Flanders (FWO) at the Catholic University of Leuven (KU Leuven).
Conflict of interest statement. No authors had a conflict of interest for this study.
References
- 1. Cawthon RM, Weiss R, Xu GF et al. . A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990;62(1):193–201. [DOI] [PubMed] [Google Scholar]
- 2. Martin GA, Viskochil D, Bollag G et al. . The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63(4):843–849. [DOI] [PubMed] [Google Scholar]
- 3. Wallace MR, Marchuk DA, Andersen LB et al. . Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249(4965):181–186. [DOI] [PubMed] [Google Scholar]
- 4. DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105(3 Pt 1):608–614. [DOI] [PubMed] [Google Scholar]
- 5. Friedman JM. Neurofibromatosis 1: clinical manifestations and diagnostic criteria. J Child Neurol. 2002;17(8):548–554; discussion 571–572, 646–651. [DOI] [PubMed] [Google Scholar]
- 6. Needle MN, Cnaan A, Dattilo J et al. . Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974–1994. J Pediatr. 1997;131(5):678–682. [DOI] [PubMed] [Google Scholar]
- 7. Waggoner DJ, Towbin J, Gottesman G, Gutmann DH. Clinic-based study of plexiform neurofibromas in neurofibromatosis 1. Am J Med Genet. 2000;92(2):132–135. [PubMed] [Google Scholar]
- 8. Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet. 1999;89(1):23–30. [DOI] [PubMed] [Google Scholar]
- 9. Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89(1):31–37. [DOI] [PubMed] [Google Scholar]
- 10. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62(5):1573–1577. [PubMed] [Google Scholar]
- 12. Uusitalo E, Rantanen M, Kallionpää RA et al. . Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34(17):1978–1986. [DOI] [PubMed] [Google Scholar]
- 13. King AA, Debaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93(5):388–392. [PubMed] [Google Scholar]
- 14. Zhou H, Coffin CM, Perkins SL, Tripp SR, Liew M, Viskochil DH. Malignant peripheral nerve sheath tumor: a comparison of grade, immunophenotype, and cell cycle/growth activation marker expression in sporadic and neurofibromatosis 1-related lesions. Am J Surg Pathol. 2003;27(10):1337–1345. [DOI] [PubMed] [Google Scholar]
- 15. Farid M, Demicco EG, Garcia R et al. . Malignant peripheral nerve sheath tumors. Oncologist. 2014;19(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123(3):295–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernthal NM, Jones KB, Monument MJ, Liu T, Viskochil D, Randall RL. Lost in translation: ambiguity in nerve sheath tumor nomenclature and its resultant treatment effect. Cancers (Basel). 2013;5(2):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beert E, Brems H, Daniëls B et al. . Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50(12):1021–1032. [DOI] [PubMed] [Google Scholar]
- 19. Dombi E, Solomon J, Gillespie AJ et al. . NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68(9):643–647. [DOI] [PubMed] [Google Scholar]
- 20. Solomon J, Warren K, Dombi E, Patronas N, Widemann B. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28(5):257–265. [DOI] [PubMed] [Google Scholar]
- 21. Mautner VF, Asuagbor FA, Dombi E et al. . Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10(4):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen R, Jett K, Harris GJ, Cai W, Friedman JM, Mautner VF. Benign whole body tumor volume is a risk factor for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. J Neurooncol. 2014;116(2):307–313. [DOI] [PubMed] [Google Scholar]
- 23. Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL. The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol. 2014;110(7):813–816. [DOI] [PubMed] [Google Scholar]
- 24. Reilly KM, Kim A, Blakely J et al. . Neurofibromatosis type 1-associated MPNST state of the science: outlining a research agenda for the future. J Natl Cancer Inst. 2017;109(8):doi: 10.1093/jnci/djx124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miettinen MM, Antonescu CR, Fletcher CDM et al. . Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.