Abstract
Testicular adrenal rest tumors (TARTs) are presumably derived from ectopic adrenocortical tissue in the testis, affecting up to 49% to 94% of males with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Few reports have described TARTs in rarer forms of CAH such as 3β-hydroxysteroid dehydrogenase type 2 deficiency (3βHSD2D). A man with 3βHSD2D presented with massive bilateral testicular tumors. He had been treated with glucocorticoids and mineralocorticoids since infancy, with difficulties in suppressing dehydroepiandrosterone sulfate. At the age of 13 years, bilateral testicular lumps were found, and a radiologic diagnosis of TARTs was proposed. Subsequent sonographic examinations showed progression, despite intensifying his glucocorticoid therapy with metabolic complications. Following an open testicular biopsy, concerns of a Leydig cell tumor and risk of malignant transformation were raised, and because the patient also had local symptoms and azoospermia, he underwent bilateral orchiectomy at age 33 years. Histopathology was consistent with bilateral TARTs, exhibiting widespread immunoreactivity for adrenocortical markers, whereas no histological features of Leydig cell tumors were seen. The distinction between TARTs and Leydig cell tumors is important but can be challenging, and in our case, orchiectomy was needed to rule out the latter diagnosis. TART should be considered a differential diagnosis also in patients with 3βHSD2D who have testicular lumps.
Keywords: 3β-hydroxysteroid dehydrogenase deficiency, congenital adrenal hyperplasia, fertility, sperm quality, testicular adrenal rest tumors
This case report is a 34-year follow-up of a patient with 3βHSD2D who had infertility due to extensive bilateral TARTs and underwent bilateral orchiectomy at age 33 years.
Congenital adrenal hyperplasia (CAH) is an inherited disorder of steroidogenesis that, in more than 95% of cases, is caused by 21-hydroxylase deficiency (1, 2). 3β-Hydroxysteroid dehydrogenase type 2 deficiency (3βHSD2D) represents a rare variant of CAH and results in a salt-wasting (SW) or non-SW phenotype with impaired steroidogenesis in the adrenals and gonads.
In patients with CAH, testicular adrenal rest tumors (TARTs) are most likely derived from ectopic adrenocortical tissue. In men with 21-hydroxylase deficiency, the reported prevalence varies between 49% and 94% (3–5). TARTs are stimulated by ACTH (and/or angiotensin II) in periods of suboptimal hormonal control, inducing hypertrophy and hyperplasia of adrenal-like cells within the testis (6, 7). Recently, mixed testicular and adrenal characteristics of TARTs have been reported, challenging the sole adrenal origin of TARTs (8). TARTs have been claimed to be the main reason for the impaired fertility seen in males with CAH (3, 6, 9). Few reports have been published about males with 3βHSD2D and only single case reports regarding TARTs and/or fertility in this group of patients (7). We describe a male with 3βHSD2D with large, bilateral TARTs and infertility.
1. Case
The patient presented in the neonatal period with severe penoscrotal hypospadias, micropenis, cryptorchidism, and bifid scrotum. The 17-hydroxyprogesterone concentrations were elevated (15 nmol/L; normal, <5 nmol/L) in infancy. After an acute adrenal crisis at 1 month of age, he was diagnosed with SW 3βHSD2D and initiated on regular doses of sodium chloride, cortisone acetate, and fludrocortisone. There was no consanguinity family history, the patient was of Swedish ethnicity, and the karyotype was 46XY. Mutation analysis of HSD3B2 revealed a rare mutation (Cys-72-Arg in homozygote form or as compound heterozygous with deletion). He was repeatedly operated on for hypospadias and retentio testis between ages 2 and 9 years.
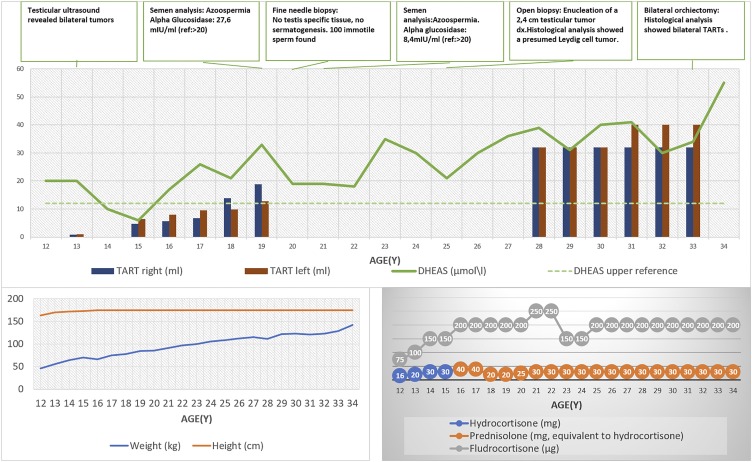
Bone maturity was advanced during childhood and adolescence [+2 standard deviations (SDS)], and his final height was 174.5 cm (−1 SDS compared with Swedish population, −2 SDS compared with his target height). The glucocorticoid and mineralocorticoid doses were successively increased over the years to control the elevated dehydroepiandrosterone sulfate (DHEAS) (Fig. 1) and renin (highest level 442 mIU/L; normal, 2.4 to 41 mIU/L), especially after the spontaneous onset of puberty. As a result, Cushing stigmata (obesity, osteoporosis, and striae) developed. During adulthood, he was treated with prednisolone (5 to 7.5 mg/d) and fludrocortisone (0.15 mg/d) with poor effects on DHEAS levels (Fig. 1). The paradoxically normal to high testosterone (30 nmol/L; normal, 10 to 30 nmol/L), androstenedione (12 nmol/L; normal, 1.2 to 5.0 nmol/L), and estradiol (164 pmol/L; normal, <130 pmol/L) concentrations were attributed to peripheral conversion of DHEAS via 3β-hydroxysteroid dehydrogenase type 1 and 17β-hydroxysteroid dehydrogenase 5 enzymes. Eventually, testosterone substitution was initiated due to successive decline in testosterone (from 30 nmol/L to 11 nmol/L in 6 years) and elevated gonadotropins [LH 15 IU/L (normal, 1.2 to 9.6 IU/L); FSH 18 IU/L (normal, 1 to 12.5 IU/L)], at age 28 years.
Figure 1.
Long-term follow up in a male with 3βHSD2D deficiency. The TARTs were revealed by age 13 years, when the patient’s height began to flatten (progressed bone age), and they gradually enlarged. Despite the depicted intensification in treatment (glucocorticoids and fludrocortisone), minimal response to DHEAS levels was observed. The increasing weight reflected the negative metabolic control. Finally, a bilateral orchiectomy was performed when the patient was 33 years old. The DHEAS concentrations were still markedly elevated at the last follow-up at 34 years of age.
At age 13 years, the testicular ultrasound revealed bilateral tumors (1.2 cm in diameter on the right side and 2 cm on the left side). They were described as hypoechogenic, well delimited, and normovascular. The tumors were considered TARTs and subsequently, over a 21-year follow up-period, advanced to larger, lobulated lesions, with blurred margins and hypervascularity, measuring finally 4 cm in diameter bilaterally. The normal testicular tissue was flattened to a minimal area in the periphery of the testes. A semen analysis performed at the age of 19 years to evaluate the fertility potential showed azoospermia.
Fine-needle testis biopsy with sperm aspiration detected no testis-specific tissue and no spermatogenesis, but ∼100 immotile sperm with abnormal morphology could be found and were cryopreserved for future fertility treatment. Due to the high risk of permanent testicular damage, an open testis biopsy was postponed. At age 25 years, an open biopsy with enucleation of a 2.4-cm testicular tumor on the right side showed a presumed benign Leydig cell tumor with possible risk for malignant transformation. The sonographic control revealed progression, and because the patient was infertile and had discomfort, he underwent bilateral orchiectomy at age 33 years.
2. Histopathology
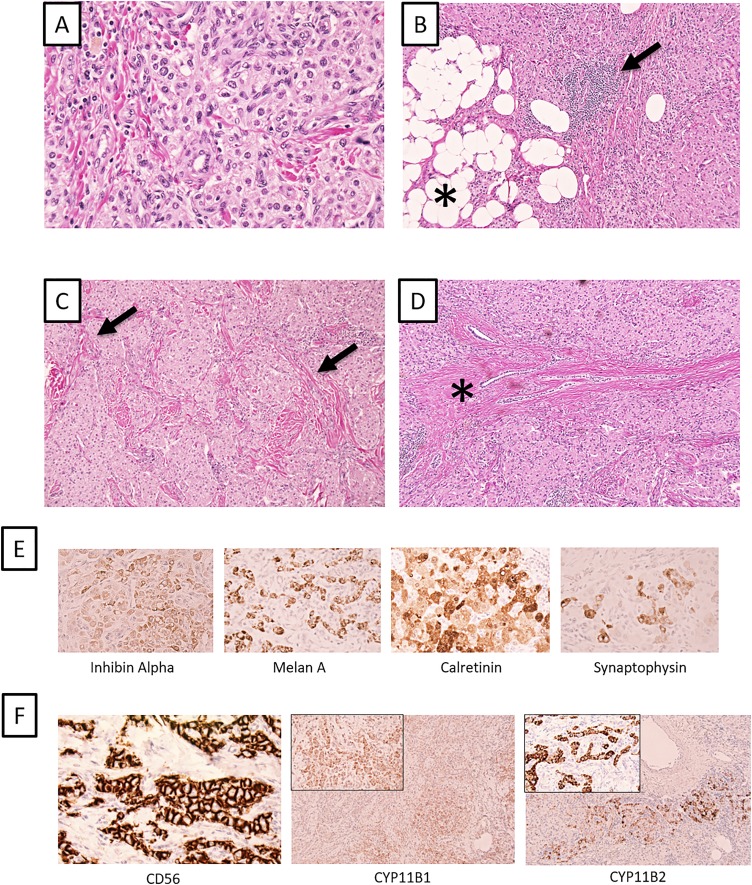
Macroscopically, the tumors were 4 cm in diameter, with a yellow to gray, lobulated to compact cut surface bilaterally. Microscopic examination showed the testicular parenchyma replaced by tumor cells with abundant, eosinophilic, slightly granulated cytoplasm and small, round to elliptical nuclei, with a loose chromatin and distinct nucleoli (Fig. 2A). No Reinke crystals were observed. The tumor cells were admixed with a hyaline stroma with local lymphocytic infiltrates and adipose tissue metaplasia (Fig. 2B and 2C), and tumor was also seen within the rete testis (the believed origin of TART; Fig. 2D). Less than one mitosis per 10 high-power field was seen, and the proliferation index (Ki-67) was 1%. Immunohistochemical stains revealed that the tumor cells were diffusely positive for adrenal markers inhibin A, Melan A, and calretinin and focally for synaptophysin (Fig. 2E). Partial immunoreactivity was seen for the androgen receptor. Negative staining was observed for EMA and OCT-3/4. Additional immunohistochemical markers (widespread immunoreactivity for CD56, focal for CYP11B1 and CYP11B2) supported the diagnosis of TARTs, stadium V (6) (Fig. 2F).
Figure 2.
Histology of extensive TARTs in a man with 3βHSD2D. (A) Routine hematoxylin and eosin staining of the left TART. The tumor cells have a large nuclear-to-cytoplasmic ratio, and tumor nuclei are round to elliptical with a loose chromatin. The cytoplasm is eosinophilic and granulated. (B) Associated TART features such as adipose tissue metaplasia (asterisk) and focal lymphocytic infiltrates (arrow) are noted. (C) The tumor is seen with a massive peritubular fibrosis and hyalinization (arrow). (D) Tumor within the rete testis (asterisk), the hypothesized origin of TARTs. (E) Immunohistochemical markers for adrenal tissue; from left to right: inhibin A, Melan A, calretinin, and synaptophysin. (F) Additional immunohistochemical markers (CD56, CYP11B1, and CYPP11B2) supporting the TART diagnosis. Widespread CD56 immunoreactivity and focal CYP11B1 and CYP11B2 positivity. All photomicrographs are magnified ×100, except A and inserts of CYP11B1/2 immunostainings (×400).
3. Discussion
To our knowledge, this is the first detailed report of a male with 3βHSD2D, extensive bilateral TARTs, infertility, and subsequent orchidectomy. TARTs can be found in children with CAH at a frequency of ~20%. Testicular ultrasounds have been recommended as the method of choice for detection and follow-up of these lesions (2). Clinical control is less sensitive to detect lesions smaller than 2 cm (3). Five stages in the development of TARTs have been proposed (6), and in our case, the regular ultrasound control showed similar morphological progress.
Autopsy data have indicated TARTs in CAH boys of a few week of age, and rest tumors may already be stimulated in utero when the ACTH levels are elevated (6). Increased glucocorticoids doses can shrink the TART volume in the early stages, but continued growth can be seen when ACTH levels are suppressed. It is unknown if this is related to angiotensin II receptor stimulation, LH rise in adolescence, or other mechanisms (6). It is known that angiotensin II has a strong trophic effect on the adrenal gland, especially on the zona glomerulosa (7, 10).
The impaired fertility is mainly related to the occurrence of TARTs (9). Glucocorticoid undertreatment leading to gonadotropin suppression due to increased adrenal androgen secretion and overtreatment also leading to gonadotropin suppression are additional reasons (3, 5). The balance between under- and overtreatment has always been a challenge in patients with CAH. Semen quality has been reported to be very poor in CAH, with 100% being pathological, if all the World Health Organization criteria are considered (3).
Although testis-sparing surgery can be considered in advanced symptomatic TARTs when the conservative therapy is ineffective, 6% of males with CAH have been found to undergo unnecessary testicular surgery (3, 6). The histological distinction between TART and Leydig cell tumor is difficult, although TARTs present bilaterally in 80% of cases, whereas Leydig cell tumors are bilateral in only 3% of cases. TARTs regularly display positivity for various adrenocortical immunohistochemical markers, which Leydig cell tumors do not. In addition, Reinke crystals are usually not seen in TARTs.
In conclusion, the clinical distinction between TARTs and Leydig cell tumors can be challenging, and it led to bilateral orchiectomy in this patient. Moreover, we show here that TARTs can be problematic in men with 3βHSD2D, a phenomenon that the practicing clinician should be aware of.
Acknowledgments
Financial Support: This project was supported by grants from the Magnus Bergvall Foundation.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 3βHSD2D
3β-hydroxysteroid dehydrogenase type 2 deficiency
- CAH
congenital adrenal hyperplasia
- DHEAS, dehydroepiandrosterone sulfate
- SDS, standard deviations
- SW
salt wasting
- TART
testicular adrenal rest tumor
References and Notes
- 1. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–2210. [DOI] [PubMed] [Google Scholar]
- 2. Falhammar H, Thorén M. Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine. 2012;41(3):355–373. [DOI] [PubMed] [Google Scholar]
- 3. Falhammar H, Nyström HF, Ekström U, Granberg S, Wedell A, Thorén M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2011;166(3):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(12):5721–5728. [DOI] [PubMed] [Google Scholar]
- 5. Engels M, Gehrmann K, Falhammar H, Webb EA, Nordenström A, Sweep FC, Span PN, van Herwaarden AE, Rohayem J, Richter-Unruh A, Bouvattier C, Köhler B, Kortmann BB, Arlt W, Roeleveld N, Reisch N, Stikkelbroeck NMML, Claahsen-van der Grinten HL; dsd-LIFE group . Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(3):285–294. [DOI] [PubMed] [Google Scholar]
- 6. Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):209–220. [DOI] [PubMed] [Google Scholar]
- 7. Güven A, Polat S. Testicular adrenal rest tumor in two brothers with a novel mutation in the 3-beta-hydroxysteroid dehydrogenase-2 gene. J Clin Res Pediatr Endocrinol. 2017;9(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engels M, Span PN, Mitchell RT, Heuvel JJTM, Marijnissen-van Zanten MA, van Herwaarden AE, Hulsbergen-van de Kaa CA, Oosterwijk E, Stikkelbroeck NM, Smith LB, Sweep FCGJ, Claahsen-van der Grinten HL. GATA transcription factors in testicular adrenal rest tumours. Endocr Connect. 2017;6(8):866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falhammar H, Frisén L, Norrby C, Almqvist C, Hirschberg AL, Nordenskjöld A, Nordenström A. Reduced frequency of biological and increased frequency of adopted children in males with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2017;102(11):4191–4199. [DOI] [PubMed] [Google Scholar]
- 10. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, Hermus AR. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab. 2007;92(9):3674–3680. [DOI] [PubMed] [Google Scholar]