Abstract
The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) was the first investigational vaccine trial conducted in Sierra Leone. STRIVE enrolled and vaccinated about 8000 healthcare and frontline workers and followed them for 6 months post-vaccination. Few in-country healthcare workers had previous experience with clinical trials. Retired nurses, veteran nurses, and recently graduated nurses were hired to fill critical study roles including study site management, screening participants for enrollment, vaccine administration, post-vaccination follow-up, and providing clinical nursing care and advice. In addition to role-specific training, nurses received general training on the study protocol and implementation, Good Clinical Practices, and documentation for clinical trials. Training in documentation and supervision was especially challenging; ongoing refresher training should be built into standard operating procedures for future similar clinical studies. Using local nurses to conduct study activities had both short- and long-term positive impacts for the study, the nurses themselves, the community, and the country’s health system.
Clinical Trials Registration
ClinicalTrials.gov [NCT02378753] and Pan African Clinical Trials Registry [PACTR201502001037220].
Keywords: nurses, Ebola, Ebola vaccine, clinical trial, Sierra Leone, workforce development
During 2014–2016 West Africa experienced the largest reported Ebola outbreak in history [1]. In Sierra Leone, by October 2015, the Ministry of Health and Sanitation (MoHS) reported almost 14000 suspected, probable, and confirmed Ebola cases and 4000 deaths, overwhelming an already fragile medical and public health infrastructure [2, 3]. In response to concerns that the outbreak might not be controlled without an Ebola vaccine, the public health community expedited the development and testing of candidate vaccines [4]. The Sierra Leone College of Medicine and Allied Health Sciences (COMAHS), the Sierra Leone MoHS, and the United States Centers for Disease Control and Prevention (CDC) initiated the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) in April 2015 [5].
Before the Ebola outbreak, Sierra Leone had a severe shortage of healthcare workers and one of the lowest healthcare worker densities in the world: 1.7–3.6 nurses and midwives and 0.2 physicians per 10000 population [6, 7]. The Ebola epidemic further exacerbated the situation. Nurses, physicians, and other healthcare professionals were overwhelmed, and healthcare work itself became extremely hazardous; Ebola incidence was more than 100-fold higher among healthcare workers than in the general population [8]. Many healthcare workers, including nurses, died or left the country during the epidemic [5, 9].
STRIVE was the first investigational vaccine trial conducted in Sierra Leone, and few healthcare workers had experience with clinical trials. This, coupled with the critical shortage of healthcare workers, severely limited the in-country availability of medical staff with any clinical trial experience. This article describes the process of identifying appropriate staff to conduct trial activities, as well as the roles that nurses played, the training they received, the challenges they faced, and lessons learned during this process.
STRIVE OVERVIEW
COMAHS, MoHS, and CDC designed STRIVE to rapidly introduce and assess the safety and immunogenicity of the candidate Ebola vaccine rVSV∆G-ZEBOV-GP (Merck) among healthcare and other frontline Ebola response workers in Sierra Leone [5]. From April through August 2015, about 8000 participants at 7 study sites in 5 study districts were enrolled and randomized to be vaccinated either at the time of enrollment (immediate group) or at 18–24 weeks after enrollment (deferred group). Participants were followed for 6 months post-vaccination to assess vaccine safety through monthly telephone calls made from 1 of the 3 participant follow-up coordination and data management centers (follow-up centers). Home visits were conducted if the participant could not be contacted. To facilitate communication, cell phones with SIM cards were provided to all study staff and participants, and calls within the study user group were free of charge. In addition, a STRIVE telephone “hotline” was answered 24 hours a day, 7 days a week for participants to report any medical or other concerns. The staff also assessed pregnancy outcomes (maternal complications, viability of infant, congenital anomalies in infant examined on or after day 28 of birth) among women who became pregnant within 2 months after vaccination, and for comparison, women in the deferred group who became pregnant within 2 months after enrollment but before vaccination.
We obtained ethical and regulatory approvals for the trial from the Sierra Leone Ethics and Scientific Review Committee, CDC Institutional Review Board, Pharmacy Board of Sierra Leone, and the US Food and Drug Administration (FDA).
We conducted the study according to Good Clinical Practices following the guidelines of the International Council for Harmonisation of Technical Requirement for Pharmaceuticals for Human Use, which are promulgated by the FDA [10, 11]. Written informed consent was obtained from all participants prior to study participation.
STRIVE AND THE SEARCH FOR STUDY PERSONNEL
Given the shortage of medical personnel, senior study staff had to be creative in identifying and recruiting other STRIVE staff members, ultimately deciding that nurses would be used for many different study roles. Retired nurses, many of whom had been in management positions (Nurse Matrons); experienced nurses who were available for STRIVE; and recently graduated nurses who had not yet entered the workforce were recruited as study staff. Approximately 115 nurses were hired to work for STRIVE, accounting for about one third of the >350 Sierra Leoneans who worked on the study. Other Sierra Leone STRIVE professional staff included physicians, medical students, and pharmacists (experienced and recently graduated) [12]. The reporting structure for STRIVE involved role-specific and geographic reporting. At the highest level, the study was overseen by the Sierra Leone primary investigator and several senior study physicians who were each responsible for different aspects of the study (eg, implementation, medical management, surveillance). Each district had a district coordinator, and there was a supervisor for each enrollment site, vaccination site, and follow-up center.
NURSING WORKFORCE IN SIERRA LEONE
The MoHS oversees nursing services, staffs government hospitals and clinics, sets standards, formulates policy, and provides nursing education. The nursing and nursing allied-professional workforce is divided into 4 categories by level of training (Table 1). In December 2015, an estimated 5300 nurses and nurse allied-professionals served a national population of more than 7 million [13, 14]. The majority of STRIVE nurses were registered nurses or had recently graduated from registered nursing school; there were some nurse midwives on the STRIVE workforce.
Table 1.
Sierra Leone Nurse and Nurse Allied Professional Workforce, 2105
| Type of Nurse | Years of Education/Training | Primary Work Activities | Estimated Number |
|---|---|---|---|
| State registered nurses | 3 years post-secondary school (additional 2–4 years for specialty, educator, midwifery training) | Nurse leaders, bedside nursing, community care, management, nurse education | 228 |
| State enrolled community health nurses | 2.5 years post-secondary school (additional 2 years for midwifery training) | Bedside nursing, community care, community education | 2465 |
| Maternal and child health aides | 3 years secondary school (junior secondary school) and 2 years classroom and community training | Basic safe maternity and under-5 pediatric services at the community level | 1750 |
| Nursing assistants | Not standardized | Assistance with bedside care and activities of daily living | 840 |
STRIVE NURSE ROLES
Nurses were assigned roles based on skills commonly used in nursing, such as taking patient histories, conducting pregnancy tests, administering vaccine, and following-up patients for medical outcomes. The specific STRIVE roles filled by nurses were as follows:
Screeners
To ensure that potential participants met eligibility criteria to participate in the study, screeners administered a brief standardized questionnaire before enrollment. They also took each participant’s oral temperature with a digital thermometer and taught women 18–49 years old how to do a urine pregnancy test, which was done on-site. Recently graduated nurses and nurses with midlevel experience filled this role
Vaccinators
These nurses administered the study vaccine to participants. A background in infection control and proper vaccination technique was critical to this role. Nurses registered with the Nurses and Midwives Board of Sierra Leone with immunization experience, such as working in the national immunization program, were used to fill this position.
Vaccination Site Managers
Site managers oversaw the day-to-day activities of the vaccination sites, including managing staff, daily and weekly scheduling, staff and participant “trouble shooting,” maintaining inventory, ensuring participants were properly reimbursed for their time and travel, and monitoring participants after vaccination. This last activity included evaluating, treating, and reporting any adverse reactions that occurred during the 1-hour post-vaccination observation period and required the nurses to have critical thinking skills and experience handling basic medical emergencies including anaphylactic shock. Retired or unemployed Nurse Matrons were hired for this role.
Surveillance Monitors
Surveillance monitors made the monthly follow-up calls to participants. Each surveillance monitor received daily assignments to call participants and inquire about any adverse events, pregnancy, or potential Ebola virus disease exposure or symptoms. Critical thinking and medical assessment skills were needed for this position, and newly graduated nurses were used in this role. This role was shared with pharmacists and medical students as many surveillance monitors were needed.
Study Nurses
Participants who reported an adverse event or pregnancy to the surveillance monitor or STRIVE hotline were referred to a study nurse for evaluation. The study nurse gathered relevant medical information via a telephone interview and made an initial assessment. Participants were either followed by the nurse until resolution of symptoms or referred to a study physician or an Ebola facility, as appropriate, at the time of the initial assessment or during follow-up. If a woman reported pregnancy within 2 months after vaccination or enrollment, the study nurses called her every 1–2 months until the outcome of the pregnancy was known; the study nurse then conducted an in-home infant examination to assess for any potential congenital anomalies and the need to have the infant evaluated by a physician. Nurses with higher levels of education and experience were chosen for this job. These study nurses reported to the study physicians in their district.
TRAINING FOR STRIVE NURSES
Training for nurses, along with all other STRIVE staff, occurred throughout the study. Sierra Leonean STRIVE staff conducted most of the training, with the assistance of international staff, especially for the initial and large-scale trainings. Before the study commenced, there was a mandatory 1-day training session in general clinical trials conduct, including human subjects protection (HSP), Good Clinical Practice, and Good Documentation Practice for all staff. A written examination was administered to ensure sufficient knowledge and understanding of HSP. All large group training was conducted in English as English is the official language of Sierra Leone, and international staff was often in attendance. For small group training, if an international person was not involved, training was conducted in a combination of English and the most common local language, Krio (an English-based Creole language).
Job-specific training then occurred in 2 stages. The first was a 3-day, large-group training held a few weeks before the study commenced. Staff members were grouped based on their study roles. Staff at each of the 7 enrollment and vaccination sites and each of the 3 follow-up centers were trained together. This training gave an overall understanding of how each nurse and other staff members would function in their specific job and how their role related to those of other study staff.
The second stage consisted of smaller trainings at each of the 7 enrollment and vaccination sites during the 2–3 days prior to opening for participant enrollment. During these trainings, each staff member learned the details of their specific tasks: interview techniques, chart documentation, administering pregnancy tests, vaccination, and treating post-vaccination emergencies such as anaphylaxis, including the use of epinephrine pens and oxygen. The small-scale trainings included dry-run activities, during which the nurses and other study staff practiced case scenarios with other staff members. A binder with the written study standard operating procedures was available at each of the study sites, and job aids describing selected processes and procedures (eg, pregnancy testing, good vaccination technique, good documentation practices) were posted on the wall or otherwise available.
Training was compressed due to the urgency of starting the study as quickly as possible. As a result, issues arose periodically that required retraining. For example, screeners were observed not allowing the pregnancy test to run for a full 3 minutes and not noting the results appropriately on the pregnancy test log. Those screeners were corrected and those tests repeated, and all screeners at that site were retrained. Retraining was also required on documentation practice standards, vaccination technique, emergency treatment protocols, and adverse event data collection procedures. Throughout the course of the study, site and role supervisors, with the assistance of senior study physicians and international staff, provided ongoing oversight and guidance, as well as periodic training sessions. These included individual and group sessions, as well as larger-scale refresher trainings prior to vaccinating the deferred participant group.
CHALLENGES AND LESSONS LEARNED
STRIVE implementation posed many challenges stemming from the country’s immediate need to respond to the Ebola outbreak, the lack of experience in conducting clinical trials, and the urgency of starting the study as quickly as possible. A typical clinical trial takes several years from conception to beginning implementation; for STRIVE, this process was compressed into <6 months. Specific challenges and lessons learned were as follows:
Training
STRIVE nurses filled a diverse array of critical study roles, and many of their duties were outside the typical bedside or community nursing roles in which they had been trained. STRIVE nurses had to be trained quickly in general clinical trials practices and role-specific duties, with little time to reinforce the learning before the study began. During the course of the study, supervisors, monitors, and auditors sometimes noticed inconsistencies and errors in study procedures, prompting the implementation of regular refresher trainings. Based on observations of senior study staff and the nurses themselves, the knowledge and experience that nurses gained through their STRIVE training and work have significantly improved their assessment techniques; vaccine expertise; and management, documentation, and communication skills. This should not only contribute to their future career success but also help build long-term national capacity for both future research studies and improved clinical practices.
Documentation
Good Clinical Practice includes stringent documentation requirements, and this was an area that needed substantial oversight. In a clinical trial, everything that is done must be meticulously documented so that monitors on behalf of the study sponsor, pharmaceutical company, and regulatory body can verify all information. Although nurses in Sierra Leone are trained to document patient care practices, the comprehensive documentation required for a clinical trial (eg, participant enrollment, participant follow-up, vaccine management, regulatory, and administrative forms) required a degree of precision and accuracy that went beyond their previous experience. Enhanced documentation is a skill that STRIVE nurses have learned and can now apply, as needed, in their bedside nursing practices, as well as in future clinical and research trials.
Communication
Communication between STRIVE nurses and with other study staff members was critical for the success of STRIVE, but the STRIVE communications network was complex. Nurses and other staff members worked different hours and shifts and were not always located in the same building. To improve communication, site supervisors initiated mandatory weekly meetings for the entire staff, implemented standard sign-out procedures, and used a central message board (a white board or flip chart) to communicate daily updates and other messages.
Maintaining communication between the nurses and participants was also a challenge. Contacting participants often required multiple attempts using both their study cell phone number and personal or other numbers (eg, those of family members) that the participant had provided at enrollment. Through refresher trainings and experience, nurses improved their interpersonal communication skills in addressing participant medical concerns. For nonmedical and administrative questions, participants communicated with the STRIVE telephone hotline staff so the study nurses could focus on medical issues.
Professional Relationships
The relationship between nurses and physicians in Sierra Leone is fairly traditional, with the physician generally making decisions about patient care and communicating them to the nurse for implementation. In STRIVE, however, study nurses usually had the first contact with the participant, made the initial medical assessment, and communicated this assessment and follow-up needs to the study physician. The STRIVE experience increased nurse confidence in communicating with the study physicians, which should benefit both nurses who plan to continue their research work as well as those returning to clinical care.
Supervision
Supervising staff during a clinical trial has unique challenges. Although some nurses had experience supervising other nurses in clinical settings, even the most experienced nurses needed guidance on how to manage clinical trial staff. Before the study began, senior staff conducted a management training session, followed by refresher trainings and one-on-one discussions. With senior staff guidance, vaccination site managers learned to institute sign-in registers and create daily schedules to ensure that staff reported for work on time and that all shifts were covered. Through one-on-one mentoring, managers learned how to review daily staff work for completeness, organize participant follow-up, plan the daily work schedule, and manage inventory.
BENEFITS OF THE STRIVE STUDY MODEL OF USING THE LOCAL NURSING WORKFORCE
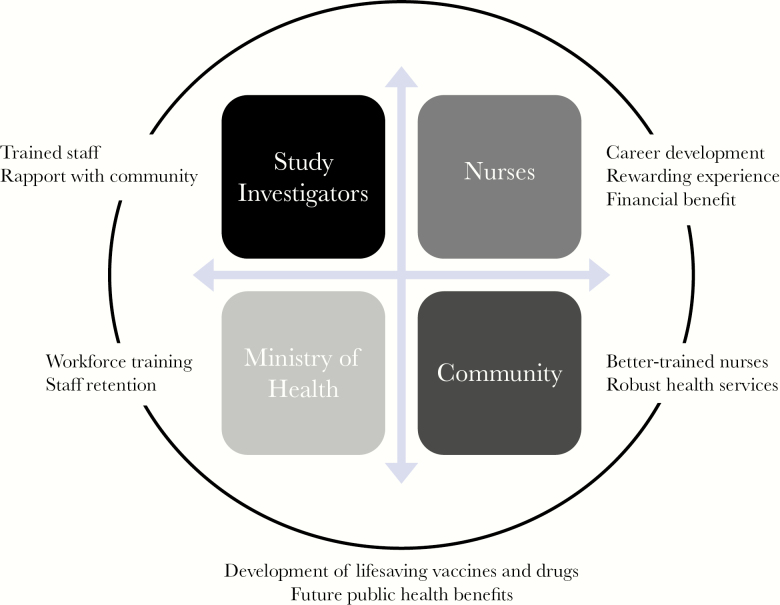
The STRIVE model for employing local nurses to conduct study activities positively affected both the local community and the country and holds promise for the future of Sierra Leone (Figure 1). For study investigators, the model has provided highly trained and trainable staff, many with years of experience, to conduct study activities. Because local nurses are trusted members of the community, they can and did help improve acceptance of the trial and communication at the local level. Nurses themselves benefited from opportunities for career advancement, financial remuneration, and the satisfaction of participating in something that contributed to the greater good of not only their families and communities but of their country as well. Over 98% of the nurses hired to work on STRIVE remained with the study for their full tenure. Members of the local community will benefit by having better trained nurses and more robust health services. Employing local nurses can strengthen the country’s health system overall by giving them high-level training, improving their communication skills, and increasing their ability to address future health emergencies. In addition, providing such opportunities to nurses can lead to better staff retention and, we believe, diminish the “brain drain” that occurs when trained personnel leave the country to seek employment elsewhere. Finally, the employment of nurses maximized the success of the study, which may lead to long-term societal and public health benefits such as improved scientific knowledge, medical breakthroughs, and development of lifesaving vaccines and drugs.
Figure 1.
Benefits of the STRIVE study model of utilizing the local nursing workforce. Abbreviation: STRIVE, Sierra Leone Trial to Introduce a Vaccine against Ebola.
CONCLUSION
In the STRIVE study, the nurses’ experience and knowledge enhanced the success in enrolling and vaccinating about 8000 participants and collecting sufficient data to evaluate the safety of an experimental Ebola vaccine. As more clinical trials are conducted in less-developed countries, investigators may find that local nurses are a valuable resource that can be tapped for study tasks. Both initial and refresher training is required to support the nurses in a clinical trial research role. Additionally, armed with the valuable training they received, study nurses may be inspired to become more involved in clinical research, help formulate healthcare policy, solve other health problems in their own or other low-income countries, and better communicate with their colleagues, thereby contributing to their communities and to medical science.
Notes
Acknowledgments. The authors wish to acknowledge the hard work and dedication all of the Sierra Leone nurses who worked on STRIVE.
Financial support. The trial was funded by the Centers for Disease Control and Prevention, the Biomedical Advanced Research and Development Authority, and the National Institutes of Health, with additional support from the CDC Foundation.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICJME Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
New affiliation: Pharmaceutical Product Development (Nashville, TN)
New position: Clinical Research Associate
References
- 1. Dahl BA, Kinzer MH, Raghunathan PL, et al. . CDC’s response to the 2014–2016 Ebola Epidemic—Guinea, Liberia, and Sierra Leone. MMWR Suppl 2016; 65:12–20. [DOI] [PubMed] [Google Scholar]
- 2. Ebola Virus Disease—Situation Report (Sit-Rep)—14 October, 2015 Government of Sierra Leone, Ministry of Health and Sanitation; 2015:1–4. Accessed 14 February 2017. [Google Scholar]
- 3. MacKinnon J, MacLaren B. Human resources for health challenges in fragile states: Evidence from Sierra Leone, South Sudan, and Zimbabwe The North-South Institute; 2012:1–18. Accessed 14 February 2017. [Google Scholar]
- 4. Interim Guidance: Potential Ebola therapies and vaccines World Health Organization; 2014:1–31. Accessed 14 February 2017. [Google Scholar]
- 5. Widdowson M-A, Schrag SJ, Carter RJ, et al. . Implementing an Ebola vaccine study—Sierra Leone. MMWR Suppl 2016;63:98–106. [DOI] [PubMed] [Google Scholar]
- 6. World Health Statistics 2015 World Health Organization, Global Health Observatory; 2015:1–164. Accessed 14 February 2017. [Google Scholar]
- 7. Kinfu Y, Dal Poz MR, Mercer H, Evans DB. The health worker shortage in Africa: are enough physicians and nurses being trained?Bull World Health Organ 2009; 87:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kilmarx PH, Clarke KR, Dietz PM, et al. ; Centers for Disease Control and Prevention (CDC) Ebola virus disease in health care workers—Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1168–71. [PMC free article] [PubMed] [Google Scholar]
- 9. Evans DK, Goldstein M, Popova A. Health-care worker mortality and the legacy of the Ebola epidemic. Lancet Glob Health 2015; 3:e439–40. [DOI] [PubMed] [Google Scholar]
- 10. Good Clinical Practices. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use; 2017. Accessed 27 July 2017.
- 11. Clinical Trials and Human Subject Protection Department of Health and Human Servies, Food and Drug Administration; 2017. Accessed 27 July 2017. [Google Scholar]
- 12. Carter RJ, Idriss A, Widdowson MA, et al. . Implementing a multi-site clinical trial in the midst of an Ebola outbreak: lessons learned from the Sierra Leone trial to introduce a vaccine against Ebola. J Infect Dis 2018; 217:s16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Designation Profile for December 2015 Sierra Leone Ministry of Health and Sanitation, 2015. Accessed 14 February 2017. [Google Scholar]
- 14. 2015 Population and Housing Census, Summary of Final Results Statistics Sierra Leone, 2016. Accessed 14 February 2017. [Google Scholar]