Abstract
Objective
This longitudinal study aimed to investigate parental distress and parenting stress in relation to parental perception of child vulnerability (PPCV) in youth with spina bifida (SB).
Methods
Parents of 140 youth with SB (ages 8–15 years at Time 1) were recruited as part of a longitudinal study; data were collected at two time points, spaced 2 years apart. Mothers and fathers completed questionnaires assessing levels of personal distress, parenting stress, and PPCV.
Results
Mothers and fathers reported similar levels of personal distress, parenting stress, and PPCV, but reports of PPCV increased over time. For mothers, both personal distress and parenting stress were significantly associated with PPCV cross-sectionally, but not longitudinally. For fathers, there were significant cross-sectional and longitudinal associations between parenting stress and PPCV. The cross-sectional association between maternal parenting stress and PPCV was moderated by age, with a significant association only for older youth.
Conclusions
For parents of youth with SB, personal distress, and parenting stress are related to parental perceptions of child vulnerability, and child age may moderate this relationship. Parental personal distress and parenting stress are important targets for future interventions.
Keywords: parenting, perception of vulnerability, spina bifida
Spina bifida (SB) is a relatively common congenital neural tube defect that results in the incomplete closure of the spinal cord during fetal development (Copp, Adzick, Chitty, Fletcher, Holmbeck, & Shaw, 2015), occurring in 3 of every 10,000 live births in the United States (National Birth Defects Prevention Network, 2010). Individuals with SB may experience neurological, orthopedic, and cognitive deficits. In addition to these difficulties, individuals with SB are at risk for secondary health complications, including bladder and bowel incontinence, urinary tract infections, and pressure sores (Copp et al., 2015), as well as psychological and social adjustment difficulties and developmental delays (Holmbeck & Devine, 2010).
Parents of youth with chronic health conditions (such as SB) face unique challenges, including the management of (and associated worry about adherence to) a child’s medical regimen, stress related to the child’s health status, and uncertainty regarding the child’s current and future level of independence (Mullins et al., 2007). Parenting a child with SB is associated with both daily and long-term stressors in addition to typical parental responsibilities; these parents also may carry larger organizational, financial, and emotional burdens than parents of typically developing (TD) children (Sawin, Bellin, Roux, Buran, Brei, & Fastenau, 2003). Parents of youth with SB have described adhering to daily medical regimen, including trusting other caretakers (e.g., school nurses) to perform these tasks (e.g., clean intermittent catheterization) appropriately to be ongoing and consistent daily stressors (Sawin et al., 2003). The medical sequelae of SB require consistent care, which may also increase parental worry.
Given these increased responsibilities and worry, parents of youth with SB are prone to experience both personal distress (Vermaes, Janssens, Bosman, & Gerris, 2005) and parenting stress (Wallander et al., 1990). Though potentially related, the constructs of personal distress and parenting stress are considered unique entities in this population (Friedman et al., 2004). Personal distress is operationalized as the psychological functioning or degree of extreme anxiety, sorrow, or pain experienced by an individual (Silver, Westbrook, & Stein, 1998). Parents of children with SB have been found to experience clinical levels of global psychological distress (e.g., depressive symptoms, anxiety, somatic complaints; Holmbeck et al., 1997). On the other hand, parenting stress is conceptualized as a state of circumstantial emotional strain or pressure resulting directly from the demands of being a parent (Deater-Deckard, Chen, & El Mallah, 2015). Parents of youth with SB have been found to experience higher levels of parenting stress than parents of TD children (Holmbeck et al., 1997). The experiences of either personal distress or parenting stress may affect how parents perceive their children with SB.
Many studies have focused solely on maternal adjustment to chronic illness (Thompson & Gustafson, 1996); fathers are infrequently included in data collections or analyses, often for logistical reasons (Cassano, Adrian, Veits, & Zeman, 2006). However, the burden of parenting children with chronic health conditions affects both parents. Further, differences may exist between mothers and fathers in their adjustment to parenting a child with a chronic illness and in their perceptions of their child (Dewey & Crawford, 2007). In families of youth with SB specifically, it has been hypothesized that mothers experience more psychological distress and parenting stress than fathers (Vermaes et al., 2005). However, another study found that fathers of children with SB, but not mothers, experienced higher levels of psychological symptoms in comparison with parents of TD children (Holmbeck et al., 1997). Thus, further research is needed to clarify the potentially different impact that parenting a child with SB has on mothers versus fathers.
Parental perception of child vulnerability (PPCV) reflects parents’ attitudes or beliefs that their child is especially at risk for or more susceptible to serious illness, injury, or harm than other children (Green & Solnit, 1964, Thomasgard & Metz, 1997). PPCV includes conscious and unconscious fears regarding their child’s health and potential premature death (Thomasgard & Metz, 1997). PPCV is especially relevant to pediatric populations, as children with chronic medical conditions are more likely to be perceived as vulnerable by parents than are their TD peers (Haverman et al., 2014; Houtzager et al., 2015). As SB is a condition that affects multiple medical/organ systems and areas of functioning (Copp et al., 2015), parents of children with SB may be especially susceptible to perceiving their children as vulnerable (e.g., owing to physical, cognitive, and social limitations). To our knowledge, PPCV has yet to be studied in families of youth with SB. However, as evidenced by research with other illness groups, PPCV may influence parenting behaviors and, subsequently, child outcomes (Anthony, Bromberg, Gil, & Schanberg, 2011; Colletti et al., 2008; Mullins et al., 2004). Therefore, it is important to understand factors that may contribute to higher PPCV.
Research in other populations has identified several non-illness-related factors that negatively influence PPCV, such as parent education and socioeconomic status (SES; Anthony et al., 2003; Houtzager et al., 2015). In addition to these sociodemographic factors, many personal and family factors can impact PPCV. While the severity of a child’s medical condition, pregnancy complications, and history of illness influence the likelihood of developing PPCV (Haverman et al., 2014; Thomasgard, 1998), evidence suggests that parent psychosocial functioning is a more potent predictor of PPCV than is a child’s health condition (Greene et al., 2016; Tallandini, Morsan, Gronchi, & Macagno, 2015). A meta-analysis of the etiology of PPCV in preterm children found that maternal anxiety and parenting stress were the strongest predictors of high PPCV levels (Tallandini et al., 2015). Increased levels of parenting stress have also been found to be associated with PPCV in other pediatric populations (e.g., cystic fibrosis: Tluczek, McKechnie, & Brown, 2011; cancer: Vrijmoet-Wiersma et al., 2010); but, to date, no studies have explored the relationships among these constructs in parents of youth with SB. As discussed previously, parents of children with SB are at risk for experiencing increased personal distress and parenting stress. It is especially important to understand the impact of these parent factors on PPCV, as they are modifiable (through intervention) while many sociodemographic and condition-related factors are not. Therefore, the present study will examine parental distress and parenting stress as predictors of PPCV in families of youth with SB.
While there is some empirical support for parental distress and parenting stress as predictors of PPCV, there is a dearth of research examining PPCV as a predictor of subsequent distress and stress. However, it is reasonable to hypothesize that high levels of PPCV could lead to increased distress and stress for parents of youth with SB, given that high levels of perceived vulnerability are likely to produce an accumulation of distress and stress over time. One study of parents of children undergoing stem cell transplantation found that higher PPCV predicted increased parenting stress for both mothers and fathers (Vrijmoet-Wiersma et al., 2010). Understanding the direction of this relationship could impact intervention targets when working with parents of youth with SB. Therefore, the present study will also examine the inverse relationship, namely, PPCV as a predictor of parental distress/parenting stress.
Lastly, research has suggested that it is normative for PPCV to decrease with child age (Houtzager et al., 2015). However, given the delay in autonomy development that has been documented in youth with SB (Devine et al., 2011), it is important to gain a better understanding of the relationship between child age and PPCV for parents of youth with SB. It is possible that the normative decrease in PPCV also lags behind in this population or that a different relationship between child age and PPCV exists. Given the atypical autonomy development of youth with SB, it is important to understand not only how PPCV changes with child age but also how child age may affect relations between parental distress/parenting stress and PPCV (i.e., age may moderate these relations).
The Current Study
Broadly, the current study aimed to examine parent personal distress and parenting stress in relation to PPCV both cross-sectionally and longitudinally in families of youth with SB. To our knowledge, this is the first study of PPCV in this population. The first objective was to determine whether the three parent factors—parent distress, parenting stress, and PPCV—change over time (as children age). Given the chronic nature of SB, it was hypothesized that all three parent factors would remain stable over time (Objective 1). Additionally, this study sought to differentiate the experiences and perceptions of mothers and fathers of youth with SB. Although the literature is mixed, it was hypothesized that mothers would report higher levels of personal distress, parenting stress, and PPCV than fathers (Objective 2). It was also hypothesized that higher levels of parental distress and parenting stress would each be associated concurrently and longitudinally with higher levels of PPCV for both parents (Objective 3a). Because the age range of youth in this sample spanned multiple developmental periods (childhood and adolescence) and our suspicion that the previously observed delay in child autonomy for youth with SB (Devine et al., 2011) might also impact PPCV, child age was included as a moderator of relations between the two parent factors and PPCV. It was predicted that parents of younger children would have higher levels of PPCV than parents of older children regardless of levels of parent distress and stress, but that parents of older children would be more likely to report increased PPCV in the presence of increased personal distress and parenting stress (Objective 3b). The current study also sought to determine whether relations between parental distress/parenting stress and PPCV are bidirectional (Objective 4). Lastly, given their potential impact on all three parent factors, the following covariates were included in analyses for Objectives 3 and 4: child age, youth IQ, family SES, and youth illness severity.
Methods
Participants
Participants were recruited from an ongoing study examining family and peer relationships, neuropsychological functioning, and psychological adjustment (Devine, Holbein, Psihogios, Amaro, & Holmbeck, 2012; Holbein et al., 2015). The current study used data from the first two time points of this longitudinal study, with each time point spaced 2 years apart (e.g., Time 1 [baseline] and Time 2 [2 years later]). Families of youth with SB were recruited from four hospitals and a statewide SB association in the Midwest. Recruitment occurred in person at regularly scheduled clinic visits and through recruitment letters. Interested families were screened by phone or in person by a trained member of the research team to determine whether their child met the following inclusion criteria: (1) a diagnosis of SB (types included myelomeningocele, lipomeningocele, and myelocystocele); (2) age 8–15 years; (3) proficiency in English or Spanish; (4) involvement of at least one primary caregiver; and (5) residence within 300 miles of the laboratory (to allow for data collection at participants’ homes).
During recruitment, 246 families were approached, out of which 163 families agreed to participate. Of these 163 families, 21 families could not be contacted or later declined to participate (owing to lack of interest, lack of time, or medical complications), and two families did not meet inclusion criteria. Therefore, the final sample included 140 families of children with SB (at Time 1, 53.6% female, Mage = 11.40; Table I). Youth of families who declined to participate did not differ from participants with respect to type of SB (myelomeningocele or other), χ2(1) = 0.0002, p > .05, shunt status, χ2(1) = 0.003, p > .05, or occurrence of shunt infections, χ2(1) = 1.08, p > .05. In terms of the household composition of participating families, 112 participants (80.0%) came from two-parent homes, with 94 (67.1%) participants living with both biological parents. Both parents were invited to participate for all two-parent households, and the final sample included 128 mothers and 102 fathers (Table I), with both parents participating for 95 families (67.9%). Families with one versus two participating parents did not differ with respect to child sex, χ2(1) = 0.47, p > .05, SES, t(128) = −1.62, p>.05, type of SB (myelomeningocele or other), χ2(1) = 0.29, p > .05, lesion level (thoracic or other), χ2(1) = 1.96, p > .05, or shunt status, χ2(1) = 0.44, p > .05.
Table I.
Youth and Parent Demographic and Condition Information at Time 1
| Youth (N = 140)M (SD) or N (%) | Mother (N = 128)M (SD) or N (%) | Father (N = 102)M (SD) or N (%) | |
|---|---|---|---|
| Gender: female | 75 (53.6%) | – | – |
| Age | 11.43 (2.46) | 40.94 (6.88) | 42.90 (6.94) |
| Race | |||
| Caucasian | 74 (52.86%) | 79 (61.72%) | 68 (65.38%) |
| African-American/Black | 19 (13.57%) | 14 (10.94%) | 7 (6.73%) |
| Hispanic | 39 (27.86%) | 29 (22.65%) | 26 (25.00%) |
| Asian | 2 (1.43%) | 1 (0.78%) | 1 (0.96%) |
| Bi-racial | 6 (4.28%) | 1 (0.78%) | 0 (0.00%) |
| Not reported | 0 (0.00%) | 4 (3.13%) | 2 (1.92%) |
| Family SES | 39.44 (15.90) | – | – |
| Two-parent household | 112 (69.6%) | ||
| IQ | 85.68 (19.68) | – | – |
| Illness severity | 7.86 (1.58) | – | – |
| Spina bifida type | |||
| Myelomeningocele | 123 (87.86%) | – | – |
| Other | 17 (12.14%) | – | – |
| Lesion level | |||
| Thoracic | 29 (20.71%) | – | – |
| Lumbar | 86 (61.42%) | – | – |
| Sacral | 18 (12.86%) | – | – |
| Unknown/not reported | 7 (5.00%) | – | – |
| Shunt: present | 109 (77.86%) | – | – |
| Ambulation | |||
| No assistance | 34 (24.28%) | – | – |
| K.F.O or A.F.O | 16 (11.43%) | – | – |
| Wheelchair | 83 (59.29%) | – | – |
| Not reported | 7 (5.00%) | – | – |
Data were collected at Time 2 for 111 (79%) of the original 140 participants. Reasons for attrition at Time 2 (n = 29) were as follows: 16 participants declined to participate, 12 participants were unable to be contacted, and 1 participant was deceased. Youth of families who did not participate at Time 2 did not differ from participants with respect to sex, χ2(1) = 0.28, p > .05, SES, t(128) = −1.86, p > .05, type of SB (myelomeningocele or other), χ2(1) = 1.19, p > .05, lesion level (thoracic or other), χ2(1) = 0.72, p > .05, or shunt status, χ2(1) = 2.73, p > .05.
Procedure
This study was approved by university and hospital institutional review boards. Trained research assistants collected data from families during two separate 3-hr home visits at Time 1 and one 3-hr home visit at Time 2. For home visits with families who primarily spoke Spanish, at least one research assistant was bilingual. Before data collection began, informed consent from parents and assent from children were obtained. Parents also filled out releases of information to permit data collection from medical charts and health professionals. During data collection, family members completed questionnaires independently, and data were attempted to be collected from both parents (mothers and fathers) of all participating youth. The questionnaires were offered in both English and Spanish and were counterbalanced to avoid order effects. Questionnaires that were only available in English were translated into Spanish by a team of research assistants who were native speakers of Spanish, using forward and back translation procedures. Research assistants read questionnaires aloud to participants when requested or when reading difficulties were observed or described by youth or parents. Additionally, research assistants completed neuropsychological testing of the child. Families received monetary compensation of $150 and small gifts (e.g., logo t-shirts, pens, water bottles) for participation.
Measures
Demographics
At Time 1, parents completed a questionnaire reporting on family and youth demographic information, including age, sex, race/ethnicity, income, education, and employment (Table I). The Hollingshead Four Factor Index of SES was computed using parent’s education and occupation (Hollingshead, 1975). Higher scores indicate higher SES.
Youth Illness Severity
At Time 1, parents completed the Medical History Questionnaire (MHQ; Holmbeck et al., 2003). This survey consists of questions regarding a variety of disease-specific medical information including ambulation method (i.e., ankle-foot orthoses (AFOs), knee-ankle-foot orthoses (KAFOs), hip-knee-ankle-foot orthoses (HKAFOs), wheelchair, or no assistance). Data were also collected from participants’ medical charts to assess type of SB (i.e., lipomeningocele, meningocele, or myelomeningocele), shunt status, and lesion level (i.e., sacral, lumbar, or thoracic). An illness severity index was calculated based on membership in a specific group for the following variables: shunt status (no = 1, yes = 2), myelomeningocele (no = 1, yes = 2), lesion level (sacral = 1, lumbar = 2, thoracic = 3), and ambulation status (no assistance/AFOs = 1, KAFOs/HKAFOs = 2, wheelchair = 3). When calculating illness severity scores, information was taken from medical charts (with the exception of ambulation method, which was based on maternal report). Medical chart information was unavailable for some participants (n = 8) because they received care at a nonparticipating site or their primary physicians declined to participate. For these participants, information from mother’s report on the MHQ was used. Illness severity scores ranged from 4 to 10, with higher scores indicating higher levels of severity (see Hommeyer, Holmbeck, Wills, & Coers, 1999).
Youth IQ
At Time 1, youth were administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). These subtests were used to estimate a Full Scale IQ score, and have demonstrated high levels of internal consistency for individuals aged 6–16 years (α = .89 for Vocabulary, α = .92 for Matrix Reasoning; Wechsler, 1999).
Parent Distress
Parent distress was assessed at both time points using the Global Severity Index (GSI) of the Symptom Checklist-90-Revised (SCL-90-R; Derogatis, Rickels, & Rock, 1976). The SCL-90-R is a 90-item measure with five possible responses: not at all = 0, a little bit = 1, moderately = 2, quite a bit = 3, or extremely = 4. Sample items include “faintness or dizziness,” “feeling no interest in things,” and “the feeling that something bad is going to happen to you.” The GSI, an average of all items on the scale, was used in this study. Higher scores on the GSI indicated higher distress, with high internal consistency for both mother and father reports (α = .95–.98).
Parenting Stress
An abbreviated version of the Parenting Stress Index (Abidin, 1990) was used to assess parenting stress at both time points. Of the 24 items on this abbreviated scale, 22 items consist of a statement about the parent–child relationship that is rated on a 4-point scale ranging from 1 (strongly disagree) to 4 (strongly agree), with some reverse-scored items. Sample items include “I enjoy being a parent,” “I feel trapped by my responsibilities as a parent,” and “I often feel that my child’s needs control my life.” The final two items are statements about how parents view themselves as parents and are rated on 5-point scales (e.g., rating oneself as a parent on a scale of “very good” to “not very good”). A parenting stress total score was computed. In creating the total score, raw item scores were converted to item-level z-scores, so that the 4- and 5-point scale items could be totaled together. Higher scores on this measure indicate higher reported parenting stress. In this study, this scale demonstrated high internal consistency for both mother and father reports (α = .85–.88).
Parent Perception of Child Vulnerability
Perception of child vulnerability was measured at both time points using parent report on the Vulnerable Child Scale (VCS; Perrin, West, & Culley, 1989; Forsyth, Horwitz, Leventhal, Bruger, & Leaf, 1996). The VCS is a 15-item measure with four possible responses: definitely true, mostly true, mostly false, and definitely false. Sample items include “In general, my child seems less healthy than other children of the same age” and “I feel anxious about leaving my child with a baby sitter.” A total score was calculated for both mother and father reports on this scale, with higher scores indicating higher PPCV. Internal consistency for mother and father report was found to be high (α = .80–.84).
Statistical Analysis
Objectives 1 and 2
Paired-samples t-tests were performed to compare parents’ reported levels of distress, parenting stress, and PPCV at Time 1 and Time 2. Separate t-tests were performed for mothers and fathers. An additional series of paired-samples t-tests were conducted to compare mothers and fathers on their reported levels of distress, parenting stress, and PPCV at both Time 1 and Time 2. Tests of these objectives were conducted using Bonferroni adjusted alpha levels of .0042 per test (0.05/12).
Objective 3
A series of hierarchical multiple regression analyses were conducted to examine parental distress and parenting stress in association with PPCV at Time 1 as well as the effect of child age as a moderator. When conducting these cross-sectional regression analyses, independent variables were entered in the following order: (Step 1) covariates (IQ, illness severity, SES, child age) entered in a forward selection fashion; (Step 2) predictors (parental distress, parenting stress) entered in a forward selection fashion, and (Step 3) age by parenting predictor interaction terms. Separate sets of regressions were run for mother and father variables.
For the longitudinal analyses, analyses were identical to the cross-sectional analyses except that Time 2 PPCV was the dependent variable and PPCV at Time 1 was controlled before entering the covariates. Again, separate regression analyses were run for maternal and paternal variables.
Objective 4
To determine whether relations between parent distress and PPCV and parenting stress and PPCV were bidirectional, inverse longitudinal regression analyses of those used to address Objective 3 (i.e., using PPCV to predict parental distress and parenting stress) were performed. Separate regressions were run for parental distress and parenting stress, and separate regression analyses were run for maternal and paternal variables.
Results
Preliminary Analyses
All variables were examined for outliers, but none were identified. Additionally, all independent and dependent variables were tested for skewness. A conservative approach to identifying skewness was used; variables were considered skewed if skewness values were >1.0 (Tabachnick & Fidell, 2013). The results indicated that four variables were positively skewed: mother-report on the SCL-90 at Time 1 (skewness value = 2.17) and Time 2 (skewness value = 2.54) and father-report on the SCL-90 at Time 1 (skewness value = 1.37) and Time 2 (skewness value = 3.39). Each of these variables was transformed using a square root transformation, and these transformations yielded variables with acceptable skewness values (e.g., <1.0).
Objective 1: Change in Parent Distress, Parenting Stress, and PPCV over Time
Results showed no significant differences in distress or parenting stress for mothers or fathers between Time 1 and Time 2 (Table II). However, results showed a significant increase in PPCV for mothers but not fathers (Table II). It should be noted that before applying the Bonferroni-adjusted alpha level, both mothers and fathers reported significantly higher levels of PPCV at Time 2 than at Time 1.
Table II.
Results of Paired-Samples t-Tests Comparing Time 1 and Time 2 Reports of Distress, Parenting Stress, and Perception of Child Vulnerability for Mothers and Fathers of Youth With Spina Bifida
| Parent variable | T1 mothersM (SD) | T2 mothersM (SD) | t | p | T1 fathersM (SD) | T2 fathersM (SD) | t | p |
|---|---|---|---|---|---|---|---|---|
| Distress | 0.52 (0.27) | 0.49 (0.28) | 1.06 | .29 | 0.42 (0.23) | 0.44 (0.23) | −0.39 | .70 |
| Parenting stress | 2.37 (0.50) | 2.36 (0.45) | 0.41 | .69 | 2.32 (0.44) | 2.36 (0.40) | −0.84 | .41 |
| Parental perception of child vulnerability | 3.12 (0.42) | 3.27 (0.41) | −3.60 | <.001 | 3.19 (0.40) | 3.29 (0.35) | −2.00 | .05 |
To determine if this increase in maternal PPCV occurred independent of child age (which ranged from 8 to 15 at Time 1 and 10 to 17 at Time 2), a repeated measures multivariate analysis of covariance was performed including the covariate of youth age. When the effect of child age was considered, the increase in PPCV from Time 1 to Time 2 was no longer significant (mothers: F(1, 92) = 0.77, p = .38; fathers: F(1, 62) = 0.77, p = .39).
Objective 2: Comparing Mothers and Fathers
Results showed no significant differences between mothers and fathers on distress, parenting stress, or PPCV at Time 1 or Time 2 (Table III).
Table III.
Results of Paired-Samples t-Tests Comparing Mother and Father Report of Distress, Parenting Stress, and Perception of Child Vulnerability at Time 1 and Time 2
| Parent variable | T1 MothersM (SD) | T1 FathersM (SD) | t | p | T2 MothersM (SD) | T2 FathersM (SD) | t | p |
|---|---|---|---|---|---|---|---|---|
| Distress | 0.49 (0.23) | 0.45 (0.24) | 1.56 | .12 | 0.51 (0.29) | 0.46 (0.23) | 0.99 | .33 |
| Parenting stress | 2.38 (0.47) | 2.33 (0.40) | 0.95 | .35 | 2.39 (0.45) | 2.36 (0.40) | 0.43 | .67 |
| Parental perception of child vulnerability | 3.16 (0.42) | 3.15 (0.44) | 0.31 | .76 | 3.32 (0.40) | 3.24 (0.37) | 1.54 | .13 |
Objective 3: Parental Distress and Parenting Stress in Association With PPCV Cross-Sectionally and Longitudinally
In terms of the included covariates, at Time 1, only family SES (Step 1) significantly predicted maternal PPCV (Table IV). For fathers, family SES (Step 1) and youth illness severity (Step 2) significantly predicted paternal PPCV (Table IV). In summary, mothers and fathers who reported lower SES and fathers of children with more serious conditions also perceived their child as being more vulnerable.
Table IV.
Summary of Cross-Sectional and Longitudinal Hierarchical Multiple Regression Analyses for Covariates and Maternal and Paternal Factors Predicting Perception of Child Vulnerability at Time 1 and Time 2.
| Time 1 (cross-sectional) | Time 2 (longitudinal) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mothers | Predictor | Step | b | β | ΔR2 | Predictor | Step | b | β | ΔR2 |
| SES | 1 | −.01 | −.44 | .20** | Time 1 PPCV | 1 | .64 | .67 | .44** | |
| Parent age | 2 | .01 | .07 | .01 | Illness severity | 2 | .04 | .16 | .02 | |
| Youth age | 3 | −.01 | −.06 | .01 | SES | 3 | −.01 | −.11 | .01 | |
| Illness severity | 4 | .01 | .04 | .01 | Youth IQ | 4 | −.01 | −.03 | .00 | |
| Youth IQ | 5 | .00 | −.01 | .00 | Parent age | 5 | .00 | .01 | .00 | |
| Distress | 6 | .68 | .40 | .14** | Youth age | 6 | −.01 | −.01 | .00 | |
| Parenting stress | 7 | .24 | .25 | .05** | Distress | 7 | .17 | .11 | .01 | |
| Parenting stress | 8 | .06 | .07 | .00 | ||||||
| Fathers | SES | 1 | −.01 | −.30 | .09** | Time 1 PPCV | 1 | .39 | .44 | .20** |
| Illness severity | 2 | .07 | .24 | .06* | Illness severity | 2 | .08 | .29 | .07 | |
| Youth age | 3 | .02 | .09 | .01 | Youth age | 3 | .03 | .18 | .03 | |
| Parent age | 4 | −.01 | −.07 | .01 | SES | 4 | .01 | .05 | .01 | |
| Youth IQ | 7 | .00 | −.01 | .00 | Parent age | 5 | −.01 | −.02 | .00 | |
| Parenting stress | 6 | .33 | .32 | .09** | Youth IQ | 6 | .00 | −.01 | .00 | |
| Distress | 7 | .23 | .12 | .01 | Parenting stress | 7 | .23 | .29 | .06* | |
| Distress | 8 | .07 | .04 | .01 | ||||||
Note: All predictor variables were measured at Time 1. The covariates of SES, parent age, youth age, illness severity, and youth IQ were entered in a block in forward-selection fashion. The predictors (distress, parenting stress) were entered in Block 2 in a forward-selection fashion. For longitudinal analyses (predicting Time 2 Parental Perception of Child Vulnerability), PPCV at Time 1 was entered at Step 1.
p < .05, **p <.01.
At Time 1, both maternal distress and maternal parenting stress were significant predictors of maternal PPCV; mothers who experienced more distress and higher levels of parenting stress also perceived their child as being more vulnerable (Table IV). In terms of fathers, Time 1, paternal parenting stress was a significant predictor of paternal PPCV, but paternal distress was not (Table IV). Fathers who reported higher levels of parenting stress also perceived their child with SB as more vulnerable.
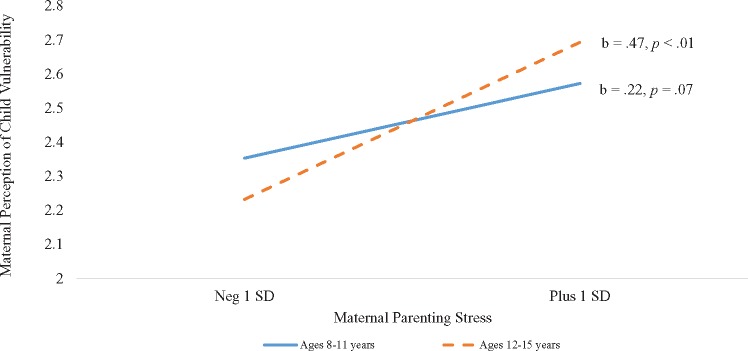
Youth age was also examined as a moderator of the relationship between the parent factors and PPCV. For mothers, the interaction between child age and maternal distress was not significant (β = .01, p = .94), but the interaction between child age and maternal parenting stress was significant (β = .17, p < .05). Post hoc probing revealed that there was not a significant relationship between maternal parenting stress and PPCV for mothers of younger children (ages 8–11; β = .22, p = .07), but that this relationship was significant for mothers of older children (ages 12–15; β = .47, p < .001; Figure 1). For mothers of older children, higher levels of parenting stress were associated with significantly higher levels PPCV. For fathers, the interactions of child age with paternal personal distress (β = −.05, p = .61) and parenting stress (β = .14, p = .14) were not significant.
Figure 1.
Simple slopes analysis of youth age as a moderator of the relationship between maternal parenting stress and maternal perception of child vulnerability.
Neither of the maternal variables were found to be significant longitudinal predictors of maternal PPCV at Time 2. Paternal personal distress at Time 1 was not a significant predictor of PPCV at Time 2. On the other hand, after controlling for PPCV at Time 1, fathers’ parenting stress at Time 1 was found to significantly predict paternal PPCV at Time 2 (Table IV). The experience of higher levels of parenting stress significantly predicted higher levels of fathers’ perception of their child with SB’s vulnerability over the next 2 years. Youth age was again examined as a moderator of the longitudinal relationships between the parent factors and PPCV, but the interactions between child age and parent factors were not significant.
Objective 4: Examining Bidirectional Relationships Among Parental Personal Distress and Parenting Stress With PPCV
For mothers, PPCV at Time 1 did not significantly predict distress (β = .10, p = .33) or parenting stress (β = .04, p = .69) 2 years later. Results were similar for paternal distress (β = .25, p = .07) and paternal parenting stress (β = .14, p = .22).
Discussion
The purpose of the current study was to determine whether parental personal distress, parenting stress, and perceived child vulnerability change over time and to identify cross-sectional and longitudinal associations between these two parent factors and PPCV for parents of youth with SB. The current study also sought to determine the moderating impact of child age on these relationships and whether these relations were bidirectional. As higher levels of PPCV may impede youth autonomy (the development of which is known to be impaired in youth with SB), it is important to identify factors (e.g., parental distress, parenting stress) that influence PPCV in this population.
The current study found that there were no differences over a 2-year time span in maternal or paternal distress and parenting stress. Additionally, there were no differences between mothers’ and fathers’ reports of distress, parenting stress, or PPCV. Still, relations among these variables differed between mothers and fathers, and so, interpretation of the findings for mothers and fathers will be considered separately.
For mothers of youth with SB, both parental distress and parenting stress were found to be significantly associated with PPCV cross-sectionally, after controlling for several covariates. With respect to maternal distress, it is possible that an intrinsic factor, such as a negative attributional process (Sanjuan, Perez, Rueda, & Ruiz, 2008), could be contributing to both maternal distress and maternal PPCV. Mothers of youth with SB have been found to favor thinking patterns that focus on the present moment and relate to pragmatic behavior (Liminana Gras, Berna, & Lopez, 2009), which may reflect an intense focus on their child’s daily needs. Disruption in daily routines, such as those caused by acute medical issues (e.g., urinary tract infections), may affect maternal thinking patterns and, subsequently, may cause increases in both maternal distress and PPCV. Therefore, interventions for mothers aimed at cognitive restructuring and changing their interpretation of these acute events could have an impact on both maternal personal distress and maternal PPCV.
In addition, maternal parenting stress was found to be significantly associated with maternal PPCV after controlling for the effects of the demographic and illness-severity factors. This relationship might also be attributable to an intrinsic process. In fact, past research on parents of youth with SB found that parents’ personality traits, rather than illness severity or demographic factors, were the strongest predictors of parenting stress (Vermaes et al., 2008). It is possible that the day-to-day demands of caring for a child with SB, which can include following a catherization schedule, facilitating transportation owing to mobility issues, and continued monitoring for secondary health complications (Copp et al., 2015), provoke a significant amount of parenting stress, regardless of their child’s current medical state (Sawin et al., 2003). A healthy child with SB must adhere to an extensive medical regimen, and this care routine could contribute to the concurrent increases in maternal parenting stress and PPCV.
Youth age was found to moderate the cross-sectional relationship between maternal parenting stress and PPCV such that this relationship was significant only for mothers of adolescents (youth ages 12–15 years). Given the developmental changes of adolescence and increasing desire for autonomy (Holmbeck & Devine, 2010), parenting an adolescent is likely more stressful for all parents. However, the stress experienced by parents of adolescents with SB may be influenced by factors unique to these families. Specifically, adolescents with SB have been found to be more dependent on their parents than are their TD peers (Lennon et al., 2015). Mothers of youth with SB have reported that managing the independence-dependence needs of their child as a major challenge (Sawin et al., 2003). Mothers of these youth may expect their child to become more independent as they progress through adolescence, as this is the “typical” developmental course. If youth do not meet this expectation, maternal parenting stress may increase. Likewise, continued need for assistance from a caretaker may be associated with increased PPCV. For both parents, PPCV was found to increase over time such that, as youth with SB grow older, their parents perceive them as more vulnerable. More research is needed to understand if this increase reflects a real increase in vulnerability for these youth—such as a worsening medical condition or increase in severity of social problems.
Interestingly, none of the longitudinal analyses involving maternal data yielded significant effects. This may indicate that mothers of youth with SB are resilient and able to adapt over time. Mothers’ present-focused cognitive styles (Liminana Gras et al., 2009) may also contribute to the lack of significant longitudinal relations of maternal distress and parenting stress with PPCV. As discussed above, maternal PPCV increased significantly from Time 1 to Time 2; as youth age, they are perceived as more vulnerable. However, this increase in PPCV does not seem to be related longitudinally to maternal factors. Future research should examine more thoroughly factors related to PPCV as youth with SB progress from childhood to adolescence and beyond.
Turning now to fathers of youth with SB, personal distress was not found to be significantly associated with PPCV cross-sectionally or longitudinally. However, paternal parenting stress was found to be associated with PPCV and to predict higher levels of PPCV 2 years later. Additionally, fathers of children with more severe conditions perceived their child as being more vulnerable. Research has shown that, in two-parent households, mothers of youth with SB are more likely to take on the role as the child’s primary medical caregiver, while fathers are more likely to take on the role of financial providers for the family (Brekke, Fruh, Kvarme, & Holmstrom, 2017). As such, fathers may spend less time and share fewer experiences with their child than do mothers, therefore experiencing fewer positive experiences with their child. Therefore, the impact of parenting stress may be chronically long-lasting for fathers but not for mothers. In fact, one study examining temporal relationships between affect and illness-related complications in parents and adolescents with type 1 diabetes found that fathers reported a longer duration of negative affect than did mothers and teens on days when adolescents reported more problems with diabetes care (Queen, Butner, Wiebe, & Berg, 2016). Paternal parenting stress is an important target of future interventions, as it affects PPCV and, therefore, has the potential to influence parenting behaviors and subsequent child outcomes.
Past research has also identified sociodemographic factors that impact PPCV in other chronic health populations, including child age (Houtzager et al., 2015) and SES (Anthony, Gil, & Schanberg, 2003). In this study of parents of youth with SB, SES was significantly associated with PPCV such that both mothers and fathers who reported lower SES perceived their child as being more vulnerable. A study using data from the National Spina Bifida Patient Registry reported that lower SES (as measured by insurance status) is associated with poorer health outcomes (Schechter et al., 2015). Families with lower SES may have less access to health care or educational resources, which may lead to a real increase in youth vulnerability. These youth may be more vulnerable to experiencing secondary health complications (e.g., obesity, urinary tract infections, pressure sores, Copp et al., 2015; Schechter et al., 2015) and have fewer opportunities to leave the home owing to ambulatory restrictions (Schechter et al., 2015). However, parents of youth with SB from lower SES families have also been found to experience increased personal distress (Malm-Buatsi et al., 2015) and parenting stress (Nomaguchi & House, 2013; Ong, Norshireen, & Chandran, 2010), which may, in turn, affect their perception of their child’s vulnerability. Further research is needed to better understand the impact of SES on real versus perceived vulnerability for youth with SB.
Limitations and Directions for Future Research
The current study had several strengths (e.g., the longitudinal nature of the study, the inclusion of both parents). However, there are also several limitations. First, it is possible that common-method variance contributed to the significant cross-sectional findings of this study, although such findings emerged even after controlling for several covariates. Second, youth included in the study may have navigated several significant transitions or developmental changes in the 2 years between study visits. SB is a condition with many life-threatening condition-related complications that could have quick or sudden onset (e.g., urinary tract infections, shunt malfunctions) and could impact parental distress, stress, and PPCV throughout the gap between study visits. The variability of family make-up (one versus two participating parents) included in the sample and the lack of a control group were additional limitations.
Future research could also be improved by extending beyond a 2-year span. This study indicates that parent factors and perception of vulnerability differ across developmental periods. It will also be important to investigate the impact of increased PPCV on parenting behaviors as well as child outcomes in youth with SB. Youth outcomes could include personal factors, such as social competence and self-esteem, as well as disease-specific factors, such as medical self-management and independence. SB is a condition that affects multiple systems, and relations between specific condition-related factors (e.g., bowel and bladder functioning, mobility, shunt status, cognitive functioning) and PPCV should be explored in future research. Finally, future research should examine outcomes in one- versus two-parent households and in two-parent households where PPCV levels are similar versus dissimilar across mothers and fathers.
Conclusions and Clinical Implications
The results of the current study have important implications for work with families of youth with SB. Parental distress and parenting stress are modifiable factors associated with parents’ perceptions of youth vulnerability in this population. As parents’ perceptions likely impact how parents interact with their children, parental distress and parenting stress are potential targets for intervention. Independence in daily functioning and managing medical routines are important goals for youth with SB that are likely to be impeded if parents perceive their child as highly vulnerable. Alleviating parental distress (e.g., by targeting distorted thinking patterns) or parenting stress (e.g., by increasing social support, self-care, and positive experiences with the family) could lower concurrent levels of PPCV and, consequently, increase parental promotion of autonomy and independence.
The current study highlights the importance of considering the differential impact of parenting a child with SB on mothers versus fathers. Both parents may experience parenting stress. Recently, there has been an increased focus on the efficacy of interventions targeting fathers in chronic health populations (Morawska, Mitchell, Burgess, & Fraser, 2017). At the very least, both parents should be considered when developing interventions for this population.
Acknowledgements
The authors thank the Illinois Spina Bifida Association as well as staff of the spina bifida clinics at Ann & Robert H. Lurie Children’s Hospital of Chicago, Shriners Hospital for Children-Chicago, and Loyola University Medical Center. They also thank the numerous undergraduate and graduate research assistants who helped with data collection and data entry. Finally, they would like to thank the parents, children, and health professionals who participated in this study.
Funding
This research was supported in part by grants from the National Institute of Nursing Research and the Office of Behavioral and Social Sciences Research (R01 NR016235), National Institute of Child Health and Human Development (R01 HD048629), and the March of Dimes Birth Defects Foundation (12-FY13-271). This report is part of an ongoing, longitudinal study.
Conflicts of interest: None declared.
References
- Abidin R. R. (1990). Parenting stress index short form. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Anthony K. K., Bromberg M. H., Gil K. M., Schanberg L. E. (2011). Parental perceptions of child vulnerability and parent stress as predictors of pain and adjustment in children with chronic arthritis. Children’s Health Care, 40, 53–69. [Google Scholar]
- Anthony K. K., Gil K. M., Schanberg L. E. (2003). Brief report: Parental perceptions of child vulnerability in children with chronic illness. Journal of Pediatric Psychology, 28, 185–190. [DOI] [PubMed] [Google Scholar]
- Brekke I., Fruh E. A., Kvarme L. G., Holmstrom H. (2017). Long-time sickness absence among parents of pre-school children with cerebral palsy, spina bifida, and down syndrome: A longitudinal study. BMC Pediatrics, 17, 26. doi: 10.1186/s12887-016-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano M., Adrian M., Veits G., Zeman J. (2006). The inclusion of fathers in the empirical investigation of child psychopathology: An update. Journal of Clinical Child and Adolescent Psychology, 35, 583–589. [DOI] [PubMed] [Google Scholar]
- Colletti C. J. M., Wolfe-Christensen C., Carpentier M. Y., Page M. C., McNall-Knapp R. Y., Meyer W. H., Chaney J. M., Mullins L. L. (2008). The relationship of parental overprotection, perceived vulnerability, and parenting stress to behavioral, emotional, and social adjustment in children with cancer. Pediatric Blood Cancer, 51, 269–274. [DOI] [PubMed] [Google Scholar]
- Copp A. J., Adzick N. S., Chitty L. S., Fletcher J. M., Holmbeck G. N., Shaw G. M. (2015). Spina bifida. Nature Reviews Disease Primers, 1, 15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K., Chen N., El Mallah S. (2015). Parenting stress. Oxford Bibliographies Online, Retrieved from: http://www.oxfordbibliographies.com/view/document/obo-9780199828340/obo-9780199828340-0142.xml. doi: 10.1093/obo/9780199828340-0142. [Google Scholar]
- Derogatis L. R., Rickels K., Rock A. F. (1976). The SCL-90 and the MMPI: A step in the validation of a new self-report scale. The British Journal of Psychiatry, 128, 280–289. [DOI] [PubMed] [Google Scholar]
- Devine K. A., Holbein C. E., Psihogios A. M., Amaro C. M., Holmbeck G. N. (2012). Individual adjustment, parental functioning, and perceived social support in Hispanic and non-Hispanic white mothers and fathers of children with spina bifida. Journal of Pediatric Psychology, 37, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine K. A., Wasserman R. M., Gershenson L. S., Holmbeck G. N., Essner B. S. (2011). Mother-adolescent agreement regarding decision-making autonomy: A longitudinal comparison of families of adolescents with and without spina bifida. Journal of Pediatric Psychology, 36, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey D., Crawford S. G. (2007). Correlates of maternal and paternal adjustment to chronic childhood disease. Journal of Clinical Psychology in Medical Settings, 14, 219–226. [Google Scholar]
- Forsyth B. W., Horwitz S. M., Leventhal J. M., Bruger J., Leaf P. J. (1996). The child vulnerability scale: An instrument to measure parental perceptions of child vulnerability. Journal of Pediatric Psychology, 21, 89–101. [DOI] [PubMed] [Google Scholar]
- Friedman D., Holmbeck G. N., Jandasek B., Zukerman J., Abad M. (2004). Parent functioning in families of preadolescents with spina bifida: Longitudinal implications for child adjustment. Journal of Family Psychology, 18, 609–619. [DOI] [PubMed] [Google Scholar]
- Greene M. M., Rossman B., Meier P., Patra K. (2016). Parental perception of child vulnerability among mothers of very low birth weight infants: Psychological predictors and neurodevelopmental sequelae at 2 years. Journal of Perinatology, in press. doi: 10.1038/jp.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Solnit A. (1964). Reactions to the threatened loss of a child: A vulnerable child syndrome. Pediatrics, 34, 58–66. [PubMed] [Google Scholar]
- Haverman L., van Oers H. A., Maurice-Stam H., Kuijpers T. W., Grootenhuis M. A., van Rossum M. A. (2014). Health related quality of life and parental perceptions of child vulnerability among parents of a child with juvenile idiopathic arthritis: Results from a web-based survey. Pediatric Rheumatology, 12, 34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein C. E., Lennon J. M., Kolbuck V. D., Zebracki K., Roache C. R., Holmbeck G. N. (2015). Observed differences in social behaviors exhibited in peer interactions between youth with spina bifida and their peers: Neuropsychological correlates. Journal of Pediatric Psychology, 40, 320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four Factor Index of Social Status. New Haven, CT: Yale University. [Google Scholar]
- Holmbeck G. N., Devine K. A. (2010). Psychosocial and family functioning in spina bifida. Developmental Disabilities Research Reviews, 16, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. N., Gorey-Ferguson L., Hudson T., Seefeldt T., Shapera W., Turner T., Uhler J. (1997). Maternal, paternal, and marital functioning in families of preadolescents with spina bifida. Journal of Pediatric Psychology, 22, 167–181. [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Westhoven V. C., Phillips W. S., Bowers R., Gruse C., Nikolopoulos T., Totura C. M. W., Davison K. (2003). A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Clinical and Consulting Psychology, 71, 782–796. [DOI] [PubMed] [Google Scholar]
- Hommeyer J. S., Holmbeck G. N., Wills K. E., Coers S. (1999). Condition severity and psychosocial functioning in pre-adolescents with spina bifida: Disentangling proximal functional status and distal adjustment outcomes. Journal of Pediatric Psychology, 24, 499–509. [DOI] [PubMed] [Google Scholar]
- Houtzager B. A., Moller E. L., Maurice-Stam H., Last B. F., Grootenhuis M. A. (2015). Parental perceptions of child vulnerability in a community-based sample. Journal of Child Health Care, 19, 454–465. [DOI] [PubMed] [Google Scholar]
- Lennon J. M., Murray C. B., Bechtel C. F., Holmbeck G. N. (2015). Resilience and disruption in observed family interactions in youth with and without spina bifida: An eight-year, five-wave longitudinal study. Journal of Pediatric Psychology, 40, 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liminana Gras R. M., Corbalan Berna J., Sanchez-Lopez M. P. (2009). Thinking styles and coping when caring for a child with severe spina bifida. Journal of Developmental and Physical Disabilities, 21, 169–183. [Google Scholar]
- Malm-Buatsi E., Aston C. E., Ryan J., Tao Y., Palmer B. W., Kropp B. P., Klein J., Wisniewski A. B., Frimberger D. (2015). Mental health and parenting characteristics of caregivers of children with spina bifida. Journal of Pediatric Urology, 11, 65.e1–65.e7. [DOI] [PubMed] [Google Scholar]
- Morawska A., Mitchell A. E., Burgess S., Fraser J. (2017). Fathers’ perceptions of change following parenting intervention: Randomized controlled trial of Triple P for parents of children with asthma and eczema. Journal of Pediatric Psychology, 42, 792–803. doi: 10.1093/jpepsy/jsw106. [DOI] [PubMed] [Google Scholar]
- Mullins L. L., Fuemmeler B. F., Hoff A., Chaney J. M., Van Pelt J., Ewing C. A. (2004). The relationship of parental overprotection and perceived child vulnerability to depressive symptomatology in children with Type 1 Diabetes Mellitus: The moderating influence of parenting stress. Children’s Health Care, 33, 21–34. [Google Scholar]
- Mullins L. L., Wolfe-Christensen C., Pai A. L., Carpentier M. Y., Gillaspy S., Cheek J., Page M. (2007). The relationship of parental overprotection, perceived child vulnerability, and parenting stress to uncertainty in youth with chronic illness. Journal of Pediatric Psychology, 32, 973–982. [DOI] [PubMed] [Google Scholar]
- National Birth Defects Prevention Network (2010). Prevalence of spina bifida and anencephaly before and after folic acid fortification, NBDPN Neural Tube Defect Ascertainment Project; 1995–2006. Retrieved from http://www.nbdpn.org/current/2010pdf/NTD%20fact%20sheet%2001-10%20for%20website.pdf
- Nomaguchi K. M., House A. N. (2013). Racial-ethnic disparities in maternal parenting stress: The role of structural disadvantages and parenting values. Journal of Health and Social Behavior, 54, 386–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong L. C., Norshireen N. A., Chandran V. (2010). A comparison of parenting stress between mothers of children with spina bifida and able bodied controls. Cerebrospinal Fluid Research, 7, S28.. [DOI] [PubMed] [Google Scholar]
- Perrin E. C., West P. D., Culley B. S. (1989). Is my child normal yet? Correlates of vulnerability. Pediatrics, 83, 355–363. [PubMed] [Google Scholar]
- Queen T. L., Butner J., Wiebe D. J., Berg C. A. (2016). A micro-developmental view of parental well-being in families coping with chronic illness. Journal of Family Psychology, 30, 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan P., Perez A., Rueda B., Ruiz A. (2008). Interactive effects of attributional styles for positive and negative events on psychological distress. Personality and Individual Differences, 45, 187–190. [Google Scholar]
- Sawin K. J., Bellin M. H., Roux G., Buran C. F., Brei T. J., Fastenau P. S. (2003). The experience of parenting an adolescent with spina bifida. Rehabilitation Nursing, 28, 173–185. [DOI] [PubMed] [Google Scholar]
- Schechter M. S., Liu T., Soe M., Swanson M., Ward E., Thibadeau J. (2015). Sociodemographic attributes and spina bifida outcomes. Pediatrics, 135, e957–e964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver E. J., Westbrook L. E., Stein R. E. (1998). Relationship of parental psychological distress to consequences of chronic health conditions in children. Journal of Pediatric Psychology, 23, 5–15. [DOI] [PubMed] [Google Scholar]
- Tabachnick B. G., Fidell L. S. (2013). Using multivariate statistics (6th ed.). Boston: Pearson. [Google Scholar]
- Tallandini M. A., Morsan V., Gronchi G., Macagno F. (2015). Systematic and meta-analytic review: Triggering agents of parental perception of child’s vulnerability in instances of preterm birth. Journal of Pediatric Psychology, 40, 545–553. [DOI] [PubMed] [Google Scholar]
- Thomasgard M. (1998). Parental perceptions of child vulnerability, overprotection, and parental psychological characteristics. Child Psychiatry and Human Development, 28, 223–240. [DOI] [PubMed] [Google Scholar]
- Thomasgard M., Metz W. P. (1997). Parental overprotection and its relation to perceived child vulnerability. American Journal of Orthopsychiatry, 67, 330–335. [DOI] [PubMed] [Google Scholar]
- Thompson R. J. Jr, Gustafson K. E. (1996). Adaptation to chronic childhood illness. Washington, DC: American Psychology Association. [Google Scholar]
- Tluczek A., McKechnie A. C., Brown R. L. (2011). Factors associated with parental perception of child vulnerability 12 months after abnormal newborn screening results. Research in Nursing & Health, 34, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaes I. P., Janssens J. M., Bosman A. M., Gerris J. R. (2005). Parents’ psychological adjustment in families of children with spina bifida: A meta-analysis. BMC Pediatrics, 5, 32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaes I. P., Janssens J. M. A., Mullaart R. A., Vinck A., Gerris J. R. (2008). Parents’ personality and parenting stress in families of children with spina bifida. Child: Care, Health, and Development, 34, 665–674. [DOI] [PubMed] [Google Scholar]
- Vrijmoet-Wiersma C. M. J., Egeler R. M., Koopman H. M., Bresters D., Norberg A. L., Grootenhuis M. A. (2010). Parental stress and perceived vulnerability at 5 and 10 years after pediatric SCT. Bone Marrow Transplantation, 45, 1102–1108. [DOI] [PubMed] [Google Scholar]
- Wallander J. L., Pitt L. C., Mellins C. A. (1990). Child functional independence and maternal psychosocial stress as risk factors threatening adaptation in mothers of physically or sensorially handicapped children. Journal of Consulting and Clinical Psychology, 58, 818–824. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1999). WASI: Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: Harcourt Assessment, Inc. [Google Scholar]