Abstract
Nontransformed cells that become detached from the extracellular matrix (ECM) undergo dysregulation of redox homeostasis and cell death. In contrast, cancer cells often acquire the ability to mitigate programmed cell death pathways and recalibrate the redox balance to survive after ECM detachment, facilitating metastatic dissemination. Accordingly, recent studies of the mechanisms by which cancer cells overcome ECM detachment–induced metabolic alterations have focused on mechanisms in redox homeostasis. The insights into these mechanisms may inform the development of therapeutics that manipulate redox homeostasis to eliminate ECM-detached cancer cells. Here, we review how ECM-detached cancer cells balance redox metabolism for survival.
Keywords: cell death, anoikis, cancer, reactive oxygen species (ROS), cell metabolism, extracellular matrix, apoptosis, redox regulation
Introduction
The metastasis of cancerous cells to distant and vital organs is responsible for an excess of 90% of cancer mortalities (1), yet the molecular mechanisms cancer cells use to successfully metastasize remain ill defined. It has become apparent that to effectively metastasize, cancer cells must inhibit anoikis, a caspase-dependent cell death pathway induced by loss of integrin-mediated attachment to extracellular matrix (ECM)2 (2, 3). Anoikis induction is not limited to ECM-detached cells as even the attachment to abnormal or foreign ECM can induce cell death (4). As such, we posit that anoikis inhibition will be critically important throughout the metastatic cascade, from local invasion and survival in the circulation to implantation at a secondary site. The deficiency of proper integrin-mediated signaling during ECM detachment has been demonstrated to alter a plethora of distinct signal transduction cascades. Oftentimes, these modifications in survival signaling converge on the Bcl-2 family of proteins that modulate the release of cytochrome c from the intermembrane space. Multiple signaling pathways regulating mitochondrial permeabilization during anoikis have tk;4been unveiled in numerous and disparate contexts (5–12). A fundamental implication of these findings is that cancer cells can employ a multifaceted arsenal of diverse and overlapping strategies to block anoikis induction leading to their survival.
Despite these recent advances in understanding anoikis, a number of pre-clinical studies have made clear that ECM detachment induces a wide variety of cellular changes that can ultimately lead to cell death and are independent of classical anoikis induction. For example, ECM-detached cells undergo autophagy (13), which involves the catabolism of intracellular organelles and can serve as both a survival mechanism under conditions of nutrient starvation (14) or an alternative form of cell death if left unchecked (15). Independent of anoikis activation, ECM detachment induces entosis, a type of programmed cell death characterized by cells being internalized inside neighboring cells and eliminated in the lysosome (16). The significance of these findings to the survival of cancer cells during the metastatic cascade is an area of active exploration (which will require additional follow-up experiments in a clinical context), but these data undoubtedly reveal that ECM detachment can induce cellular alterations that impact viability independent of anoikis induction.
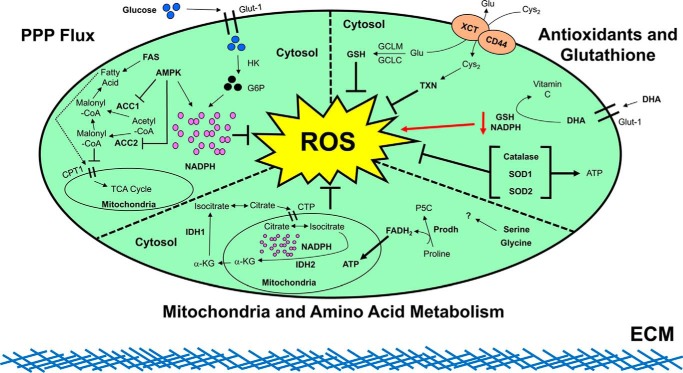
Additionally, ECM detachment is now well-established to cause a host of catastrophic metabolic alterations, including defective glucose uptake, diminished pentose phosphate pathway (PPP) flux, reduced cellular ATP levels, and a robust increase in reactive oxygen species (ROS) (7, 17, 18). This elevated ROS during ECM detachment and the consequences on the viability of cells are now areas of dynamic investigation in a number of different contexts. As an example, recent research has unveiled alterations in citrate metabolism, initiated by reductive carboxylation, and consequent elimination of ROS as a contributor to redox homeostasis during ECM detachment (19). Furthermore, additional studies have revealed that upon detachment of nontransformed mammary epithelial cells from the ECM, they up-regulate pyruvate dehydrogenase kinase 4 (PDK4), which stalls the flux of glycolytic carbon into mitochondrial oxidation (20). As a consequence, the production of ROS due to mitochondrial oxidation is attenuated, and thus ECM-detached cells are more effectively able to survive during ECM detachment. These findings raise the possibility that antagonizing the electron transport chain with inhibitors like metformin may facilitate survival of ECM-detached cells. In support of this possibility, a recent study discovered that metformin treatment could promote the detachment of viable breast cancer cells from the ECM (21). Despite these elegant studies, the precise manner in which ROS are modulated during ECM detachment remain incompletely understood. That being said, taken together, these studies on redox metabolism raise the possibility that cancer cells need to both inhibit anoikis and appropriately regulate ROS to survive during the metastatic cascade (2, 3). Herein, we review the current body of work regarding the understanding of how redox metabolism is controlled during detachment from the ECM (summarized in Fig. 1).
Figure 1.
Summary of ROS modulation during ECM detachment. As shown in the upper left panel, it has been revealed in detached, nontransformed cells that glucose uptake via GLUT1 is diminished during ECM detachment. This deficiency in glucose uptake leads to a low level of glucose 6-phosphate (G6P) and limited flux through the pentose phosphate pathway (PPP). Subsequently, the diminished PPP flux causes a decrease in NADPH leading to an increase in ROS levels, ultimately resulting in cell death. Upon expression of the oncogene ERBB2 in these cells, defective glucose uptake is restored, leading to abundant glucose 6-phosphate production, rescue of PPP flux, and subsequent NADPH generation to fortify ROS defenses. Continuing in the upper left panel, it has been shown that when the PPP is antagonized during ECM detachment, AMPK activation blocks cell death by maintaining NADPH levels through fatty acid oxidation-induced NADPH production and by inhibiting NADPH consumption during fatty-acid synthesis (FAS) (via antagonizing ACC1 and ACC2). Progressing to the upper right panel, it has been discovered that synthesis of GSH, driven by the glutamate cysteine ligase modifier (GCLM), is necessary for primary tumor formation. Loss of GCLM prevented a tumor's ability to drive the metastatic cascade, where survival in the absence of ECM attachment is imperative. These data indicate that at the later stages of the metastatic cascade, GSH becomes dispensable due to compensation from alternative antioxidant pathways. Indeed, thioredoxin (TXN) levels were increased when GSH synthesis was blocked. Combinatorial inhibition of TXN and GSH leads to a synergistic block on cancer cell survival, leading to their ultimate death both in vitro and in vivo. Other studies have discovered that high doses of vitamin C, which has well-documented antioxidant activity, can lead to an elevation in intracellular ROS levels in cells with activating mutations in K-Ras or B-Raf. The increase in ROS is first initiated by cellular uptake of oxidized vitamin C (dehydroascorbate (DHA)) via the glucose transporter, GLUT1. This uptake of DHA leads to a significant increase in ROS levels as intracellular DHA is reduced back to vitamin C at the expense of GSH. Additional studies have explored the role of catalase, SOD1, and SOD2 in mitigating ROS levels to enhance the survival of ECM-detached cells. It was discovered that antagonizing catalase or SOD2 did not impact the viability of ECM-attached cells but specifically compromised the survival of ECM-detached cells. Expanding upon this further, antagonizing catalase or SOD2 attenuated tumor burden in the lungs of immunocompromised mice following tail vein injection. Additional studies demonstrated that SOD2 expression is elevated in human breast cancer metastases compared with primary tumors, a finding consistent with a role for SOD2 in facilitating the survival of ECM-detached cancer cells. Finally, proceeding to the middle panel, it was recently discovered that ECM-detached lung carcinoma cells utilized IDH1 to reductively decarboxylate glutamine to citrate in the cytosol. The newly derived citrate subsequently enters the mitochondria via the citrate transporter protein (CTP) where it participates in oxidative metabolism. The activity of mitochondrion-located IDH2 functions to synthesize NADPH and neutralize mitochondrial ROS in a fashion that promotes survival. Other research has found that proline catabolism via proline dehydrogenase (Prodh) supports growth of breast cancer cells grown in 3D culture but not in 2D culture. The breakdown of proline by Prodh supports mitochondrial ATP production by feeding electrons, in the form of FADH2, into the electron transport chain to ultimately balance redox homeostasis in favor of metastasis formation. Finally, the particular contributions of serine and glycine metabolism to balancing redox homeostasis during ECM detachment remain an interesting topic for future exploration.
Pentose phosphate pathway (PPP)
In a three-dimensional (3D) cell culture model of mammary acini, previous studies have revealed that antagonizing anoikis induction is not sufficient to inhibit the death of ECM-detached cells in the luminal space, suggesting that an anoikis-independent mechanism is involved in lumen generation (22). Given that these centrally located, ECM-bereft cells were populated with autophagic vesicles (23), it was postulated that these cells were nutrient-starved and under significant (potentially lethal) metabolic stress. Indeed, it was discovered that these ECM-detached mammary epithelial cells had a number of substantial metabolic alterations (e.g. deficient glucose uptake and diminished cellular ATP levels) and that rectifying these alterations, independent of modulating anoikis, could promote survival of ECM-detached cells in the luminal space of the mammary acini (17, 18).
These data provoked additional questions regarding the precise link between altered glucose metabolism and the viability of ECM-detached cells. Upon further examination of the consequences of metabolic alterations during ECM detachment, a striking elevation in the levels of intracellular ROS (independent of anoikis induction) was unearthed. Interestingly, cancer cells seemed to benefit from the elimination of ECM detachment-mediated ROS production as overexpression of the ERBB2 oncogene led to luminal filling and diminished ROS levels (18). In addition, ErbB2 signaling also restored robust glucose uptake in ECM-detached cells; a finding that suggested a possible link between glucose metabolism and the generation of ROS. To more directly assess a possible link between oncogenic ErbB2 signaling, glucose metabolism, and antioxidant activity, the investigators turned their attention to the NADPH-generating PPP. Strikingly, inhibition of the PPP compromised the capacity of ErbB2 to promote survival during ECM detachment. Moreover, it subsequently became clear that deficient glucose uptake and diminished flux through the PPP, because of inadequate NADPH production by the PPP, was an important and significant contributor to ROS elevation during ECM detachment (18). These surprising findings motivated the hypothesis that PPP-derived NADPH functions as a significant source of antioxidant activity to promote the survival of ECM-detached cells. As confirmation of these conclusions, it was discovered that treatment of mammary acini with antioxidant compounds (in the absence of any alterations that restore glucose uptake and PPP flux) was sufficient to promote the survival of ECM-detached cells in the luminal space. These findings suggest that antioxidant activity is sufficient and critically important for the survival of ECM-detached cells. Although Schafer et al. (18) discovered a link between ECM detachment-induced ROS and deficient oxidation of fatty acids for ATP generation, the precise mechanism by which this elevated ROS functions to eliminate ECM-detached cells remains elusive.
Other studies have unveiled additional mechanisms to maintain NADPH production and limit ROS generation in circumstances where the PPP is inhibited. During times of energy stress, a signaling pathway that is critically involved in metabolic adaptation is the liver kinase B1 (LKB1)–AMP-activated protein kinase (AMPK) pathway (24). AMPK is activated when cells detach from the ECM, which is dependent on LKB1 and Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) (25). Furthermore, when cells are re-attached to the matrix, re-adhesion subsequently results in dephosphorylation and inactivation of AMPK emphasizing the importance of ECM attachment in activation of AMPK signaling. Moreover, during ECM detachment (where glucose-mediated PPP flux is limited), AMPK activation prolongs cell survival through redox regulation (26). More specifically, AMPK activates additional pathways to maintain NADPH and prolong survival. Interestingly, investigators have discovered that AMPK signaling can inhibit acetyl-CoA carboxylases ACC1 and ACC2 and thus stabilize NADPH levels by diminishing NADPH consumption during fatty-acid synthesis. Furthermore, inhibition of ACC1 or ACC2 functions to elevate NADPH levels through activation of fatty-acid oxidation. Antagonizing ACC1 or ACC2 phenocopies AMPK activation and subsequent NADPH production and promotes anchorage-independent growth. In support of a role for this pathway in tumors, the inhibition of ACC1 or ACC2 promotes solid tumor formation in vivo, while the activation of ACC1 or ACC2 blocks tumor growth. Taken together, AMPK functions as a critical regulator of NADPH generation that is imperative for cancer cell survival during ECM detachment (26).
Interestingly, although flux through the PPP has been demonstrated to be critical for limiting ROS downstream of ErbB2, its function is quite distinct in K-Ras–transformed cells lacking ECM attachment. In this case, although flux through the PPP is critical for K-Ras-induced anchorage-independent growth, its importance seems to be linked to biosynthetic pathways rather than the elimination of ROS (27). However, ROS do play a role in the survival of ECM-detached, K-Ras–transformed cells. In this case, glutamine conversion into the tricarboxylic acid (TCA) cycle intermediate α-ketoglutarate (through glutaminase and alanine aminotransferase) results in robust TCA cycle flux. Strikingly, this mitochondrial metabolism facilitates the survival of K-Ras–transformed cells via the generation of ROS. The foremost source of ROS generation necessary for ECM-detached survival is the Qo site of mitochondrial complex III. Mechanistically, mitochondrial ROS function to promote anchorage-independent growth and the survival of ECM-detached cells via the stimulation of the ERK-MAPK–signaling pathway. Remarkably, if mitochondrial function is inhibited by loss of the mitochondrial transcription factor A (TFAM) gene, tumor formation in an oncogenic K-Ras-driven mouse model of lung cancer is compromised (27). Thus, it seems clear that ROS can have both deleterious and beneficial effects on ECM-detached cells depending on the oncogenic background. However, additional studies are necessary to further elucidate the context(s) in which ROS activate cell death pathways or initiate productive signal transduction in ECM-detached cells.
Antioxidant enzymes and glutathione
The above findings represent some of the initial forays into studying the complicated relationship between redox metabolism and the survival of ECM-detached cells. Subsequent studies have focused on understanding the relationship between endogenous antioxidant enzyme programs and redox metabolism during ECM-detached conditions. For example, it seems reasonable to extrapolate the aforementioned findings regarding deficient PPP flux during ECM detachment to the fact that the activity of the endogenous antioxidant program would be disproportionately important for mitigating oxidative stress. Intriguingly, it was discovered that overexpression of catalase (CAT) or superoxide dismutase 2 (SOD2 also known as manganese superoxide dismutase or MnSOD) is sufficient to promote luminal filling by promoting the survival of centrally located, ECM-detached cells in 3D cultures of mammary acini (17). Furthermore, antagonizing catalase or SOD2 expression did not impact the viability of ECM-attached cells but specifically compromised the survival of ECM-detached cells. When expanding on these findings in vivo, Davison et al. (17) assessed whether inhibiting antioxidant enzymes (e.g. catalase) could compromise tumor formation in an experimental metastasis assay. Indeed, a reduction of catalase levels in breast cancer cells substantially attenuated tumor burden in the lungs of immunocompromised mice following tail vein injection (17). Additional studies have demonstrated that SOD2 expression is elevated in human breast cancer metastases compared with primary tumors, a finding entirely consistent with a role for SOD2 in facilitating the survival of ECM-detached cancer cells. Furthermore, expression of SOD2 correlates with histologic tumor grades in human breast cancer and contributes to cancer cells' evasion of anoikis (28). Taken together, these data highlight an important role for the endogenous antioxidant programs in facilitating the survival of cancer cells during ECM detachment.
Additional studies examining antioxidant activity during metastasis further underscore the importance of maintaining redox metabolism during ECM detachment. For example, animal models of lung cancer and malignant melanoma have shown that dietary supplementation of antioxidants and genetic manipulation of either glutathione synthesis or antioxidant activity can result in enhanced distant metastases (29–31). Although these studies do not focus on the behavior of cells during ECM detachment per se, they are broadly consistent with the aforementioned data suggesting that ECM-detached cancer cells benefit from antioxidant activity. Supporting this supposition, studies have demonstrated a role for NAD(P)H/quinone oxidoreductase 1 (NQO1) in the survival of ECM-bereft cancer cells (32). Antagonizing NQO1 signaling in lung carcinoma cells led to a deleterious increase in ROS levels, halted anchorage-independent growth, increased anoikis, and blocked the invasion of tumor cells grown in spheroids. Furthermore, NQO1 deficiency decreased both cell proliferation and lung tumor growth in mouse xenograft studies thereby underscoring the importance of NQO1's enzymatic activity for the survival of cancer cells. Other studies exploring the endogenous cellular antioxidant pathways have focused on glutathione (GSH), the most abundant antioxidant present in human cells (33). Using a mouse model of breast cancer, investigators discovered that synthesis of GSH, driven by the glutamate cysteine ligase modifier (GCLM), is necessary for primary tumor formation. Loss of GCLM prevented a tumor's ability to drive the metastatic cascade, where survival in the absence of ECM attachment is imperative. These results can be reproduced using an inhibitor of GSH synthesis but strikingly only if the inhibitor is delivered prior to tumor onset, suggesting that antagonizing GSH solely is effective prior to tumor formation. Additionally, these data indicate that at the later stages of the metastatic cascade, GSH becomes dispensable due to compensation from alternative antioxidant pathways. Indeed, this was the case as thioredoxin (TXN) levels were increased when GSH synthesis was blocked (34). Intriguingly, combinatorial inhibition of TXN and GSH leads to a synergistic block on cancer cell survival, leading to their ultimate death both in vitro and in vivo. Thus, aspiring cancer cells can be eliminated by restraining a single antioxidant pathway, but fully transformed cancer cells can thwart this restraint and utilize additional, redundant antioxidant pathways to promote their survival. These findings are entirely consistent with data demonstrating that ECM-detached cells can be compromised through manipulation of their endogenous antioxidant defense systems.
Similar to the complexity surrounding ROS signaling during ECM detachment, a study on colorectal cancer emphasizes the complex nature of understanding how antioxidant activity impacts cancer cell survival. Unexpectedly, high doses of vitamin C, which has well-documented antioxidant activity, can lead to an elevation in intracellular ROS levels in cells with activating mutations in K-Ras or B-Raf (35). The surprising increase in ROS is first initiated by cellular uptake of oxidized vitamin C (dehydroascorbate (DHA)) via the glucose transporter, GLUT1. This uptake of DHA leads to a significant increase in ROS levels as intracellular DHA is reduced back to vitamin C at the expense of GSH. As a consequence of diminished GSH pools, ROS levels increase and subsequently inactivate glyceraldehyde-3-phosphate dehydrogenase. This hindrance of glyceraldehyde-3-phosphate dehydrogenase activity in highly glycolytic KRAS or BRAF mutant cells leads to a bio-energetic crisis and subsequent cell death. Although the precise impact that high doses of vitamin C can have on the viability of ECM-detached cells was not assessed in these studies, ECM-detached cells could be disproportionately eliminated by high doses of vitamin C given their already compromised redox defenses. Furthermore, these findings provide compelling evidence that the precise impact of certain compounds on ROS levels can be highly context-dependent as small molecules with antioxidant properties can enhance rather than reduce harmful ROS when administered at high doses (35).
Mitochondrial and amino acid metabolism
Balancing redox homeostasis during ECM detachment extends to mitochondrial and amino acid metabolism. In times of glucose deprivation, such as during ECM detachment, cancer cells often shift their metabolism disproportionately to utilize glutamine. For example, in order for cancer cells to survive glucose deprivation, glutamate dehydrogenase activity increases to support the synthesis of TCA cycle intermediates that can be utilized for macromolecular and nucleotide synthesis (36, 37). More salient for the topic at hand, glutamine metabolism is critically important for the production of glutathione and NADPH, both of which function to detoxify ROS in a fashion that is required for cancer cell survival (19, 37–44). Moreover, certain types of cancers appear to have a specific mechanism to utilize glutamine to effectuate their progression. For example, a noncanonical pathway of glutamine metabolism transpires in pancreatic ductal adenocarcinoma (PDAC). In PDAC cells, glutamine-derived aspartate can translocate to the cytosol and be converted into oxaloacetate by aspartate transaminase (GOT1). The newly derived oxaloacetate is then synthesized into malate followed by pyruvate, which functions to ameliorate any elevations in cellular ROS (45). In addition, a recent study in lung carcinoma cells explored how glutamine is metabolized to specifically facilitate anchorage-independent growth. This study discovered that ECM-detached lung carcinoma cells utilized IDH1 to reductively decarboxylate glutamine to citrate in the cytosol. The newly derived citrate subsequently enters the mitochondria via citrate transporter protein where it participates in oxidative metabolism. More specifically, the activity of mitochondrial IDH2 functions to generate NADPH and neutralize mitochondrial ROS in a fashion that promotes survival (19). These data are in alignment with previous anchorage-independent growth studies whereby mitigation of ROS is required to promote ATP generation and survival (18).
Glutamine is far from the only amino acid that can be metabolized in a fashion that impacts redox regulation during ECM detachment. Recently, a number of studies have assessed the significance of serine and glycine metabolism in cancer cells. The complex, cyclical nature of serine and glycine metabolism can occur through cytoplasmic and mitochondrial pathways, both of which have been demonstrated to be up-regulated in cancer cells (46–50). Additionally, serine and glycine metabolism converges on the formation of folate compounds, a target of chemotherapy for over 60 years (51–53). The significance of serine and glycine metabolism does not halt at the support of nucleotide synthesis. Serine and glycine contribute to ATP production through one-carbon metabolism using methylenetetrahydrofolate dehydrogenase (MTHFD1) (54). In poorly vascularized gliomas, high serine hydroxymethyltransferase 2 (SHMT2) activity and glycine cleavage are required for cancer cell survival (55). Additionally, when MYC-transformed cells are exposed to hypoxic conditions, they require the activity of SHMT2 to break down serine and thus maintain NADPH production and redox balance necessary for tumor cell survival (56). Expanding upon these findings, serine metabolism has been shown to contribute to the regeneration of mitochondrial NADPH in lung cancer cells (57). Overall, these data indicate that serine and glycine metabolism are not only required for nucleotide synthesis but also for promoting ATP generation, redox homeostasis, and cancer cell survival during periods of stress. To the best of our knowledge, no studies to date have elucidated the specific contribution of serine or glycine metabolism to the maintenance of appropriate redox metabolism during ECM detachment. However, additional studies focused on addressing this question would substantially improve our understanding of cancer cell survival during ECM detachment, when cells experience nutrient deficiency and are subject to elevated oxidative stress.
Although the specific contributions of serine and glycine metabolism to balancing redox homeostasis during ECM detachment remain unexplored, a recent, elegant study shed light on the contribution of proline metabolism to the survival of ECM-detached cells. Interestingly, proline catabolism via proline dehydrogenase (Prodh) supports growth of breast cancer cells grown in 3D culture but not in 2D culture. The breakdown of proline by Prodh supports mitochondrial ATP production by feeding electrons, in the form of FADH2, into the electron transport chain. Furthermore, PRODH expression and proline catabolism are increased in metastases compared with primary breast cancers of patients and mice. Strikingly, antagonizing Prodh is sufficient to abrogate formation of lung metastases in two independent mouse models, without affecting healthy tissue or organ function (58). In aggregate, Prodh holds promise as a novel drug target to tip the balance of redox homeostasis in favor of blocking metastasis formation.
Concluding remarks
The numerous pre-clinical studies in distinct cell lines and cancers discussed here emphasize the importance of maintaining redox balance during ECM detachment (see summary in Fig. 1). For cancer cells to successfully metastasize to a secondary site, they must inhibit anoikis-dependent and -independent signaling in addition to detoxifying damaging ROS levels. As is evident from the studies discussed here, cancer cells utilize a throng of distinct approaches to influence redox metabolism and promote survival. These include oncogene-mediated signal transduction, the promotion of PPP flux, the abundance and activity of endogenous antioxidant programs, modifications in enzymes that regulate glucose flux, activation of reductive decarboxylation of glutamine, and stimulation of proline catabolism. Although the ability of ROS to compromise ECM-detached cells is now well understood, the precise impact of these alterations on various stages of tumor progression will need to be further assessed. Additional studies using more sophisticated animal models that more faithfully recapitulate disease will facilitate the translation of these findings into a clinical context. It also remains a mystery as to the molecular mechanism by which ECM-detached cells are eliminated in an anoikis-independent fashion when exposed to elevated ROS. One intriguing avenue that may be explored in the future is the role of ferroptosis in antagonizing the survival of ECM-bereft cells. Ferroptosis is novel type of programmed cell death that is dependent upon intracellular iron and is morphologically, biochemically, and genetically distinct from apoptosis, necrosis, and autophagy (59, 60). Mechanistically, ferroptosis is activated when glutathione biosynthesis or the glutathione-dependent antioxidant enzyme glutathione peroxidase 4 (GPX4) is inhibited. Consequently, halting either of these functions leads to prevention of cystine uptake by system Xc− creating a void in the antioxidant fortifications of the cell and culminates in iron-dependent, oxidative cell death (59–62). Considering the substantial increase in ROS levels during ECM detachment and the importance of the loss of glutathione levels in activating ferroptosis, it seems plausible that ferroptosis is activated when cells detach from the ECM. That being said, manipulation of other endogenous antioxidant enzymes (independent of GPX4) has not been described to lead to the induction of ferroptosis during ECM detachment. Given this, additional studies that assess ferroptotic cell death as a consequence of distinct manipulations of the antioxidant machinery will be important to better understand whether this pathway is important for the elimination of ECM-detached cells.
Acknowledgments
We thank Veronica Schafer and all the members of the Schafer lab for helpful comments and support. We apologize to all authors whose work we were not able to highlight in this Minireview.
This work was supported by The American Cancer Society, Susan G. Komen, Phi Beta Psi Sorority, National Science Foundation, Boler-Parseghian Center for Rare and Neglected Diseases, Coleman Foundation, and funds from Ron and Rosemarie Malanga. This is the sixth article in the Thematic Minireview Series “Redox metabolism and signaling.” The authors declare that they have no conflicts of interest with the contents of this article.
- ECM
- extracellular matrix
- ROS
- reactive oxygen species
- PPP
- pentose phosphate pathway
- AMPK
- AMP-activated protein kinase
- TXN
- thioredoxin
- DHA
- dehydroascorbate
- PDAC
- pancreatic ducal adenocarcinoma
- GCLM
- glutamate cysteine ligase modifier.
References
- 1. Nguyen D. X., and Massagué J. (2007) Genetic determinants of cancer metastasis. Nat. Rev. Genet. 8, 341–352 10.1038/nrg2101 [DOI] [PubMed] [Google Scholar]
- 2. Buchheit C. L., Rayavarapu R. R., and Schafer Z. T. (2012) The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin. Cell Dev. Biol. 23, 402–411 10.1016/j.semcdb.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 3. Buchheit C. L., Weigel K. J., and Schafer Z. T. (2014) Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat. Rev. Cancer 14, 632–641 10.1038/nrc3789 [DOI] [PubMed] [Google Scholar]
- 4. Horbinski C., Mojesky C., and Kyprianou N. (2010) Live free or die: tales of homeless (cells) in cancer. Am. J. Pathol. 177, 1044–1052 10.2353/ajpath.2010.091270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buchheit C. L., Angarola B. L., Steiner A., Weigel K. J., and Schafer Z. T. (2015) Anoikis evasion in inflammatory breast cancer cells is mediated by Bim-EL sequestration. Cell Death Differ. 22, 1275–1286 10.1038/cdd.2014.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Z., Li H., Derouet M., Berezkin A., Sasazuki T., Shirasawa S., and Rosen K. (2006) Oncogenic Ras inhibits anoikis of intestinal epithelial cells by preventing the release of a mitochondrial pro-apoptotic protein Omi/HtrA2 into the cytoplasm. J. Biol. Chem. 281, 14738–14747 10.1074/jbc.M508664200 [DOI] [PubMed] [Google Scholar]
- 7. Mason J. A., Davison-Versagli C. A., Leliaert A. K., Pape D. J., McCallister C., Zuo J., Durbin S. M., Buchheit C. L., Zhang S., and Schafer Z. T. (2016) Oncogenic Ras differentially regulates metabolism and anoikis in extracellular matrix-detached cells. Cell Death Differ. 23, 1271–1282 10.1038/cdd.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Owens T. W., Valentijn A. J., Upton J. P., Keeble J., Zhang L., Lindsay J., Zouq N. K., and Gilmore A. P. (2009) Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK. Cell Death Differ. 16, 1551–1562 10.1038/cdd.2009.102 [DOI] [PubMed] [Google Scholar]
- 9. Rayavarapu R. R., Heiden B., Pagani N., Shaw M. M., Shuff S., Zhang S., and Schafer Z. T. (2015) The role of multicellular aggregation in the survival of ErbB2-positive breast cancer cells during extracellular matrix detachment. J. Biol. Chem. 290, 8722–8733 10.1074/jbc.M114.612754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valentijn A. J., and Gilmore A. P. (2004) Translocation of full-length Bid to mitochondria during anoikis. J. Biol. Chem. 279, 32848–32857 10.1074/jbc.M313375200 [DOI] [PubMed] [Google Scholar]
- 11. Valentijn A. J., Metcalfe A. D., Kott J., Streuli C. H., and Gilmore A. P. (2003) Spatial and temporal changes in Bax subcellular localization during anoikis. J. Cell Biol. 162, 599–612 10.1083/jcb.200302154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weigel K. J., Jakimenko A., Conti B. A., Chapman S. E., Kaliney W. J., Leevy W. M., Champion M. M., and Schafer Z. T. (2014) CAF-secreted IGFBPs regulate breast cancer cell anoikis. Mol. Cancer Res. 12, 855–866 10.1158/1541-7786.MCR-14-0090 [DOI] [PubMed] [Google Scholar]
- 13. Fung C., Lock R., Gao S., Salas E., and Debnath J. (2008) Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabinowitz J. D., and White E. (2010) Autophagy and metabolism. Science 330, 1344–1348 10.1126/science.1193497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galluzzi L., Morselli E., Vicencio J. M., Kepp O., Joza N., Tajeddine N., and Kroemer G. (2008) Life, death and burial: multifaceted impact of autophagy. Biochem. Soc. Trans. 36, 786–790 10.1042/BST0360786 [DOI] [PubMed] [Google Scholar]
- 16. Florey O., Kim S. E., and Overholtzer M. (2015) Entosis: cell-in-cell formation that kills through entotic cell death. Curr. Mol. Med. 15, 861–866 10.2174/1566524015666151026100042 [DOI] [PubMed] [Google Scholar]
- 17. Davison C. A., Durbin S. M., Thau M. R., Zellmer V. R., Chapman S. E., Diener J., Wathen C., Leevy W. M., and Schafer Z. T. (2013) Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Cancer Res. 73, 3704–3715 10.1158/0008-5472.CAN-12-2482 [DOI] [PubMed] [Google Scholar]
- 18. Schafer Z. T., Grassian A. R., Song L., Jiang Z., Gerhart-Hines Z., Irie H. Y., Gao S., Puigserver P., and Brugge J. S. (2009) Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113 10.1038/nature08268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang L., Shestov A. A., Swain P., Yang C., Parker S. J., Wang Q. A., Terada L. S., Adams N. D., McCabe M. T., Pietrak B., Schmidt S., Metallo C. M., Dranka B. P., Schwartz B., and DeBerardinis R. J. (2016) Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 10.1038/nature17393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamarajugadda S., Stemboroski L., Cai Q., Simpson N. E., Nayak S., Tan M., and Lu J. (2012) Glucose oxidation modulates anoikis and tumor metastasis. Mol. Cell. Biol. 32, 1893–1907 10.1128/MCB.06248-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bizjak M., Malavašič P., Dolinar K., Pohar J., Pirkmajer S., and Pavlin M. (2017) Combined treatment with metformin and 2-deoxy glucose induces detachment of viable MDA-MB-231 breast cancer cells in vitro. Sci. Rep. 7, 1761 10.1038/s41598-017-01801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Debnath J., Mills K. R., Collins N. L., Reginato M. J., Muthuswamy S. K., and Brugge J. S. (2002) The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111, 29–40 10.1016/S0092-8674(02)01001-2 [DOI] [PubMed] [Google Scholar]
- 23. Mills K. R., Reginato M., Debnath J., Queenan B., and Brugge J. S. (2004) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl. Acad. Sci. U.S.A. 101, 3438–3443 10.1073/pnas.0400443101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shackelford D. B., and Shaw R. J. (2009) The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9, 563–575 10.1038/nrc2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sundararaman A., Amirtham U., and Rangarajan A. (2016) Calcium-oxidant signaling network regulates AMP-activated protein kinase (AMPK) activation upon matrix deprivation. J. Biol. Chem. 291, 14410–14429 10.1074/jbc.M116.731257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeon S. M., Chandel N. S., and Hay N. (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 10.1038/nature11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberg F., Hamanaka R., Wheaton W. W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G. M., Budinger G. R., and Chandel N. S. (2010) Mitochondrial metabolism and ROS generation are essential for K-Ras-mediated tumorigenicity. Proc. Natl. Acad. Sci. U.S.A. 107, 8788–8793 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamarajugadda S., Cai Q., Chen H., Nayak S., Zhu J., He M., Jin Y., Zhang Y., Ai L., Martin S. S., Tan M., and Lu J. (2013) Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 4, e504 10.1038/cddis.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Le Gal K., Ibrahim M. X., Wiel C., Sayin V. I., Akula M. K., Karlsson C., Dalin M. G., Akyürek L. M., Lindahl P., Nilsson J., and Bergo M. O. (2015) Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 7, 308re8 10.1126/scitranslmed.aad3740 [DOI] [PubMed] [Google Scholar]
- 30. Piskounova E., Agathocleous M., Murphy M. M., Hu Z., Huddlestun S. E., Zhao Z., Leitch A. M., Johnson T. M., DeBerardinis R. J., and Morrison S. J. (2015) Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 10.1038/nature15726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sayin V. I., Ibrahim M. X., Larsson E., Nilsson J. A., Lindahl P., and Bergo M. O. (2014) Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 6, 221ra15 10.1126/scitranslmed.3007653 [DOI] [PubMed] [Google Scholar]
- 32. Madajewski B., Boatman M. A., Chakrabarti G., Boothman D. A., and Bey E. A. (2016) Depleting tumor-NQO1 potentiates anoikis and inhibits growth of NSCLC. Mol. Cancer Res. 14, 14–25 10.1158/1541-7786.MCR-15-0207-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meister A., and Anderson M. E. (1983) Glutathione. Annu. Rev. Biochem. 52, 711–760 10.1146/annurev.bi.52.070183.003431 [DOI] [PubMed] [Google Scholar]
- 34. Harris I. S., Treloar A. E., Inoue S., Sasaki M., Gorrini C., Lee K. C., Yung K. Y., Brenner D., Knobbe-Thomsen C. B., Cox M. A., Elia A., Berger T., Cescon D. W., Adeoye A., Brustle A., et al. (2015) Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 10.1016/j.ccell.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 35. Yun J., Mullarky E., Lu C., Bosch K. N., Kavalier A., Rivera K., Roper J., Chio I. I., Giannopoulou E. G., Rago C., Muley A., Asara J. M., Paik J., Elemento O., Chen Z., et al. (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396 10.1126/science.aaa5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choo A. Y., Kim S. G., Vander Heiden M. G., Mahoney S. J., Vu H., Yoon S. O., Cantley L. C., and Blenis J. (2010) Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol. Cell 38, 487–499 10.1016/j.molcel.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang C., Sudderth J., Dang T., Bachoo R. M., Bachoo R. G., McDonald J. G., and DeBerardinis R. J. (2009) Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 69, 7986–7993 10.1158/0008-5472.CAN-09-2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeBerardinis R. J., and Chandel N. S. (2016) Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 10.1126/sciadv.1600200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeBerardinis R. J., and Cheng T. (2010) Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 10.1038/onc.2009.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DeBerardinis R. J., Lum J. J., Hatzivassiliou G., and Thompson C. B. (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 10.1016/j.cmet.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 41. DeBerardinis R. J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., and Thompson C. B. (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U.S.A. 104, 19345–19350 10.1073/pnas.0709747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mullen A. R., Hu Z., Shi X., Jiang L., Boroughs L. K., Kovacs Z., Boriack R., Rakheja D., Sullivan L. B., Linehan W. M., Chandel N. S., and DeBerardinis R. J. (2014) Oxidation of α-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 7, 1679–1690 10.1016/j.celrep.2014.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mullen A. R., Wheaton W. W., Jin E. S., Chen P. H., Sullivan L. B., Cheng T., Yang Y., Linehan W. M., Chandel N. S., and DeBerardinis R. J. (2011) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vander Heiden M. G., and DeBerardinis R. J. (2017) Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 10.1016/j.cell.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Son J., Lyssiotis C. A., Ying H., Wang X., Hua S., Ligorio M., Perera R. M., Ferrone C. R., Mullarky E., Shyh-Chang N., Kang Y., Fleming J. B., Bardeesy N., Asara J. M., Haigis M. C., et al. (2013) Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Labuschagne C. F., van den Broek N. J., Mackay G. M., Vousden K. H., and Maddocks O. D. (2014) Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 10.1016/j.celrep.2014.04.045 [DOI] [PubMed] [Google Scholar]
- 47. Locasale J. W., Grassian A. R., Melman T., Lyssiotis C. A., Mattaini K. R., Bass A. J., Heffron G., Metallo C. M., Muranen T., Sharfi H., Sasaki A. T., Anastasiou D., Mullarky E., Vokes N. I., Sasaki M., et al. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 10.1038/ng.890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maddocks O. D., Labuschagne C. F., Adams P. D., and Vousden K. H. (2016) Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol. Cell 61, 210–221 10.1016/j.molcel.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Possemato R., Marks K. M., Shaul Y. D., Pacold M. E., Kim D., Birsoy K., Sethumadhavan S., Woo H. K., Jang H. G., Jha A. K., Chen W. W., Barrett F. G., Stransky N., Tsun Z. Y., Cowley G. S., et al. (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 10.1038/nature10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang W. C., Shyh-Chang N., Yang H., Rai A., Umashankar S., Ma S., Soh B. S., Sun L. L., Tai B. C., Nga M. E., Bhakoo K. K., Jayapal S. R., Nichane M., Yu Q., Ahmed D. A., et al. (2012) Glycine decarboxylase activity drives nonsmall cell lung cancer tumor-initiating cells and tumorigenesis. Cell 148, 259–272 10.1016/j.cell.2011.11.050 [DOI] [PubMed] [Google Scholar]
- 51. Amelio I., Cutruzzolá F., Antonov A., Agostini M., and Melino G. (2014) Serine and glycine metabolism in cancer. Trends Biochem. Sci. 39, 191–198 10.1016/j.tibs.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hosios A. M., Hecht V. C., Danai L. V., Johnson M. O., Rathmell J. C., Steinhauser M. L., Manalis S. R., and Vander Heiden M. G. (2016) Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 36, 540–549 10.1016/j.devcel.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Locasale J. W. (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 10.1038/nrc3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tedeschi P. M., Markert E. K., Gounder M., Lin H., Dvorzhinski D., Dolfi S. C., Chan L. L., Qiu J., DiPaola R. S., Hirshfield K. M., Boros L. G., Bertino J. R., Oltvai Z. N., and Vazquez A. (2013) Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 4, e877 10.1038/cddis.2013.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim D., Fiske B. P., Birsoy K., Freinkman E., Kami K., Possemato R. L., Chudnovsky Y., Pacold M. E., Chen W. W., Cantor J. R., Shelton L. M., Gui D. Y., Kwon M., Ramkissoon S. H., Ligon K. L., et al. (2015) SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520, 363–367 10.1038/nature14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ye J., Fan J., Venneti S., Wan Y. W., Pawel B. R., Zhang J., Finley L. W., Lu C., Lindsten T., Cross J. R., Qing G., Liu Z., Simon M. C., Rabinowitz J. D., and Thompson C. B. (2014) Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 10.1158/2159-8290.CD-14-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lewis C. A., Parker S. J., Fiske B. P., McCloskey D., Gui D. Y., Green C. R., Vokes N. I., Feist A. M., Vander Heiden M. G., and Metallo C. M. (2014) Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 10.1016/j.molcel.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elia I., Broekaert D., Christen S., Boon R., Radaelli E., Orth M. F., Verfaillie C., Grünewald T. G., and Fendt S. M. (2017) Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 8, 15267 10.1038/ncomms15267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cao J. Y., and Dixon S. J. (2016) Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–2209 10.1007/s00018-016-2194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., Patel D. N., Bauer A. J., Cantley A. M., Yang W. S., Morrison B. 3rd., Stockwell B. R. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao M., Monian P., Pan Q., Zhang W., Xiang J., and Jiang X. (2016) Ferroptosis is an autophagic cell death process. Cell Res. 26, 1021–1032 10.1038/cr.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim S. E., Zhang L., Ma K., Riegman M., Chen F., Ingold I., Conrad M., Turker M. Z., Gao M., Jiang X., Monette S., Pauliah M., Gonen M., Zanzonico P., Quinn T., et al. (2016) Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 11, 977–985 10.1038/nnano.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]