Abstract
We previously demonstrated that p15RS, a newly discovered tumor suppressor, inhibits Wnt/β-catenin signaling by interrupting the formation of β-catenin·TCF4 complex. However, it remains unclear how p15RS helps exert such an inhibitory effect on Wnt signaling based on its molecular structure. In this study, we reported that dimerization of p15RS is required for its inhibition on the transcription regulation of Wnt-targeted genes. We found that p15RS forms a dimer through a highly conserved leucine zipper–like motif in the coiled-coil terminus domain. In particular, residues Leu-248 and Leu-255 were identified as being responsible for p15RS dimerization, as mutation of these two leucines into prolines disrupted the homodimer formation of p15RS and weakened its suppression of Wnt signaling. Functional studies further confirmed that mutations of p15RS at these residues results in diminishment of its inhibition on cell proliferation and tumor formation. We therefore concluded that dimerization of p15RS governed by the leucine zipper–like motif is critical for its inhibition of Wnt/β-catenin signaling and tumorigenesis.
Keywords: Wnt signaling, dimerization, tumor suppressor gene, cell proliferation, β-catenin (B-catenin), T-cell factor (TCF), leucine zipper-like, p15RS
Introduction
Wnt signaling pathway regulates a variety of biological events including cell proliferation and organogenesis (1, 2). Based on extensive studies, the Wnt signaling pathway has been classified into canonical and noncanonical categories. The canonical Wnt pathway starts from the binding of Wnt proteins as ligands to receptors Frizzled and LRP5/6, which activates Dishevelled proteins (Dvls) in the cytoplasm. Dvls bridge the recruitment of a destructive complex containing APC·Axin·GSK3β·CK1 to the receptors, allowing cytoplasmic β-catenin proteins free from degradation. The aggregated β-catenin proteins then translocate into the nucleus to interact with T cell–specific factor (TCF)3/LEF and therefore initiates the transcription of Wnt-targeted genes (3). Aberrant accumulation of β-catenin in the nucleus promotes the transcription of numerous oncogenes such as c-MYC and CCND1. As a result, the Wnt signaling pathway contributes to tumorigenesis of several cancers, including colon cancer (4), lung cancer (5), and melanoma (6, 7).
p15RS, also known as RPRD1A, is a p15INK4b-related gene discovered by a differential display experiment in cells overexpressing p15INK4b (8). Recent studies revealed that p15RS is evolutionarily homologous to yeast Rtt103 and human CREPT (9–11), whereas its function in the regulation of cell proliferation is proved to be opposite to CREPT (9, 11, 12). p15RS was initially observed to inhibit cell proliferation, as down-regulation of p15RS expression resulted in an up-regulation of cyclin D and cyclin E in A375 cells (8). It was also found that p15RS down-regulates the mRNA levels of proteins known to promote cell invasion, such as cathepsin B and MMP-9 (13). Furthermore, p15RS was identified to participate in the regulation of gene transcription, as it was associated with RNA polymerase II (14, 15). p15RS seemed to predominantly interact with phosphorylated RNA polymerase II, leading to the reduction of CTD S5- and S7-phosphorylation during transcription elongation (16). In particular, we have reported that p15RS attenuates the transcriptional regulation of Wnt/β-catenin signaling. p15RS was found to not only block the complex formation of β-catenin·TCF4 (12) but also partially recruit HDAC2 to the TCF4-binding region of targeted promoters to reduce the level of acetylated histone H3 (17).
Protein dimerization is crucial in the functional regulation of proteins such as transcription factors (18). Dimerization of transcription-associated factors facilitates the formation of multiprotein complexes required for transcription initiation by enabling proteins to bind simultaneously to different partners with overlapping binding sites (19). As these multiprotein complexes are dynamic, protein dimerization also acts as a reversible switch to control the off/on status of the information flow during signal transduction (20). Previous studies revealed that one of the most important motifs mediating protein dimerization is the leucine zipper, a parallel coiled-coil domain (21). A typical leucine zipper consists of a series of heptads, a seven-amino acid motif, with the presence of a leucine residue in each heptad (22). Previous studies observed that the CCT (coiled-coil terminus) domain of p15RS owns a potential to form homodimer (14, 15). In this study, we attempt to examine whether the dimerization of p15RS via a leucine zipper–like motif affects the complex formation of β-catenin·TCF4 and thereafter inhibits Wnt signaling during tumorigenesis.
Results
p15RS forms homodimer via its CCT domain
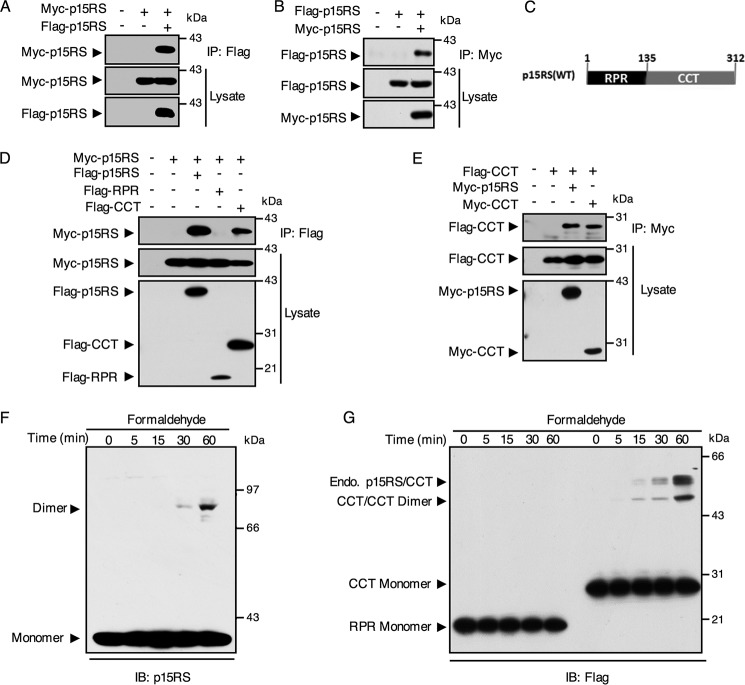
A previously resolved crystal structure suggested that the CCT domain of p15RS forms dimers (14, 15). To examine whether full-length p15RS dimerizes in vitro, we performed a reciprocal immunoprecipitation (IP) experiment, co-expressing Myc-p15RS and Flag-p15RS in HEK293T cells. The results showed that Flag-p15RS and Myc-p15RS interacted with each other, as precipitated by either an anti-FLAG (Fig. 1A) or an anti-Myc antibody (Fig. 1B). As p15RS contains a RPR (regulation of nuclear pre-mRNA) domain and a CCT domain (Fig. 1C), we questioned the exact domain responsible for p15RS dimerization. For this purpose, different domains of FLAG-tagged p15RS were co-expressed with full-length Myc-p15RS in HEK293T cells for an IP assay. The result indicated that the CCT domain (Fig. 1D, fifth lane) interacted with full-length p15RS but the RPR domain (Fig. 1D, fourth lane) lost this ability. Another IP experiment showed that the CCT domain formed a homodimer as well (Fig. 1E, fourth lane). These data suggested that the CCT domain of p15RS is responsible for its dimeric interaction in vitro.
Figure 1.
The CCT domain of p15RS mediates its dimerization in vitro. A and B, homologous interaction of p15RS in vitro. Lysates of HEK293T cells transfected with Myc-tagged and FLAG-tagged p15RS were immunoprecipitated by anti-FLAG antibody (A) or anti-Myc antibody (B) and subjected to Western blotting with the indicated antibodies. C, graphic representation of p15RS protein structure: RPR domain from amino acids 1 to 135 and CCT domain from amino acids 136 to 312. D, CCT domain is responsible for the dimerization of p15RS. FLAG-tagged full-length p15RS, RPR, or CCT domains were co-expressed with Myc-tagged full-length p15RS in HEK293T cells. Cell lysates were incubated with an anti-FLAG antibody for the IP assay. E, the CCT domain of p15RS per se dimerizes. Myc-tagged full-length (Myc-p15RS) or CCT domain of p15RS (Myc-CCT) were co-expressed in HEK293T cells with the FLAG-tagged CCT domain of p15RS (Flag-CCT). An anti-Myc antibody was used for immunoprecipitation. F, p15RS forms a homodimer. HEK293T cells transiently overexpressing Flag-p15RS were cross-linked by 1% formaldehyde for the indicated times at room temperature 24 h after transfection. An anti-p15RS antibody was used to detect the 39-kDa monomer and the 78-kDa dimer of Myc-p15RS. G, the CCT domain of p15RS determines dimerization, whereas the RPR domain stays as monomer. HEK293T cells transfected with FLAG-tagged RPR or CCT domains of p15RS were subjected to cross-linking with 1% formaldehyde for the indicated times. The monomers and dimers were revealed by Western blotting using an anti-FLAG antibody. Note that dimers of endogenous p15RS with the CCT domain are also marked.
To further confirm whether full-length p15RS forms a homologous dimer, we performed formaldehyde cross-linking assays in HEK293T cells transfected with FLAG-tagged full-length, RPR or CCT domain of p15RS. Western blot analysis of the cross-linked cells transfected with full-length p15RS demonstrated the presence of an additional band of about 80 kDa, twice the size of a p15RS monomer (about 39 kDa with tag) (Fig. 1F). This result suggested that full-length p15RS dimerizes. We also observed that the CCT domain per se formed homodimers, whereas the RPR domain failed to dimerize (Fig. 1G), corresponding to the IP results. Interestingly, we observed two bands around the dimerized position when the CCT domain was expressed (Fig. 1G). We speculated that the higher band represented dimers of the CCT domain with the endogenous full-length p15RS and the lower band represented dimers of the homo CCT domain. This result was consistent with observations from the IP experiments, showing that the CCT domain interacts with the CCT domain and full-length p15RS (Fig. 1, D and E). Taken together, these results indicated that full-length p15RS dimerizes due to its CCT domain.
Mutation of the leucine zipper–like motif impairs p15RS dimerization
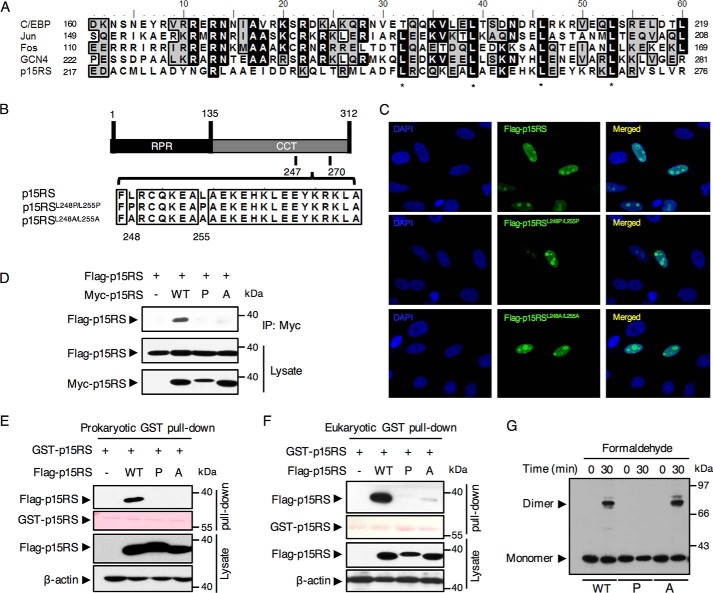
To reveal the exact motif responsible for the homologous interaction of p15RS, we analyzed the amino acid sequence in the CCT domain. Interestingly, we identified that from residue 217 to 276, the amino acid sequence of p15RS shares a high similarity with the leucine zipper motif, based on an alignment analysis of p15RS with typical leucine zipper family proteins including C/EBP, Jun, Fos, and GCN4 (Fig. 2A) (23–25). In particular, p15RS was observed to contain 4 typical leucine zipper heptads from residue Leu-245. This finding suggested that p15RS contains a leucine zipper–like motif in its CCT domain.
Figure 2.
Dimerization of p15RS depends on the leucine zipper–like motif within its CCT domain. A, a similarity analysis of amino acid sequences of p15RS with typical leucine zipper–containing proteins by an alignment using Bioedit software. Identical amino acids were back-colored in black, whereas residues sharing similar characteristics were back-colored in gray. *, indicates critical leucines in the heptad structure. B, a schematic diagram of the mutation in the leucine zipper–like motif of p15RS. p15RSL248P/L255P (referred hereafter to as P): Leu-248 and Leu-255 double-substituted into prolines; p15RSL248A/L255A (referred hereafter to as A): Leu-248 and Leu-255 double-substituted into alanines. Full-length p15RS is referred hereafter as WT. C, mutations failed to affect p15RS localization in the nucleus. MCF-7 cells expressing Flag-p15RS, Flag-p15RSL248P/L255P, and Flag-p15RSL248A/L255A were fixed and stained with an anti-FLAG antibody followed by an anti-mouse IgG conjugated with FITC. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Cells were observed by a confocal microscope with a 400-fold magnification. D, p15RSL248P/L255P no longer dimerizes. Myc-tagged full-length p15RS, p15RSL248P/L255P, or p15RSL248A/L255A were co-expressed with FLAG-tagged p15RS in HEK293T cells. Cell lysates were incubated with an anti-Myc antibody and subjected to Western blotting by an anti-FLAG antibody. E and F, leucines 248/255 of p15RS are required for the dimeric interaction in vivo. GST pulldown assays were performed with purified prokaryotic (E) or eukaryotic (F) GST-p15RS proteins and FLAG-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A proteins expressed in HEK293T cells. Cell lysates were incubated with GST beads and subjected to Western blotting by an anti-FLAG antibody. G, p15RSL248P/L255P remains as monomer, whereas p15RSL248A/L255A forms dimer. HEK293T cells transfected with FLAG-tagged full-length p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A were subjected to cross-linking and detected by Western blotting using an anti-FLAG antibody.
As leucine zipper motif is well-recognized to specifically regulate protein dimerization (21), we speculated that it is this leucine zipper–like motif within the p15RS CCT domain that mediate p15RS dimerization. To clarify this, point mutations were introduced to substitute the first two heptadic leucines at residues 248 and 255 into prolines (p15RSL248P/L255P) or alanines (p15RSL248A/L255A) (Fig. 2B) (26, 27). An immunostaining experiment showed that both mutants were located in the nucleus, similar to the WT p15RS (Fig. 2C), suggesting that the leucine zipper–like motif had no effect on protein distribution.
To determine whether this leucine zipper–like motif regulates p15RS dimerization, we performed an immunoprecipitation co-expressing WT Flag-p15RS with Myc-tagged mutants (Fig. 2D). Intriguingly, we observed that Flag-p15RS failed to interact with Myc-p15RSL248P/L255P but weakly interacted with Myc-p15RSL248A/L255A. Furthermore, GST pulldown experiments using purified proteins from either Escherichia coli (Fig. 2E) or mammalian cells (Fig. 2F) showed that p15RSL248P/L255P no longer interacted with WT p15RS, whereas p15RSL248A/L255Aremained a weak dimeric interaction in mammalian cells. A cross-linking assay strengthened these findings, showing that p15RSL248P/L255P lost the ability to form dimers (Fig. 2G, P), whereas p15RSL248A/L255A maintained dimerization ability when it was subjected to a sufficient formaldehyde treatment (Fig. 2G, A), consistent with the eukaryotic GST pulldown results (Fig. 2F). Taken together, these data revealed that mutations of the leucines into prolines at the leucine zipper–like motif abolishes the dimerization of p15RS.
Homologous interaction of p15RS is critical for its inhibition on Wnt signaling
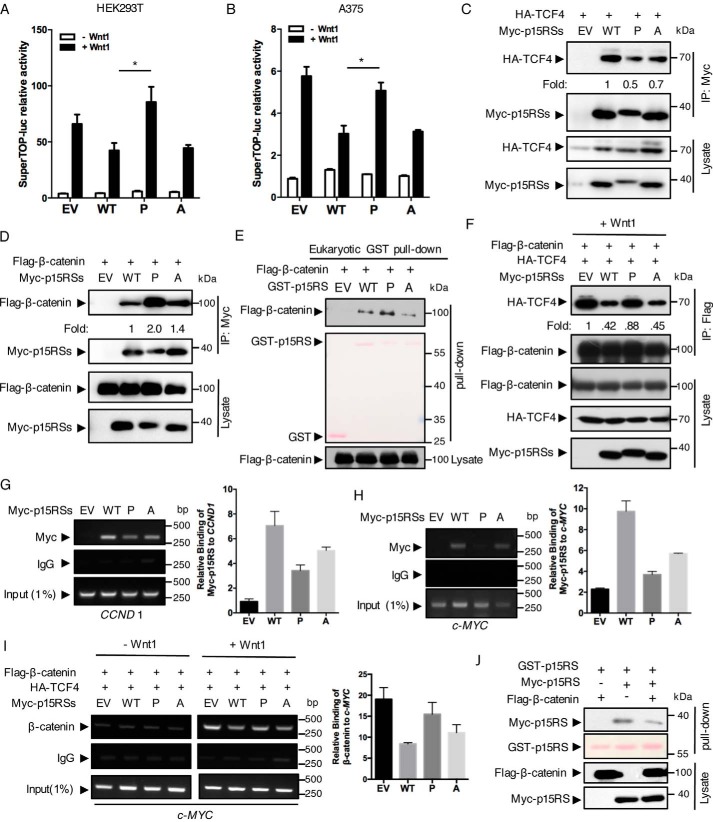
p15RS was previously reported as an intrinsic inhibitor of Wnt signaling (12). To address whether dimerization contributes to the inhibitory effect of p15RS on Wnt signaling, we examined the transcriptional activity of Wnt-targeted genes using the SuperTop-luciferase reporter. Luciferase assays in both HEK293T (Fig. 3A) and A375 cells (Fig. 3B) showed that expression of p15RSL248P/L255P failed to inhibit the Wnt1-stimulated luciferase activities, whereas p15RSL248A/L255A, similar to WT p15RS, remained to suppress Wnt signaling. These results suggested that dimerization of p15RS is crucial for its inhibition on the Wnt signaling pathway.
Figure 3.
The dimeric ability of p15RS is required for its inhibitory role on Wnt signaling. A and B, dimerization of p15RS participates in the inhibition of Wnt1-stimulated transcriptional activity. Luciferase assays were performed using HEK293T (A) or A375 (B) cells with transient expression of Myc-tagged p15RS (referred hereafter to as WT), p15RSL248P/L255P (referred hereafter to as P), or Flag-p15RSL248A/L255A (referred hereafter to as A) together with a SuperTop-luciferase reporter and pRL-TK (as an internal control). EV indicates empty vector as a control. Wnt1 expression was generated by transfection of a Wnt1 plasmid. Relative luciferase activities were normalized with the internal control. Results are presented from three independent experiments, and data are represented as mean ± S.D. (n = 3). Asterisk indicates a statistically significant difference. *, p < 0.05. C, p15RSL248P/L255P interacts with TCF4 with a decreased affinity. Myc-tagged p15RS, p15RSL248P/L255P, or p15RSL248A/L255A were co-expressed with HA-TCF4 in HEK293T cells. Cell lysates were incubated with an anti-Myc antibody and subjected to Western blotting by an anti-HA antibody. Relative binding affinity was represented as fold-change based on the level of the HA-TCF4 and Myc-p15RS. D and E, decreased dimerization leads to tighter bond between p15RS and β-catenin. Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A were co-expressed with FLAG-β-catenin in HEK293T cells. Cell lysates were incubated with an anti-Myc antibody and subjected to Western blotting by an anti-FLAG antibody. Cell lysates expressing FLAG-β-catenin were incubated with eukaryotic purified GST-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A proteins, together with GST beads, and then subjected to Western blotting by an anti-FLAG antibody (E). F, diminished dimerization of p15RS enhances the interaction of β-catenin and TCF4. HA-TCF4 and FLAG-β-catenin were co-expressed with Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A in HEK293T cells. The cell lysates were subjected to an IP experiment with an anti-FLAG antibody and examined by Western blotting using the indicated antibodies. G and H, dimerization of p15RS is critical for its occupancy on the TBS of Wnt-targeted gene promoters. HEK293T cells were transfected with Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A and cross-linked to perform a ChIP assay using an anti-Myc antibody. The co-immunoprecipitated DNA was examined by PCRs or quantitative PCRs using specific primers for TBS of CCND 1 (G) or c-MYC (H). I, dimerization of p15RS is crucial for its blockage of the TCF4·β-catenin complex to occupy on the TBS of c-MYC. HA-TCF4 and FLAG-β-catenin were co-expressed with Myc-tagged p15RS, p15RSL248P/L255P, or Flag-p15RSL248A/L255A in HEK293T cells. Cells were cross-linked to perform a ChIP assay using an anti-β-catenin antibody. Primers specifically recognizing the TBS of c-MYC were used to detect and quantify the precipitated DNAs. J, β-catenin weakens the dimeric interaction of p15RS. HEK293T cells transfected with GST-p15RS, Myc-p15RS, or FLAG-β-catenin were precipitated by GST beads and the dimerized p15RS was examined by Western blotting using an anti-Myc antibody.
p15RS was proved to interact with β-catenin and TCF4 (12). To address whether the dimeric interaction of p15RS alters its affinity in forming a complex with β-catenin or TCF4, we performed IP experiments to examine the association of TCF4, β-catenin, and p15RS mutants. The results showed that the interaction of p15RSL248P/L255P with TCF4 was decreased compared with the affinity of WT p15RS with TCF4 (Fig. 3C, P versus WT). However, the interaction of p15RSL248P/L255P with β-catenin was dramatically increased compared with WT p15RS and p15RSL248A/L255A (Fig. 3D, P). These results suggested that dimerized p15RS (WT) prefers to interact with TCF4, whereas p15RS monomer (L245P/L255P mutant) tends to associate with β-catenin. Consistently, a GST-pulldown assay using proteins purified from eukaryotic cells confirmed that p15RSL248P/L255P interacted with β-catenin with a higher affinity (Fig. 3E). To further address whether dimerization of p15RS affects the complex formation of β-catenin·TCF4, we examined the interaction of β-catenin and TCF4 under overexpression of WT or mutated p15RS. An IP result showed that the interaction of FLAG-β-catenin with HA-TCF4 was recovered when p15RSL248P/L255P was co-expressed (Fig. 3F, P), whereas WT p15RS and p15RSL248A/L255A decreased the interaction compared with the positive control (Fig. 3F, WT and A versus EV). This result indicated that WT p15RS and p15RSL248A/L255Ablocked the interaction of β-catenin and TCF4, whereas p15RSL248P/L255P failed to maintain this blockage. All these data further suggested that dimerization of p15RS participates in interrupting TCF4·β-catenin complex formation.
To investigate whether the dimerization of p15RS is required for its occupancy on promoters of Wnt-targeted genes (12), we performed ChIP assays using primers recognizing a TCF4-binding sequence (TBS) within promoters of CCND1 and c-MYC, two typical Wnt downstream genes. The results showed that unlike WT p15RS and p15RSL248A/L255A, occupancies of p15RSL248P/L255P on the TBS of CCND1 (Fig. 3G) or c-MYC (Fig. 3H) were weakened, suggesting that aberrant dimerization of p15RS impairs its occupancy on the promoters of Wnt-targeted genes. Furthermore, we observed that both WT p15RS and p15RSL248A/L255A significantly inhibited the binding of β-catenin and TCF4 on the TBS, whereas p15RSL248P/L255P failed to block their occupancy (Fig. 3I). These results suggested that dimerization of p15RS facilitates its inhibition on Wnt downstream genes transcription.
To examine whether β-catenin affects the dimerization of p15RS, we performed a GST pulldown assay using cells overexpressing GST-tagged and Myc-tagged p15RS together with β-catenin (Fig. 3J). The result showed that the presence of β-catenin decreased homo-interaction of p15RS, indicating that β-catenin might disassociate the dimerized p15RS. Further analyses revealed that the RPR domain of p15RS exhibited an enhanced interaction with β-catenin compared with full-length p15RS, whereas the CCT domain failed to interact with β-catenin (Fig. S1A). We also confirmed that a region from residue 407 to 533 in β-catenin was required for its interaction with p15RS (Fig. S1, B and C). Based on these observations, we proposed that β-catenin may compete with p15RS through its high affinity with the p15RS monomer and prevent p15RS from inhibiting the transcription of Wnt-targeted genes.
Dimerization is required for p15RS to inhibit tumor cell proliferation and invasion
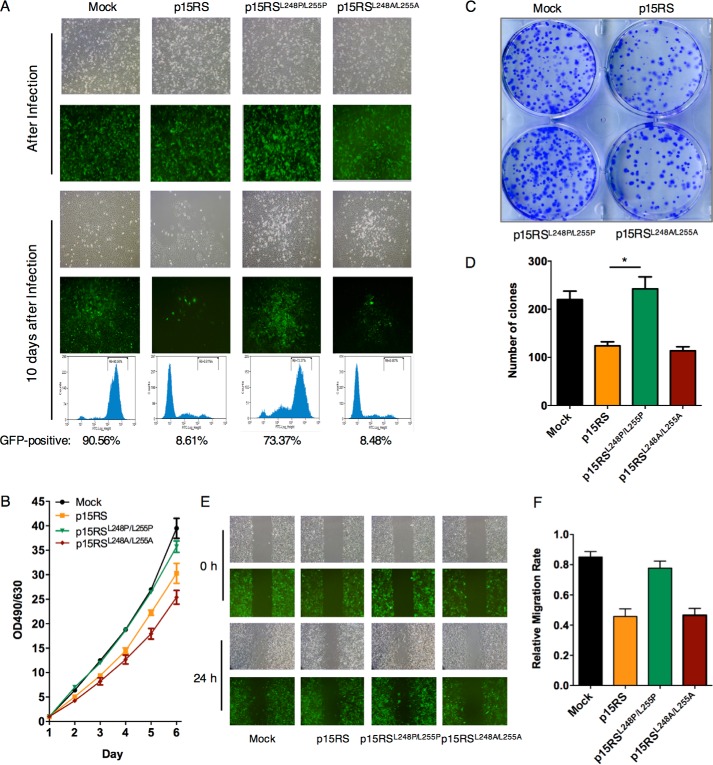
To address whether dimerization of p15RS affects cell growth through its participation in the Wnt signaling pathway, we examined cell proliferation in metastatic melanoma A375 cells lines stably overexpressing pLVX-GFP-p15RS, pLVX-GFP-p15RSL248P/L255P, or pLVX-GFP-p15RSL248A/L255A. Right after lentivirus infection, the same number of GFP-positive cells among groups were seeded (Fig. 4A, top panels). Along cell passages, we were surprised to observe that the amounts of GFP-positive cells overexpressing p15RS or p15RSL248A/L255A decreased dramatically, whereas p15RSL248P/L255P overexpression exerted no inhibitory effect in cell growth, similar with the negative control cell line overexpressing pLVX vector (Fig. 4A, bottom panels). FACS analyses indicated that GFP-positive cells account for 90.56 and 73.37% in the mock and p15RSL248P/L255Pgroups but decreased to 8.61 and 8.49% in the groups overexpressing p15RS and p15RSL248A/L255A (Fig. 4A, FACS). Consistently, a MTT assay confirmed that p15RSL248P/L255P loses the ability to inhibit cell proliferation (Fig. 4B). Together, these results suggested that the ability of p15RS to inhibit cell proliferation was weakened by aberrant dimerization.
Figure 4.
p15RS dimerization participates in the inhibition of cell proliferation. A, p15RSL248P/L255P failed to inhibit cell proliferation. A375 cells were infected with pLVX/GFP-tagged p15RS, p15RSL248P/L255P, or p15RSL248A/L255A lentivirus packaged by pLVX plasmid system. The same efficiency of infection was set for overexpression of p15RS and its mutants. Brightfield and fluorescence were shown. After a 10-day culture, GFP-positive cells were counted by FACS. B, dimerization of p15RS is important for cell growth inhibition. 1 × 103 A375 cells stably overexpressing GFP-tagged p15RS, p15RSL248P/L255P, or p15RSL248A/L255A by pLVX lentivirus were seeded in triplicate on 96-well plates and cultured for the indicated time periods. Cell densities were measured at OD490 nm/OD630 nm. C and D, disabled dimerization loses the ability to inhibit cell clone formation. A375 stable cell lines were seeded on 6-well plates at a density of 1 × 103 cells/well and maintained for 2 weeks for the colony formation. Cells were fixed with methanol and stained with 0.1% crystal violet (C). Colony numbers were counted from three independent experiments (D). Data are presented as mean ± S.D. *, p < 0.05. E and F, the effect of dimerization on cell migration. Monolayer A375 cells stably overexpressing GFP-tagged p15RS, p15RSL248P/L255P, or p15RSL248A/L255A were scratched with sterile pipette tips. Cell migration was observed and photographed by microscopy 24 h later (E). The migration rates were measured by the software ImageJ (F). Data are presented as mean ± S.D. **, p < 0.01.
To examine whether dimerization of p15RS influences the colony formation ability of tumor cells, we performed a clone formation experiment in the A375 cells. The result showed that stable overexpression of p15RS or p15RSL248A/L255A inhibited clone formation but p15RSL248P/L255P overexpressing cells formed more colonies (Fig. 4C). A statistics analysis confirmed that the colony numbers of p15RSL248P/L255P cells were significantly increased compared with other groups (Fig. 4D). To address whether p15RS dimerization affects the invasion of tumor cells, we performed a wound-healing experiment. The data showed that overexpression of p15RSL248P/L255P failed to inhibit cell migration (Fig. 4, E and F). Taken together, these results suggested that p15RS loses its ability to inhibit tumor cell proliferation and invasion when its dimerization ability is broken.
Dimerization of p15RS plays a crucial role in tumor suppression
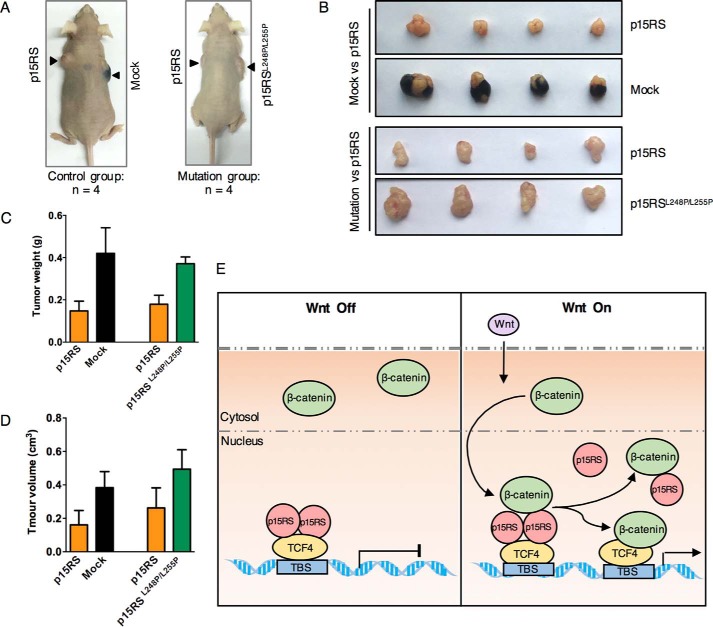
To investigate whether the dimeric ability of p15RS is involved in its inhibition on tumor growth, we performed a tumorigenesis assay by injecting A375 overexpressing cell lines into BALB/c nude mice through their armpits (Fig. 5A). The results showed that p15RS suppressed tumor formation compared with mock cells, whereas p15RSL248P/L255P-overexpressing cells exhibited a similar ability to form tumors compared with the mock cells (Fig. 5B). Consistently, we observed that the weight (Fig. 5C) and size (Fig. 5D) of the tumors were dramatically decreased by the overexpression of WT p15RS, whereas restored, although not completely, by p15RSL248P/L255P. All these results suggested that dimerization of p15RS is critical for tumor suppression.
Figure 5.
Dimerization of p15RS plays a critical role in tumor suppression. A–D, p15RSL248P/L255P exhibits little inhibitory effect on tumor formation. Tumorigenesis assay was performed by injecting 2 × 105 of the indicated A375 cells into BALB/c nude mice. Each mouse was injected with control cells and testing cells bilaterally into the armpit (A). Tumors were cultured to reach a diameter of about 1.5 cm (B). The weight (C) and size (D) of the tumors were collected and calculated. Data are presented as mean ± S.D. E, a proposed model of how dimerization of p15RS participates in Wnt signaling inhibition. Under Wnt quiescence conditions (Off), dimerized p15RS associates with TCF4 to suppress its transcription. When Wnt signaling is activated (On), β-catenin is accumulated and translocated into nucleus, where β-catenin disassociates the dimerized p15RS by preferably interacting with monomer p15RS. In such a way, β-catenin competes with dimerized p15RS and then forms a complex with TCF4 to activate transcription.
Based on all the results, we proposed that dimerization of p15RS is critical for its down-regulation of Wnt signaling and thus for tumor suppression (Fig. 5E). Dimerization of p15RS facilitates its association with TCF4 and thereafter maintains TCF4 in an inactive status without Wnt signaling. However, when Wnt signaling is activated, nuclear β-catenin binds with p15RS monomer with increased affinity so that it may break down the dimerized p15RS. Due to decreased association between monomer p15RS and TCF4, dimerized p15RS is removed from TCF4 so as to allow β-catenin to associate with TCF4, and to initiate transcription of Wnt-targeted genes.
Discussion
Dimerization is proved to be a key process in the activity regulation of proteins, including enzymes, ion channels, receptors, and transcription factors (18). Previous crystal structure analyses demonstrated that the CCT domain of p15RS owns a potential to form homodimer (14, 15). However, the function of the homodimerization of full-length p15RS remained unknown. In this study, we revealed that dimerization of p15RS is required for its inhibitory role on Wnt signaling. We used biochemistry experiments to confirm the dimerization of full-length p15RS, similar to the CCT domain. In particular, we identified several leucine residues responsible for the dimeric interaction. We speculated that the leucine zipper–like motif within the CCT domain holds two p15RS molecules together to form an increased association interface with TCF4. Because the size of the association interface tends to correlate with the affinity of interaction between two proteins (28), we proposed that the dimerized p15RS has an increased affinity to interact with TCF4. Therefore, dimerized p15RS exhibits a stronger ability to inhibit the transcriptional activity of TCF4 and to maintain Wnt signaling in a quiescent status. Accordingly, blocking the interface by de-dimerization of p15RS appears to impair the association of p15RS with TCF4. Our study has provided compelling evidence for the association of p15RS with TCF4 using the mutants that lost the ability of dimerization. Furthermore, we identified that β-catenin, by interacting with the monomer p15RS, interrupts the dimerization of p15RS, leading to release of p15RS from TCF4. Our model illustrated that β-catenin eventually interacts with TCF4 to activate the transcription of Wnt-targeted genes after dimerized p15RS is broken and released from the promoter (Fig. 5E). This study provided a detailed molecular mechanism of how β-catenin compete with dimerized p15RS during its binding to TCF4 and thus to activate transcription of Wnt-targeted genes (12).
Our model is supported by the mutation of the leucine zipper–like motif where Leu-248 and Leu-255 are critical residues. Indeed, mutation of leucines 248 and 255 into prolines or alanines impaired the dimeric interaction (Fig. 2, D–F). Interestingly, mutation of leucine 248 and 255 into alanines appeared with equally strong dimerized interaction by the formaldehyde cross-linking experiment (Fig. 2G) and weak interaction by the IP experiment in mammalian cells (Fig. 2, D and F), whereas with no interaction by the GST pulldown experiment using proteins purified from E. coli (Fig. 2E). Functional studies showed that mutation into proline completely lost, but mutation into alanine remained, the ability of p15RS to inhibit cell proliferation and invasion (Fig. 4). These results were also consistent to the luciferase analyses on the transcription activity by the Wnt reporter (Fig. 3, A and B) and the IP experiments for the effect of p15RS on β-catenin·TCF4 complex formation (Fig. 3F). Therefore, we concluded that mutation of leucine 248 and 255 into proline lost the dimerization ability and thereafter impaired the ability to inhibit Wnt signaling. However, we could not exclude the role of the other two leucines, which also fit the heptad structure in the leucine zipper–like motif, on the formation of p15RS dimer.
We have proved our model that dimerized WT p15RS interacts strongly with TCF4 but the mutated monomer (L248P/L255P) diminishes the interaction with TCF4 (Fig. 3C). It echoes to the strong interaction of β-catenin with monomer p15RS (L248P/L255P) (Fig. 3, D and E, and Fig. S2). Furthermore, we observed that dimerized p15RS was dramatically reduced in the presence of β-catenin (Fig. 3J). Consistently, we found that the RPR domain, in a monomer status, interacted with residues 407–533 of β-catenin with a higher affinity, compared with full-length p15RS (Fig. S1A). Together with the results of the occupancy of p15RS, TCF4, and β-catenin (Fig. 3, G–I), we proposed that β-catenin, after being translocated into the nucleus upon Wnt stimulation, breaks down the dimerized p15RS into monomer, to abrogate the ability of p15RS to associate with TCF4. However, because the association of proteins is a dynamic process, we could not exclude the possibility that β-catenin may block the dimer formation by sequestering monomer p15RS. Further analyses are needed to decipher whether β-catenin disrupts the dimerized p15RS or blocks the formation of dimer from monomer p15RS in the nucleus (29, 30). Also, our previous studies revealed that p15RS also associated with HDAC2 (17) and its homologues CREPT interacted with HDAC1 (10). Whether the dimerization of p15RS is critical for its association with HDAC2 remains to be unveiled.
Previous studies demonstrated that p15RS has malignancy-inhibitory functions. In this study, we confirmed that p15RS suppresses cell proliferation and invasion. We further identified that dimerization of p15RS is critical for its inhibitory role in tumor cell growth and formation. Due to the participation of p15RS in the Wnt signaling pathway, we therefore speculated that p15RS inhibits cell proliferation via down-regulating the Wnt signaling pathway. Because aberrant Wnt signal regulations always cause a wide spectrum of cancers (31), we speculate that p15RS may be a potent tumor suppressor. This study validated that p15RS dimerization is required for p15RS to fulfill its inhibitory effects on tumor cells. We have identified that Leu-248/Leu-255 are critical residues to maintain the dimerization and the inhibition role of p15RS on tumorigenesis. We speculate that loss of p15RS dimerization may act as a key to trigger tumorigenesis. However, it remains unclear whether the Leu-248/Leu-255 mutation occurs in human cancers. Our preliminary mutagenesis analyses in cBioPortal for Cancer Genomics based on the TCGA database showed that most of the single amino acid mutations in p15RS in cancer patients are located in the CCT domain, although not Leu-248/Leu-255 (data not shown). Nevertheless, we expect that, along with the emergence of techniques like PROTAC (32), facilitating the dimerization of p15RS may become a way to cure cancers.
Experimental procedures
Plasmids and reagents
Myc-p15RS, Myc-p15RS/RPR, Myc-p15RS/CCT, Wnt1, FLAG-β-catenin, and HA-TCF4 plasmids were constructed or obtained previously (12, 15, 17). Flag-p15RS, Flag-p15RS/RPR, Flag-p15RS/CCT, prokaryotic GST-p15RS expressing construct, and eukaryotic GST-p15RS were generated by inserting the PCR-amplified fragments into a pCDNA3.1/Flag vector, a pGEX-4T-1, or a pXJ40 vector. Site-directed mutations were generated by two pairs of primers (Sangon Biotech Co. Ltd., Shanghai, China) designed to mutate the target amino acids Leu-248 and Leu-255: L248P/L255P (assigned as p15RSL248P/L255P), 5′-AAGGAAGCCCCCGCAGAGAAA-3′ and 5′-TTGACAACGGGGAAAATCTGCT-3′; L248A/L255A (assigned as p15RSL248A/L255A), 5′-AAGGAAGCCGCTGCAGAGAAA-3′ and 5′-TTGACAACGAGCAAAATCTGCT-3′. SuperTop-luciferase reporter was a gift from Dr. Wei Wu (School of Life Science, Tsinghua University, China). Anti-β-actin (AC-15) and anti-FLAG (M2) were from Sigma. Anti-Myc (9E10), anti-β-catenin, anti-TCF4, anti-p15RS, and anti-HA (F-7) were purchased from Santa Cruz Biotechnology. Fluorescent secondary antibodies (goat anti-rabbit IgG and goat anti-mouse IgG) were purchased from Jackson ImmunoResearch Laboratories.
Cell culture and transfection
HEK293T cells were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum and penicillin (100 units/ml)/streptomycin (100 units/ml). A375 cells were cultured in RPMI medium 1640 basic (×1) (Gibco) with 10% fetal bovine serum and penicillin (100 units/ml)/streptomycin (100 units/ml). All cells were grown at 37 °C with 5% CO2. Serum were purchased from Invitrogen. Stable cell lines were selected by FACS after being infected with specific lentivirus. Cells were transfected with plasmids as indicated using Vigofect (Vigorous Inc., Beijing, China) according to the manufacturer's protocols.
Protein extraction and purification
Prokaryotic GST-p15RS was expressed in E. coli BL21 for 4 h at 30 °C and induced with 0.5 mm isopropyl 1-thio-β-galactopyranoside at 30 °C for 4 h. E. coli cells were harvested and sonicated. Glutathione S-transferase (GST) fusion proteins were purified by GST–Sepharose 4B (GE Healthcare) according to the manufacturer's instructions. Eukaryotic GST fusion proteins were purified by adding GST–Sepharose 4B beads into HEK293T cell lysates expressing pXJ40/p15RS, pXJ40/p15RSL248P/L255P, and pXJ40/p15RSL248A/L255.
Immunoprecipitation assay (IP), GST pulldown, and Western blotting
For immunoprecipitation assays, HEK293T cells were plated in 60-mm dishes transfected with the indicated plasmids. Cells were harvested in RIPA buffer (50 mm Tris-Cl, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1% SDS, pH 8.0) together with protease inhibitors after 24–48 h transfection. Whole cell lysates were incubated with the indicated antibodies and protein G/A-Sepharose beads at 4 °C overnight. After washing the beads with lysis buffer (50 mm, pH 7.6, Tris-Cl, 150 mm NaCl, 0.5 m EDTA, 0.05% Nonidet P-40) for 4 times, precipitates were eluted with 2× SDS-PAGE sample buffer from the beads and analyzed by Western blotting with the indicated antibodies. GST pulldown was performed by adding prokaryotic or eukaryotic purified GST–tagged proteins into cell lysates expressing targeted proteins and then following the IP procedures after incubation.
Cross-linking assay
Transiently transfected HEK293T cells were cross-linked by 1% formaldehyde for 30 min at 37 °C and 0.25 m glycine was added for 5 min at room temperature to stop the cross-linking reaction. Cells were then collected and lysed directly in 2× SDS-PAGE sample buffer and analyzed by Western blotting with specific antibodies.
Immunofluorescent analysis
Cells were plated and incubated overnight on glass coverslips placed in 6-well dishes ahead of use. 24 h after being transfected with specific plasmids, cells were rinsed with PBS, fixed with 4% paraformaldehyde for 15–20 min at room temperature, and permeated with 0.3% Triton X-100 for 10 min. After blocking with 10% goat serum for 1 h at room temperature, cells were incubated with thr indicated primary antibodies at 4 °C overnight. Secondary antibodies conjugated with FITC (green) were added for 1 h at room temperature to detect bound primary antibodies. 4′,6-Diamidino-2-phenylindole were applied to indicate the nucleus. Stained cells were analyzed by a laser scanning confocal microscopy (Zeiss780) at ×40 oil lens.
Luciferase assay
HEK293T and A375 cells were plated in 24-well dishes and transfected with the indicated plasmids together with a SuperTOP-luciferase reporter plasmid and an internal control pRL-TK vector (Promega, Madison, WI). 24 h after transfection, the luciferase activity was determined by the Dual-Luciferase Assay System (Vigorous Inc., Beijing, China). The firefly luciferase activity was normalized by Renilla luciferase activity and presented as mean ± S.D. from triplicate experiments.
Chromatin immunoprecipitation (ChIP) assay
Cells were cross-linked by 1% formaldehyde at 37 °C for 10–15 min and then treated with 125 mm glycine for 5 min. Cells were collected and suspended in 500 μl of ChIP/SDS lysis buffer. The mixture was then sonicated for 10 s and pulsed with a 30-s interval at 4% output power to yield DNA fragments of 200 to 500 bp in size. After centrifugation, lysates containing protein·DNA complexes were diluted 10-fold and immunoprecipitated with the indicated antibodies. The precipitated complexes were de-associated by 5 m NaCl and treated with protein kinase K to remove proteins. DNAs were eluted by phenol/chloroform extraction and analyzed by PCR. The fragment corresponding to the TCF4-binding site in the CCND1 promoter was amplified by primers (33): 5′-CACCTCCACCTCACCCCCTAAATCC-3′ and 5′-ACTCCCCTGTAGTCCGTGTGACG-TT-3′, and that from c-MYC promoter was amplified with 5′-TTGCTGGGTTATTTTAATCAT-3′ and 5′-ACTGTTTGACAAACCGCATCC-3′ (34).
Real time-PCR
Real time-PCR was performed to quantify samples from ChIP assays, using a Real-MasterMix (SYBR Green) kit (TIANGEN Biotech, Beijing, China) under the following conditions: denature, 95 °C, 20 s; annealing, 60 °C, 20 s; and extension, 72 °C, 30 s. Primers were the same as those used in ChIP assays.
MTT assay and wound-healing assay
For the MTT assay, 1 × 103 A375 stable cells were seeded in triplicate in 96 wells. After culture for the indicated times, 20 μl of MTT (5 mg/ml) was added for 4 h. Cells were then dissolved in 150 μl of DMSO for 10 min in the dark. OD490 and OD630 were read using a Bio-Rad model 680 microplate reader. Data were collected for 7 days. For the wound-healing assay, monolayer cells were wounded by a sterile plastic tip. Cell migration was observed by microscopy 24 h later.
Colony formation assay
Cells were seeded on 6-well plates at a density of 500 cells/well. After being cultured for 2 weeks, cells were fixed with methanol for 10 min and incubated with 0.1% crystal violet (Sigma) for 10 min at room temperature. The number of colonies was counted and presented as mean ± S.D.
Tumorigenesis assay
A375 cells (2 × 105 cells/mouse) were injected subcutaneously into 6-week-old BALB/c nude mice, with control cells and tested cells bilaterally at the armpit of each mouse. When tumors were about 1.5 cm in diameter, mice were sacrificed and tumor tissues were collected, weighted, and measured in size. This protocol follows the institutional guidelines and regulations on the animal health and ethics, approved by the animal health and ethics committee in Tsinghua University.
Statistical analyses
All experiments were repeated at least 3 times. Data were presented as mean ± S.D. Significant differences between groups were determined using a Student's t test.
Author contributions
X. F., J. Z., F. R., Y. W., Y. S., J. L., J. N., B. J., Y. L., and Z. C. conceptualization; X. F., F. R., Y. W., Y. F., L. D., B. J., Y. L., and Z. C. resources; X. F., J. Z., F. R., Y. W., Y. F., L. D., L. Z., Y. S., J. L., J. N., B. J., Y. L., and Z. C. data curation; X. F., J. Z., F. R., L. D., L. Z., and Y. S. software; X. F., J. Z., F. R., Y. W., Y. F., L. D., L. Z., Y. S., J. L., J. N., B. J., Y. L., and Z. C. formal analysis; X. F., F. R., Y. W., Y. F., J. L., J. N., B. J., Y. L., and Z. C. supervision; X. F., B. J., Y. L., and Z. C. funding acquisition; X. F., J. Z., F. R., Y. W., Y. F., L. D., L. Z., Y. S., J. L., B. J., Y. L., and Z. C. validation; X. F., J. Z., Y. W., Y. F., Y. S., J. L., J. N., B. J., Y. L., and Z. C. investigation; X. F., J. Z., F. R., L. D., L. Z., Y. S., B. J., Y. L., and Z. C. visualization; X. F., J. Z., F. R., Y. W., Y. F., L. D., L. Z., Y. S., J. L., J. N., B. J., Y. L., and Z. C. methodology; X. F. and J. Z. writing-original draft; X. F., F. R., Y. W., Y. F., L. D., B. J., Y. L., and Z. C. project administration; X. F., J. Z., F. R., Y. W., Y. F., L. D., L. Z., B. J., Y. L., and Z. C. writing-review and editing.
Supplementary Material
Acknowledgments
We thank Dr. Robert N. Eisenman, Fred Hutchinson Cancer Research Center, for suggestions and support to this project. We thank Dr. Xi He, Harvard University, and Dr. Wei Wu, Tsinghua University, for the Wnt-related plasmids they have kindly provided. We thank Dr. John M. Denu from University of Wisconsin for editing our manuscript.
This work was supported by Chinese National Major Scientific Research Program Grant 2016YFA0500301 and National Natural Science Foundation of China Grants 81230044, 81372167, 81572729, 81572728, 81402293, and 81372372. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1 and S2.
- TCF
- T cell–specific factor
- CCT
- coiled-coil terminus
- RPR
- regulation of nuclear pre-mRNA
- TBS
- TCF4-binding sequence
- GST
- glutathione S-transferase
- IP
- immunoprecipitation
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
References
- 1. Logan C. Y., and Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 2. Shang S., Hua F., and Hu Z. W. (2017) The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8, 33972–33989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacDonald B. T., Tamai K., and He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deitrick J., and Pruitt W. M. (2016) Wnt/β catenin-mediated signaling commonly altered in colorectal cancer. Prog. Mol. Biol. Transl. Sci. 144, 49–68 10.1016/bs.pmbts.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 5. He B., and Jablons D. M. (2006) Wnt signaling in stem cells and lung cancer. Ernst Schering Found. Symp. Proc. 27–58 [DOI] [PubMed] [Google Scholar]
- 6. Kaur A., Webster M. R., and Weeraratna A. T. (2016) In the Wnt-er of life: Wnt signalling in melanoma and ageing. Br. J. Cancer 115, 1273–1279 10.1038/bjc.2016.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xue G., Romano E., Massi D., and Mandalà M. (2016) Wnt/β-catenin signaling in melanoma: preclinical rationale and novel therapeutic insights. Cancer Treat Rev. 49, 1–12 10.1016/j.ctrv.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 8. Liu J., Liu H., Zhang X., Gao P., Wang J., and Hu Z. (2002) Identification and characterization of P15RS, a novel P15(INK4b) related gene on G1/S progression. Biochem. Biophys. Res. Commun. 299, 880–885 10.1016/S0006-291X(02)02684-0 [DOI] [PubMed] [Google Scholar]
- 9. Lu D., Wu Y., Wang Y., Ren F., Wang D., Su F., Zhang Y., Yang X., Jin G., Hao X., He D., Zhai Y., Irwin D. M., Hu J., Sung J. J., Yu J., Jia B., and Chang Z. (2012) CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell 21, 92–104 10.1016/j.ccr.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y., Liu C., Duan X., Ren F., Li S., Jin Z., Wang Y., Feng Y., Liu Z., and Chang Z. (2014) CREPT/RPRD1B, a recently identified novel protein highly expressed in tumors, enhances the β-catenin:TCF4 transcriptional activity in response to Wnt signaling. J. Biol. Chem. 289, 22589–22599 10.1074/jbc.M114.560979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin K., Chen H., Zuo Q., Huang C., Zhao R., Yu X., Wang Y., Zhang Y., Chang Z., and Li B. (2018) CREPT and p15RS regulate cell proliferation and cycling in chicken DF-1 cells through the Wnt/β-catenin pathway. J. Cell. Biochem. 119, 1083–1092 10.1002/jcb.26277 [DOI] [PubMed] [Google Scholar]
- 12. Wu Y., Zhang Y., Zhang H., Yang X., Wang Y., Ren F., Liu H., Zhai Y., Jia B., Yu J., and Chang Z. (2010) p15RS attenuates Wnt/β-catenin signaling by disrupting β-catenin-TCF4 interaction. J. Biol. Chem. 285, 34621–34631 10.1074/jbc.M110.148791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X., Cao Q., Liu X., Liu S., Wang J., Sun S., Wang O., Tian Z., Liu H., Kuang J., and Zhang W. (2012) Cellular and molecular evidence for malignancy-inhibitory functions of p15RS. Cell Cycle 11, 1988–1998 10.4161/cc.20400 [DOI] [PubMed] [Google Scholar]
- 14. Ni Z., Xu C., Guo X., Hunter G. O., Kuznetsova O. V., Tempel W., Marcon E., Zhong G., Guo H., Kuo W. W., Li J., Young P., Olsen J. B., Wan C., Loppnau P., El Bakkouri M., et al. (2014) RPRD1A and RPRD1B are human RNA polymerase II C-terminal domain scaffolds for Ser5 dephosphorylation. Nat. Struct. Mol. Biol. 21, 686–695 10.1038/nsmb.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mei K., Jin Z., Ren F., Wang Y., Chang Z., and Wang X. (2014) Structural basis for the recognition of RNA polymerase II C-terminal domain by CREPT and p15RS. Sci. China Life Sci. 57, 97–106 10.1007/s11427-013-4589-7 [DOI] [PubMed] [Google Scholar]
- 16. Ni Z., Olsen J. B., Guo X., Zhong G., Ruan E. D., Marcon E., Young P., Guo H., Li J., Moffat J., Emili A., and Greenblatt J. F. (2011) Control of the RNA polymerase II phosphorylation state in promoter regions by CTD interaction domain-containing proteins RPRD1A and RPRD1B. Transcription 2, 237–242 10.4161/trns.2.5.17803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C., Zhang Y., Li J., Wang Y., Ren F., Zhou Y., Wu Y., Feng Y., Zhou Y., Su F., Jia B., Wang D., and Chang Z. (2015) p15RS/RPRD1A (p15INK4b-related sequence/regulation of nuclear pre-mRNA domain-containing protein 1A) interacts with HDAC2 in inhibition of the Wnt/β-catenin signaling pathway. J. Biol. Chem. 290, 9701–9713 10.1074/jbc.M114.620872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marianayagam N. J., Sunde M., and Matthews J. M. (2004) The power of two: protein dimerization in biology. Trends Biochem. Sci. 29, 618–625 10.1016/j.tibs.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 19. Beckett D. (2001) Regulated assembly of transcription factors and control of transcription initiation. J. Mol. Biol. 314, 335–352 10.1006/jmbi.2001.5134 [DOI] [PubMed] [Google Scholar]
- 20. Amoutzias G. D., Robertson D. L., Van de Peer Y., and Oliver S. G. (2008) Choose your partners: dimerization in eukaryotic transcription factors. Trends Biochem. Sci. 33, 220–229 10.1016/j.tibs.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 21. Vinson C., Acharya A., and Taparowsky E. J. (2006) Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim. Biophys. Acta 1759, 4–12 10.1016/j.bbaexp.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 22. Landschulz W. H., Johnson P. F., and McKnight S. L. (1988) The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240, 1759–1764 10.1126/science.3289117 [DOI] [PubMed] [Google Scholar]
- 23. Hattori T., Ohoka N., Inoue Y., Hayashi H., and Onozaki K. (2003) C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene 22, 1273–1280 10.1038/sj.onc.1206204 [DOI] [PubMed] [Google Scholar]
- 24. Halazonetis T. D., Georgopoulos K., Greenberg M. E., and Leder P. (1988) c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 55, 917–924 10.1016/0092-8674(88)90147-X [DOI] [PubMed] [Google Scholar]
- 25. Hope I. A., and Struhl K. (1987) GCN4, a eukaryotic transcriptional activator protein, binds as a dimer to target DNA. EMBO J. 6, 2781–2784 DOI not found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yaku H., and Mizuno T. (1997) The membrane-located osmosensory kinase, EnvZ, that contains a leucine zipper–like motif functions as a dimer in Escherichia coli. FEBS Lett. 417, 409–413 10.1016/S0014-5793(97)01329-X [DOI] [PubMed] [Google Scholar]
- 27. Xie W., Wen H., Chu F., Yan S., Xie W., Lin B., Chen Y., Li Z., Ren G., Song Y., Zhao L., and Wang Z. (2015) Mutations in the leucine zipper–like motif of the human parainfluenza virus 3 fusion protein impair fusion activity. Intervirology 58, 297–309 10.1159/000441978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matthews J. M., and Sunde M. (2012) Dimers, oligomers, everywhere. Adv. Exp. Med. Biol. 747, 1–18 10.1007/978-1-4614-3229-6_1 [DOI] [PubMed] [Google Scholar]
- 29. Poy F., Lepourcelet M., Shivdasani R. A., and Eck M. J. (2001) Structure of a human Tcf4-β-catenin complex. Nat. Struct. Biol. 8, 1053–1057 10.1038/nsb720 [DOI] [PubMed] [Google Scholar]
- 30. Sampietro J., Dahlberg C. L., Cho U. S., Hinds T. R., Kimelman D., and Xu W. (2006) Crystal structure of a β-catenin/BCL9/Tcf4 complex. Mol. Cell 24, 293–300 10.1016/j.molcel.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 31. Johnson M. L., and Rajamannan N. (2006) Diseases of Wnt signaling. Rev. Endocr. Metab. Disord. 7, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neklesa T. K., Winkler J. D., and Crews C. M. (2017) Targeted protein degradation by PROTACs. Pharmacol. Ther. 174, 138–144 10.1016/j.pharmthera.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 33. Togi S., Ikeda O., Kamitani S., Nakasuji M., Sekine Y., Muromoto R., Nanbo A., Oritani K., Kawai T., Akira S., and Matsuda T. (2011) Zipper-interacting protein kinase (ZIPK) modulates canonical Wnt/β-catenin signaling through interaction with Nemo-like kinase and T-cell factor 4 (NLK/TCF4). J. Biol. Chem. 286, 19170–19177 10.1074/jbc.M110.189829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xiong B., Rui Y., Zhang M., Shi K., Jia S., Tian T., Yin K., Huang H., Lin S., Zhao X., Chen Y., Chen Y. G., Lin S. C., and Meng A. (2006) Tob1 controls dorsal development of zebrafish embryos by antagonizing maternal β-catenin transcriptional activity. Dev. Cell 11, 225–238 10.1016/j.devcel.2006.06.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.