Abstract
Human epidermal growth factor receptor 2 (HER2) is an oncogenic driver, and a well-established therapeutic target in breast and gastric cancers. Using functional and genomic analyses of patient-derived xenografts, we previously showed that a subset (approximately 5%) of metastatic colorectal cancer (CRC) tumors is driven by amplification or mutation of HER2. This paper reviews the role of HER2 amplification as an oncogenic driver, a prognostic and predictive biomarker, and a clinically actionable target in CRC, considering the specifics of HER2 testing in this tumor type. While the role of HER2 as a biomarker for prognosis in CRC remains uncertain, its relevance as a therapeutic target has been established. Indeed, independent studies documented substantial clinical benefit in patients treated with biomarker-driven HER2-targeted therapies, with an impact on response rates and duration of response that compared favorably with immunotherapy and other examples of precision oncology. HER2-targeted therapeutic strategies have the potential to change the treatment paradigm for a clinically relevant subgroup of metastatic CRC patients.
Keywords: biomarker, colorectal cancer, HER2, targeted therapies
Key Message
Identification of a subset of CRC patients with HER2 abnormalities represents an opportunity for precision oncology. Trastuzumab combined with lapatinib/pertuzumab inhibited growth of HER2-amplified CRC cells/xenografts, paving the way for clinical translation. The clinical benefit of anti-HER2 therapy has been shown in HER2-amplified CRC patients, most of whom are resistant to anti-EGFR therapy.
Introduction
Few clinically actionable genetic abnormalities have been identified in primary tumors, metastases, or blood of patients with colorectal cancer (CRC) [1–3]. While the role of human epidermal growth factor receptor 2 (HER2) as a biomarker for prognosis in CRC remains uncertain, its role as a therapeutic target is rising [4–8]. HER2 is also emerging as a negative predictor of response to epidermal growth factor receptor (EGFR)-targeted treatments [9]. Hence, patients with HER2-positive CRC might have fewer treatment options and carry an inferior prognosis. In this review, we consider the role of HER2 in CRC as an oncogenic driver and prognostic and predictive biomarker, present HER2 testing methods utilized specifically in patients with CRC, and discuss clinical diagnostic and therapeutic data supporting HER2 as a novel therapeutic target in CRC.
HER2 as an oncogenic driver in CRC
HER2 is the only member of the EGFR family that does not bind ligands; it is activated via heterodimerization with other ligand-bound receptors [10], with the strongest mitogenic signals created by HER2–HER3 heterodimers. HER2 overexpression, usually caused by gene amplification, allows HER2 activation even in the absence of ligand bound to the other partners [11]. Overexpression or amplification of HER2 has been reported in 13%–20% of breast cancers [12], 7%–34% of gastric cancers [13], and 1.9%–14.3% of lung carcinomas [14]. Diverse rates of HER2 overexpression have been reported in CRC (Table 1 [9, 15–38]), with rates of membranous expression ranging from 2% to 11% [16]. A number of factors may account for these differing rates, including small study populations, different antibodies for immunohistochemistry (IHC), analysis of distinct subgroups of patients with heterogeneous clinico-pathologic CRC characteristics, and application of diverse scoring systems [18]. More recent studies consistently indicate that HER2 overexpression accounts for approximately 2% of all CRCs [9, 16, 18], increasing up to 5% in stage III [9] or IV KRAS exon 2 wild-type tumors [6, 16, 17].
Table 1.
Incidence of HER2 overexpression and association with prognosis in CRC
| HER2 testing by IHC/ISH | ||||||
|---|---|---|---|---|---|---|
| References | Patients, N | Stage | Monoclonal antibody | HER2 3+, % | Location | Prognostic role |
| Ni et al. [15] | 4913 | – | – | 1.4 | Colorectal | Yes |
| Laurent-Puig et al. [9] | 1795 | III | 4B5 Ventana | 1.4 (n=21/1457) | Colon | Yes for IHC/FISH only for RFS but not OSa |
| Richman et al. [16] | 1914 | II–III | A 0485 Dako | 1.3 | Colorectal | No |
| 1342 | IV | A 0485 Dako | 2.2 | No | ||
| Valtorta et al. [17] | 1086 | – | 4B5 Ventana/ Hercep-Test | 4.1 | Colorectal | Not assessed |
| Ingold Heppner et al. [18] | 1645 | I-IV | Clone SP3 | 0.5 (1.6 total CISH+) | Colorectal | Trend toward worse survival |
| Song et al. [19] | 106 | pT1, pT2, pT3 | 4B5 | 7.5 (2/3+) | Colorectal | No |
| SP3 | 3.8 (2/3+) | No | ||||
| Conradi et al. [20] | 264 | II/III rectal | PATHWAY (Ventana) | 5.9 (n=10/169 pretreatment biopsies) | Rectal | Yes |
| Kruwszewski et al. [21] | 202 | II–IV | A 0485 | 15.3 | Colorectal | No |
| Kountourakis et al. [22] | 106 | – | NCL-CB11 | 2.8 membranous | Colorectal | No |
| Schuell et al. [23] | 77 | I–IV | Hercep-Test | 3 | Colorectal | Trend toward worse survival |
| Essapen et al. [24] | 170 | II–III | HM64.13 | 54.1 (2+, cytoplasmic) | Colorectal | Yes (cytoplasmic, stage III) |
| 40.0 (2+, membranous) | ||||||
| McKay et al. [25] | 249 | I–IV | NCL-CB11 | 3.2 | Colorectal | No |
| Rossi et al. [26] | 156 | I–III | – | 4 (2/3+) | Colorectal | No |
| Osako et al. [27] | 146 | – | Nichirei | 4.5 (HER2 amplification) | Colorectal | Yes |
| Kapitanović et al. [28] | 221 | – | Ab-3 | 43 (n=67/155 adenocarcinomas) | Colorectal | Yes |
|
Molecular profiling of HER2 | ||||||
|---|---|---|---|---|---|---|
| References | Patients, N | Stage | Method | HER2 amplification, % | Location | Prognostic role |
| Ross et al. [29] | 8874 | – | CGS | 4.9 (and/or short variant alterations) | Colorectal | Not assessed |
| Shimada et al. [30] | 201 | I–IV | CGS | 5.0 | Colorectal | Not assessed |
| Gong et al. [31] | 138 | – | NGS | 5.1 | Colorectal | Not assessed |
| Schrock et al. [32] | 143 | – | ctDNA | 4 (≥1 HER2 activating mutation or amplification) | Colorectal | Not assessed |
| Laurent-Puig et al. [9] | 1795 | III | NGS | 2.9 (5.6 alterations KRAS wild type) | Colorectal | For RFS and OS on anti-EGFR-based first-line therapy |
| Takegawa et al. [33] | 18 | – | ctDNA | 22 (HER2 gene copy number ratio 1.25) | Colorectal | Not assessed |
| Edenfield et al. [34] | 4110 | – | NGS, IHC/ISH | 1.8 | Colorectal | Not assessed |
|
HER2 by primary tumor location | ||||||
|---|---|---|---|---|---|---|
| References | Patients, N | Stage | Method | HER2 amplification, % by location | Prognostic role | |
| Weinberg et al. [35] | 1602 | – | CISH | 5.7 left colon (patients ≤45 years) | Not assessed | |
| 2.1 left colon (patients ≥65 years) | ||||||
| Marshall et al. [36] | 496 | – | CISH | 5.4 rectum | Not assessed | |
| 3.4 descending colon | ||||||
| 1.3 right colon | ||||||
| Siena et al. [37] | 33 (all HER2+) | IV | IHC/FISH | 64 distal colon | Not assessed | |
| 21 rectal | ||||||
| 15 proximal colon | ||||||
| Raghav et al. [38] | 97 | – | NGS, IHC/ISH | 9.3 distal | For PFS on anti-EGFR-based therapy in 2nd/3rd line; not for OS | |
| 5.2 proximal | ||||||
| Ingold Heppner et al. [18] | 1645 | I–IV | IHC | 2.1 sigmoid colon–rectum | Trend toward worse survival | |
| 0.9 cecum–descending colon | ||||||
For FISH and NGS, RFS and OS analyses performed by pooling HER2 amplifications and mutations.
CGS, comprehensive genomic sequencing; CISH, chromogenic in situ hybridization; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; NGS, next-generation sequencing; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival.
HER2 testing in CRC
Although routinely used in breast and gastric cancer, IHC and fluorescent in situ hybridization (ISH; FISH) or silver-enhanced ISH (SISH) techniques have not been modified for assessment of HER2 overexpression/amplification in CRC. Table 2 [13, 17, 39, 40] and supplementary Figure S1, available at Annals of Oncology online, summarize current IHC/ISH testing guidelines in breast, gastric, and CRC.
Table 2.
Guidelines for HER2 testing in breast, gastric, and CRC
| Negative | Equivocal | Positive | |
|---|---|---|---|
| Breast cancer (Wolff et al. [39]) |
|
|
|
| Gastric cancer surgical specimen (Rüschoff et al. [13]) |
|
|
|
| Gastric cancer biopsy (Bartley et al. [40]) |
|
|
|
| Colorectal cancer VENTANA (based on Rüschoff et al. [13]) |
|
|
|
| Colorectal cancer HERACLES (Valtorta et al. [17]) |
|
|
At least five consecutive cells.
If additional scoring does not allow a definitive result to be rendered, multiple options are feasible: (1) consultation between scorer and pathologist regarding selection of malignant cells or tumor areas for scoring, (2) use of an alternative chromosome 17 probe in a retest to calculate the ratio, (3) selecting a different tumor block, (4) using genomics or an alternative analytic method to evaluate HER2 amplification.
Circumferential, basolateral, or lateral.
CEP, chromosome enumeration probe.
Valtorta and colleagues conducted a diagnostic study to define specific IHC/ISH criteria to determine HER2 positivity in CRC, and to accurately select patients with HER2-positive, KRAS wild-type metastatic CRC (mCRC) for enrollment in the phase II HERACLES (HER2 Amplification for ColorectaL Cancer Enhanced Stratification) trial of HER2-targeted therapy [17].
HER2 protein expression was assessed manually by IHC using the HercepTest antibody (Dako A/S Glostrup, Glostrup, Denmark), and automatically using the VENTANA 4B5 antibody on the BenchMark ULTRA platform (Ventana Medical Systems, Inc. Tucson, AZ, USA). HER2 amplification was evaluated by FISH using a PathVysion HER2 DNA Probe Kit (Abbott Laboratories, Abbott Park, IL), and by SISH with a VENTANA 4B5 Inform HER2 dual-color assay on the BenchMark ULTRA platform [17]. All samples were centrally scored. HER2 positivity was defined as tumors with HER2 3+ score in ≥50% of cells by IHC, or HER2 2+ score and HER2: CEP17 ratio ≥2 in ≥50% of cells by FISH [17]. Referred to as the HERACLES Diagnostic Criteria, these are more stringent than those adopted for defining HER2 positivity in breast and gastric tumors.
In an archival cohort of 256 patients tested by an international panel of HER2 expert pathologists, and a clinical validation cohort of 1277 patients, 5% of patients with KRAS wild-type mCRC had HER2-positive tumors according to HERACLES Diagnostic Criteria [17, 37]. In recent CRC studies, applying scoring consistent with these criteria, the rate of HER2 positivity (IHC 2+/3+, or HER2 ISH amplification) ranged from 1.6% to 6.3% [18, 41], in contrast to the wide-ranging values previously reported (Table 1 [9, 15–38]).
HER2 amplification in CRC has also been explored using molecular techniques such as next-generation sequencing (NGS) and comprehensive genomic sequencing (CGS), with rates ranging from 1.8% to 22.0% (Table 1 [9, 29–34]). Molecular profiling using NGS, IHC, and chromogenic ISH (CISH)/FISH in a large dataset of patients with HER2-overexpressing CRC revealed a 1.8% (81/4110 patients) incidence of HER2 overexpression, with 97% concordance between HER2 protein expression and gene amplification [34]. Shimada and colleagues retrospectively assessed the HER2 status of 201 patients with stages I–IV CRC using IHC and FISH compared with using CGS [30]. Ten patients (5%) whose tumors were diagnosed as HER2 positive by HERACLES Diagnostic Criteria also had HER2 amplifications according to CGS. HER2 status and HER2 amplifications at the primary site were identical in all patients analyzed (P < 0.001), indicating the utility of CGS for detecting HER2-positive CRC.
The use of liquid biopsies to determine HER2 status was first explored using blood samples from patients with breast cancer [42, 43] and was recently applied in patients with mCRC [32, 33]. Takegawa and colleagues analyzed circulating tumor DNA (ctDNA) from 18 patients with cetuximab-resistant mCRC, of which four (22%) were classified as HER2 positive [33]. Concordance of HER2 amplification between plasma ctDNA and tissue samples was demonstrated by rebiopsy of the metastatic lesion of one of these four patients. In a separate analysis, Schrock and coworkers isolated ctDNA from 143 patients with CRC and identified five patients (4%) with HER2 activating mutations or amplification [32].
IHC is readily available and successful trials of therapeutic HER2 blockade have been based on IHC results. However, it is likely that in the near future, molecular screening using NGS may replace IHC. Although NGS is now more expensive, it has the advantage of capturing a wider range of genome abnormalities including HER2-activating mutations (see section ‘Are HER2 mutations actionable therapeutic targets in mCRC?’) and allowing quantitation of gene copy number.
Distribution and prognostic effect of HER2 in CRC
Clinical and pathologic features of HER2-positive CRC
Tumors originating in the right or left side of the colon and rectum differ in their epidemiology, pathology, mutation profile, and presentation, likely due to distinct embryologic origins of the proximal and distal colon [44]. Proximal, or right-sided, tumors are more likely to be hypermethylated or to have microsatellite instability (MSI) than distal tumors [5]. Right-sided tumors are also more common in women and the elderly [45]. Recent meta-analyses showed a consistent and significant worsening in overall survival (OS) in mCRC tumors originating in the right versus the left side of the colon [46–48].
A number of CRC studies have reported differential expression related to the occurrence of HER2 amplification based on clinical and pathologic features of the tumor, including primary location (Table 1). Analysis of gene expression and DNA copy number data for patients with CRC in the PETACC-3 adjuvant chemotherapy trial revealed that distal carcinomas (splenic flexure, descending colon, rectum) were more likely to be HER2 or EGFR amplified than proximal carcinomas (cecum, ascending, hepatic flexure, transverse colon) [49]. Similar results were reported in advanced CRC, with HER2 amplifications identified using SISH correlating with a distal location [50]. A retrospective analysis identified higher frequencies of HER2 overexpression/amplification in rectal cancers compared with descending colon or right colon cancers [36]. A similar trend for more frequent HER2 overexpression in tumors of the sigmoid colon-rectum than the cecum-descending colon was reported in a large series of primary CRC cases [18]. In the HERACLES-A trial, among 33 patients with HER2-positive mCRC, 64% had distal tumors and 21% had rectal tumors [37]. Retrospective data from the phase II EXPERT-C trial, in patients with high-risk, locally advanced rectal cancer receiving neoadjuvant capecitabine and oxaliplatin plus chemoradiotherapy with or without cetuximab, showed a 4.3% prevalence of HER2 overexpression/amplification using IHC/ISH [51]. These findings are in line with the 5.4% HER2-positivity rate for rectal cancers described by Marshall and colleagues [36].
In contrast to studies reporting a correlation between HER2 amplification and tumor location, other researchers have found no such association. In the PETACC-8 FOLFOX-based adjuvant stage III colon cancer study, no significant differences were seen between patient groups on the basis of tumor location [9]. A retrospective analysis of two independent cohorts of patients with mCRC observed no significant difference in HER2 expression between right- and left-sided primary tumors [38]. A trend toward a decreasing frequency of HER2-positive tumors by IHC was noted from colon to rectum in 3/77 (4%) HER2-positive specimens, but this was not statistically significant (P = 0.251) [23]. Several studies have considered the relationship between KRAS status and HER2 amplification in CRC. In a meta-analysis of 3256 patients with mCRC, HER2 amplification using FISH and gene copy number variation was associated with KRAS/BRAF wild-type status at all disease stages: 5.2% wild type versus 1.0% mutated tumors (P < 0.0001) in stage IV, and 2.1% versus 0.2%, respectively, in stages II–III (P = 0.01) [16]. Similarly, in the PETACC-8 study, HER2 alterations determined using NGS, IHC, and FISH were detected in 42 (5.6%) patients with KRAS wild-type tumors compared with 22 (2.4%) patients with RAS mutation (P < 0.001) [9]. HER2 amplifications according to SISH were also correlated with KRAS wild-type status in 191 patients with mCRC and distant metastases, but only with borderline significance (P = 0.052) [50].
In a meta-analysis of 30 studies enrolling 4942 patients with CRC, HER2 expression assessed by IHC was significantly higher in patients with Duke’s stage C/D tumors compared with Duke’s stage A/B tumors [odds ratio (OR) 0.335, 95% confidence interval (CI) 0.198–0.568; P < 0.001], and in patients with versus without lymph node metastasis (OR 1.987, 95% CI 1.209–3.265; P = 0.007) [52]. However, no significant association was found between HER2 expression and CRC location (rectal versus colon; OR 1.123, 95% CI 0.858–1.468; P = 0.399).
Prognostic role of HER2 in CRC
The prognostic role of HER2 in CRC remains uncertain. A negative prognostic impact of HER2 overexpression was proposed in earlier studies [27, 28], but more recent trials have found no association between HER2 amplification and outcome (Table 1 [16, 19, 21, 22, 25]). However, in one of the largest study cohorts examined (1645 patients with stages I–IV CRC), a trend toward worse OS was reported for the 26 (1.6%) patients with HER2-positive disease compared with those with HER2-negative disease [18]. HER2 was also identified as a poor prognostic indicator in the PETACC-8 study in patients with stage III colon cancer [9]. HER2 alterations were present in 66/1689 evaluated patients (3.9%). HER2 concordant amplification-positive status by both NGS and FISH, and HER2 mutation status determined by NGS, were associated with shorter time to recurrence [hazard ratio (HR) 1.9, 95% CI 1.1–3.2; P = 0.03] and shorter OS (HR 1.7, 95% CI 0.9–3.2; P = 0.045). This prognostic value was maintained after adjustment for age, treatment, RAS mutation, histologic grade, tumor location, pT and pN status, bowel obstruction or perforation, and vascular or lymphatic invasion. Assessment of the potential prognostic effect of HER2 amplifications in CRC is hindered by the low incidence of these alterations, potentially explaining the inconsistent results of studies addressing this question. Nevertheless, based on available data, it appears that the negative prognostic effect of HER2 is not as marked as that of other alterations such as BRAF mutation.
HER2 as a negative biomarker for EGFR-targeted treatments in CRC
CRCs are a molecularly heterogeneous group of tumors that often harbor mutations in KRAS, BRAF, or PIK3CA, as well as HER2 amplifications. These genetic alterations confer resistance to EGFR-targeted therapies in patients with CRC [1, 53, 54], although currently only selection based on RAS status is recommended for excluding patients from anti-EGFR treatment [55–57]. Using data generated from patient-derived mCRC xenografts, Bertotti and coworkers identified HER2 amplification as a negative predictor of response to cetuximab [1, 4]. Two retrospective clinical series supported that activation of HER2 signaling causes resistance to cetuximab [58, 59]. Yonesaka and colleagues evaluated the clinical impact of de novo HER2 amplification in 233 cetuximab-treated patients [58]. Median progression-free survival (PFS) and OS were almost halved in patients with HER2-amplified (n = 13) versus nonamplified tumors (n = 220): PFS 89 versus 149 days, respectively; OS 307 versus 515 days, respectively; P = 0.0013, log-rank test [58]. Survival outcomes were negatively influenced by HER2 gene copy number in a second series of 170 patients with KRAS wild-type mCRC treated with cetuximab or panitumumab alone or in combination with chemotherapy [59]. Raghav and coworkers analyzed the impact of HER2 amplification on the efficacy of anti-EGFR monoclonal antibody therapy in RAS and BRAF wild-type mCRC [38]. They tested HER2 amplification in a first cohort of 97 patients using IHC and dual ISH (HER2: CEP17 ≥ 2.2), and validated their findings in a second cohort of 99 patients, which comprised 37 cases of HER2 amplification identified by NGS (HER2 gene copy number ≥ 4) and 62 HER2 nonamplified control patients pretreated with anti-EGFR antibodies. Median PFS on anti-EGFR therapy was significantly shorter in patients with HER2 amplification versus non-HER2-amplification (2.9 months versus 8.1 months, HR 5.0, P < 0.0001). These findings were confirmed in the validation cohort, in which 69 patients received anti-EGFR treatment after first-line therapy; median PFS was significantly shorter for patients harboring HER2-amplified versus nonamplified tumors (2.8 months versus 9.3 months, HR 6.6, P < 0.0001). Notably, these subgroups had a similar OS (P = 0.86) and PFS while on first-line therapy (P = 0.62) [38]. Finally, in the HERACLES-A study, conducted exclusively in HER2-positive mCRC, Sartore-Bianchi and colleagues reported that patients who had previously received panitumumab or cetuximab, evaluable according to rigorous clinical criteria, were resistant to such therapy [6].
As summarized in Table 3 [4, 6, 37, 38, 58, 59], the results from these experimental and clinical studies concur that HER2 activation substitutes for EGFR dependence in a fraction of patients with CRC. From a clinical perspective, this notion could potentially impact on optimal therapeutic sequence; however, it should be interpreted with caution since the data are retrospective and need to be validated in prospective clinical studies of patients treated with cetuximab or panitumumab.
Table 3.
Studies addressing the predictive role of HER2 to EGFR-targeted therapies in mCRC
| Reference | Patients, N | HER2 amplification, % of patients | Method | Prediction of resistance to cetuximab or panitumumab |
|---|---|---|---|---|
| Bertotti et al. [4] | 85 |
|
IHC/FISH (LSI HER2/CEP17 PathVysion probe) | HER2 amplification or overexpression in 6/44 (13.6%) patients with KRAS WT tumors without objective response to cetuximab or panitumumab versus 0/45 (0%) patients with objective response (P<0.05)a |
| Yonesaka et al. [58] | 233 (182 KRAS WT) |
|
FISH (LSI HER2 SO/CEP17 PathVysion probe) | Median OS longer in HER2 nonamplified versus HER2-amplified (515 versus 307 days, P=0.0013)b |
| Martin et al. [59] | 162 (KRAS WT) |
|
FISH (LSI HER2-neu/CEP17 probe) | Median PFS longer in HER2 FISH+ versus HER2 FISH– (7.4 versus 3.9 months, HR 2.00, 95% CI 1.42–2.83, P<0.0001)c |
| Sartore-Bianchi et al. [6] and Siena et al. [37] | 33 [36] | 100 | IHC/FISH | No objective response to cetuximab or panitumumab in 15/27 patients [6] with HER2-positive tumors assessed for sensitivity to cetuximab or panitumumab according to rigorous criteria |
| Raghav et al. [38] |
|
14% cohort 1; RAS/BRAF-WT cohort 2; not reported |
|
Data supported by a molecularly annotated platform of patient-derived xenografts.
Acquired HER2 amplification in two cases of secondary resistance to cetuximab.
HER2 FISH+: HER2 gene copy number gain (presence of ≥4 copies of the HER2 gene in ≥40% of cells) and HER2-amplified (HER2 gene amplification defined as HER2: CEP17 ≥2 in ≥10% of cells).
HER2 amplification defined as HER2: CEP17 ≥ 2.2.
HER2 amplification defined as HER2 ≥ 4 copies.
HR, hazard ratio; mCRC, metastatic colorectal cancer; WT, wild type.
HER2 as a novel therapeutic target in CRC
HER2 has been investigated as a therapeutic target in mCRC in several small studies during the last decade, but with differing outcomes (Table 4 [7, 37, 60, 61]). A phase II study assessed the combination of trastuzumab and FOLFOX therapy as second- or third-line treatment of patients with mCRC [61]. Notably, patients with IHC HER2 2+ tumors were eligible for enrollment and no ISH testing was planned. Overall, 26 of 653 (4%) of screened tumor blocks scored HER2 ≥ 2+. Among 21 evaluable patients, 5 (24%) achieved a partial response. However, the low rate of HER2 positivity precluded completion of the trial. Results of a subsequent phase II study of first- or second-line trastuzumab plus irinotecan in nine patients with HER2-overexpressing advanced CRC were also reported [60]. HER2 overexpression by IHC was evident in 11 of 138 (8.0%) screened tumors (HER2 2+ in 5 patients and HER2 3+ in 6 patients). Partial responses were observed in five of seven evaluable patients (71%), with responses maintained for ≥6 weeks in four patients. The median survival time was 14 months. The study was closed prematurely due to lack of accrual.
Table 4.
Clinical studies exploiting HER2 as a target for mCRC
| Reference | Phase | Patients, N | HER2 overexpression, % | Treatment | Line of treatment | Objective response rate, % |
|---|---|---|---|---|---|---|
| Published studies | ||||||
| Ramanathan et al. [60] | II | 9a | 3.6 (2+) | Trastuzumab and irinotecan | 1st/2nd | 71 |
| 4.3 (3+) | ||||||
| Clark et al. [61] | II | 21b | 4.0 (2+/3+) | 5-FU, leucovorin, oxaliplatin and trastuzumab | 2nd/3rd | 24 |
| HERACLES-A; Siena et al. [37] | II | 33 | 21 (2+) | Trastuzumab and lapatinib | Salvage | 30.3 |
| 79 (3+) | ||||||
| MyPathway; Hainsworth et al. [8] | II | 37 | 100c | Trastuzumab and pertuzumab | Salvage | 38 |
| Ongoing studies | ||||||
| HERACLES-B; Siena et al. [37] | II | 30 | 100 (17, 2+; 83, 3+) | Pertuzumab and T-DM1 | 2nd/3rd | Not reported |
| HERACLES-RESCUE; Siena et al. [37] | II | 9 | 100 | T-DM1 | Salvage | Not reported |
| S1613 (NCT03365882) | II | Not available | Not reported | Trastuzumab and pertuzumab or cetuximab and irinotecan | 2nd or later | Not reported |
| MODUL (NCT02291289) | II | Not available | Not reported | Capecitabine, trastuzumab, and pertuzumab | Biomarker-driven maintenance therapy after first-line FOLFOX + bevacizumab induction | Not reported |
| NCT03384940 | II | Not available | Not reported | DS-8201 (investigational trastuzumab conjugated with deruxtecan) | 3rd | Not reported |
The study was prematurely closed due to low accrual.
The low rate of HER2 positivity precluded completion of the trial.
Patients with HER2 mutations were also eligible; see text for details.
DS-8201, trastuzumab deruxtecan; 5-FU, 5-fluorouracil; T-DM1, trastuzumab emtansine.
A case report was also published of two patients with mCRC and liver metastases who responded to capecitabine, oxaliplatin, and lapatinib while on a clinical trial, but HER2 status was not determined in these patients [62].
The inconclusive efficacy findings and poor accruals observed in these studies most likely relate to the absence of a number of important prerequisites in study design, including a mechanistically based HER2-targeted preclinical strategy, patient selection using a validated HER2 scoring system, and a preplanned sample size based on the actual estimated incidence of HER2 amplification. Moreover, due to concomitant chemotherapy in some studies, it is difficult to establish the specific contribution of HER2 blockade on therapeutic outcome.
Using xenografts derived from patients with mCRC in preclinical therapeutic trials, Bertotti and colleagues identified amplified HER2 as an effective therapeutic target in cetuximab-resistant CRC [1, 4]. Patient-derived mCRC xenografts with HER2 amplification were sensitive to HER2-blockade with trastuzumab in combination with lapatinib, but not to either agent alone. These preclinical data formed a strong rationale for clinical trials targeting HER2 genetic alterations in patients with mCRC [1, 4, 63], paving the way for the HERACLES studies. Recently, results of the HERACLES-A phase II trial of dual HER2-targeted therapy (trastuzumab plus lapatinib) in CRC were presented [6, 37]. This proof-of-concept trial was conducted in patients with KRAS wild-type, HER2-positive mCRC who were refractory to standard-of-care treatments, including cetuximab or panitumumab. The HER2 status of the patients was determined using the CRC-specific HERACLES Diagnostic Criteria [17]. At the data cutoff of 28 February 2017, 33 patients had been enrolled and were evaluable for response. Complete responses were observed in two patients (6.1%) and partial responses in eight patients (24.2%), giving an overall response rate (ORR) of 30.3% (Table 4) [37]. Patients with HER2 gene copy number ≥9.6 had significantly longer time to progression (median 26.6 weeks versus 13.4 weeks; P = 0.0001) and longer OS (median 53.1 weeks versus 34.0 weeks; P = 0.0058) than those with HER2 gene copy number <9.6 [37]. The combination regimen was well tolerated in this heavily pretreated population (median of five prior regimens), with no grade 4/5 adverse events and no withdrawals due to patient request [37]. Novel methods of generating additional evidence to make this regimen widely available in clinical practice are needed.
Based on preliminary data from the phase II MyPathway trial, investigating agents targeting the HER2, EGFR, BRAF, or Hedgehog pathways in tumors for which these therapies are not currently indicated, the CRC cohort was expanded to enroll 37 patients with HER2-amplified/overexpressed mCRC who had exhausted standard treatments [8]. Patients received a combination of trastuzumab and pertuzumab. The ORR was 38% (95% CI 22.2–56.4) (Table 4). Four (11%) patients had stable disease for >4 months [8]. Median PFS was 4.6 months (95% CI 1.6–9.8) and median OS was 10.3 months (95% CI 7.2–22.1) [7]. ORR was higher in patients with wild-type versus mutated KRAS (52.0% versus 0%, respectively), and in patients with left-sided colon cancer (42.9%) or rectal cancer (45.5%) versus right-sided colon cancer (12.5%) [7].
In support of the available preclinical and clinical data, anecdotal case reports of patients with HER2-positive mCRC who have achieved substantial clinical benefit with targeted anti-HER2 therapy have recently been published [64–66].
Challenges and open issues toward clinical application
Are HER2 mutations actionable therapeutic targets in mCRC?
A number of HER2 activating mutations, sometimes in concomitance with HER2 amplification, have been identified in CRC. These mutations are present in approximately 7% of CRCs, based on TCGA data, and may co-exist with HER2 gene amplification in around 20% of cases [63]. They are similar to those seen in breast cancer and include kinase domain single nucleotide variations such as V842I, V777L, and L755S, and extracellular domain mutations such as S310F [1, 5, 63]. Introduction of these four mutations into immortalized mouse colon epithelial cells triggered HER2 signaling pathways, with increases in HER2, MAPK, and AKT phosphorylation noted relative to HER2 wild-type transduced cells [63]. These HER2 mutations also dramatically increased the number of colonies formed in soft agar, demonstrating enhanced anchorage-independent growth. Furthermore, these HER2-activating mutations produced resistance to the EGFR monoclonal antibodies, cetuximab or panitumumab, when transfected into two cetuximab-sensitive CRC cell lines [63]. In patient-derived CRC xenografts containing the HER2-activating mutations S310Y or L866M, treatment with trastuzumab, neratinib, or lapatinib alone delayed tumor growth, but after 30 days, the mice developed large tumors. However, dual HER2-targeted therapy with trastuzumab plus either neratinib or lapatinib produced durable tumor regression, similar to that observed in HER2-amplified mCRC [63]. Lack of effect of neratinib monotherapy in HER2-mutant CRC was recently confirmed in a basket trial of 125 patients with different tumors harboring HER2 mutations, including 12 patients with CRC [67]. In the latter cohort, there were no objective responses and median PFS was just 1.8 months. In the MyPathway trial, eight patients with mCRC had tumors that harbored HER2 mutations and one of these (12.5%) achieved an investigator-assessed objective response with the combination of pertuzumab and trastuzumab (H. Hurwitz, personal communication). Overall, these data indicate that monotherapy with HER2 small molecule inhibitors is ineffective. It remains to be determined whether HER2-directed combination therapy with monoclonal antibodies or monoclonal antibodies plus small molecule inhibitors can be effective in HER2-mutated tumors.
What are the determinants of resistance to HER2-directed therapies?
Understanding the mechanisms of primary and secondary resistance to HER2 blockade is a priority to develop more effective and additional lines of therapy, because 40%–50% of patients treated within the HERACLES-A and MyPathway trials did not achieve partial response or prolonged stable disease despite HER2 amplification [6, 8]. Moreover, even in patients displaying disease control, secondary resistance occurs in almost all cases. Preclinical models of HER2 therapeutic blockade were carried out in quadruple negative mCRC (i.e. KRAS, NRAS, PIK3CA, and BRAF wild-type) since aberrations in one or more of these effectors could impact on and compensate for the inhibition exerted by HER2-directed therapy [4, 6, 7]. Based on these findings, the HERACLES-A trial was performed in KRAS exon 2 wild-type patients, thus positive clinical results can be applied to this subset of patients only. Anecdotally, we treated two patients harboring KRAS exon 2 mutations off-study, and neither obtained a partial response (S. Siena and A. Sartore-Bianchi, personal communication). The MyPathway study included patients with KRAS-mutated CRC, but none of these patients responded to HER2-directed therapy [7], confirming the biologic rationale underlying resistance.
Extensive studies utilizing pre- and post-treatment tissue samples are ongoing to define the molecular basis of primary and secondary resistance to trastuzumab and lapatinib in HER2-positive mCRC. Preliminary data obtained by molecular profiling of plasma samples collected during the HERACLES-A trial suggest that alterations affecting HER2 parallel pathways controlled by receptor tyrosine kinases, and/or downstream effectors such as RAS and PI3KA, are implicated in primary and secondary resistance to HER2 blockade (G. Siravegna, unpublished data). Once completed, these analyses may have important implications for the selection of patients with mCRC most likely to benefit from anti-HER2 therapies, as well as providing the basis for designing new treatments in patients who experience disease progression.
When to test for HER2 and how an HER2-directed therapy may integrate in the treatment algorithm of mCRC
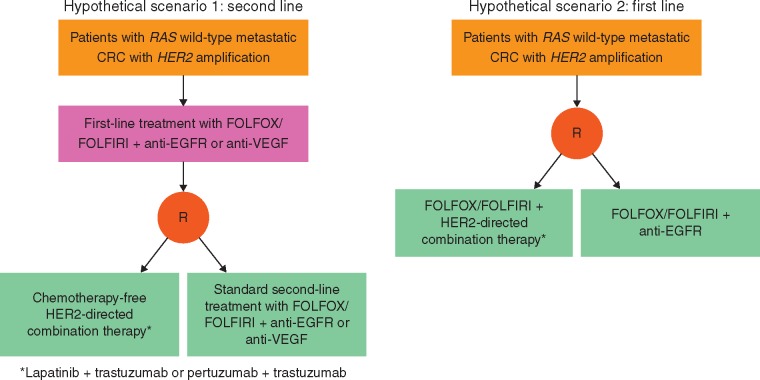
Based on the consistent therapeutic actionability of dual HER2 inhibition in refractory mCRC [6, 8], it is feasible that a similar effect may be obtained by targeting HER2 in earlier lines of treatment and testing for HER2 alongside other biomarkers being assessed at the time of diagnosis of metastatic disease [68]. HER2 amplification in mCRC can be considered an orphan molecular entity. Difficulties in developing a targeted treatment of this cancer, up to the level of regulatory approval, are being addressed with tools developed in the context of international initiatives [69]. While speculative in the absence of randomized data, hypotheses can be made regarding clinical trials with HER2-directed therapies aimed at defining optimal positioning and combination partners in the treatment algorithm of mCRC (Figure 1). National and international collaborations and translational studies designed to understand mechanisms of resistance to HER2 inhibition will be of paramount importance to the successful development of these trials. Based on available results [6, 8], these studies should be restricted to KRAS exon 2 wild-type tumors. Although data regarding the potential impact on sensitivity exerted by other RAS mutations outside KRAS exon 2 are lacking, application of expanded RAS wild-type criteria might be more realistic. Finally, since HER2 amplification might act as a negative predictor of response to EGFR-targeted treatments, HER2-directed therapy before cetuximab or panitumumab in the continuum of care of these patients should be considered a reasonable option.
Figure 1.
Scenarios for positioning of clinical trials with HER2-targeted treatments in mCRC.
Available data suggest a ≥30% ORR with dual HER2 inhibition, which compares favorably with results of second-line standard treatment options in patients with RAS wild-type CRC previously treated with a first-line regimen including an anti-EGFR component. In patients who do not receive an anti-EGFR in first-line, second-line chemotherapy plus anti-EGFR (especially in RAS and BRAF selected cases [70]) might produce good responses, but given the potential negative predictive role of HER2 amplification, this is unlikely to be the case in this patient population. In light of these considerations, and of the expected lower toxicity of a chemotherapy-free regimen, a dual HER2-targeted combination might be an optimal choice for a trial in this setting, either after FOLFOX/FOLFIRI with an anti-EGFR or a vascular endothelial growth factor (VEGF)-targeted component, and compared with standard second-line options.
A hypothetical alternative might be starting with an HER2-targeted component during first-line treatment. However, it is uncertain whether a chemotherapy-free HER2-targeted regimen would perform better than standard FOLFOX/FOLFIRI or FOLFOXIRI+/– EGFR- or VEGF-targeted options, which produce ORRs of 59%–65% in this setting [71, 72]. Therefore, a combination of HER2-directed treatment with chemotherapy should be considered, taking into account safety issues for the combination and the fact that HER2 might act as a negative predictive biomarker of response to EGFR-targeted treatment. For the latter reason, comparison between a FOLFOX/FOLFIRI backbone with an HER2-directed agent versus the same chemotherapy with an anti-EGFR agent might offer a straight-forward answer on the optimal sequencing in RAS wild-type tumors, and whether or not to offer an anti-EGFR drug upfront to these patients.
Conclusion
HER2 amplification is a clinically relevant genetic alteration in mCRC as documented by the HERACLES [6, 37] and MyPathway [7, 8] studies. This biomarker can be screened for with established diagnostic tools [16, 17], occurs in a sizable 5% of patients with KRAS wild-type mCRC, and can potentially act as a predictor of lack of benefit to anti-EGFR monoclonal antibodies [4].
HER2-targeted therapy compares favorably with emerging therapeutic strategies for mCRC such as BRAF-directed therapy and immunotherapy with checkpoint inhibitors. HER2 amplification displays an incidence similar to that of MSI-high (MSI-H) tumors (5%) [73] and lower than that of BRAF mutations (10%); however, compared with BRAF-directed combinations (ORR 16%–21%; median PFS 4.2 months [74]), responses achieved so far in clinical studies with HER2-directed therapies are higher (ORR 30%–38% [6, 8]) and more durable (median PFS 5.2 months [6]), resembling results obtained with checkpoint inhibitors in MSI-H tumors [75]. The toxicity of HER2-targeted combinations is also less than BRAF- or MSI-H-directed therapeutics [74, 75]. Thus, HER2-directed therapies appear to reconcile the merits of precision medicine (rapid and deep induction of tumor shrinkage) with those of immunotherapy (durable responses and better tolerability).
Although evidence from phase III trials with HER2-targeted agents is lacking, randomized studies will take a long time to achieve results in such a selected population [69]. The strong underlying biologic rationale [4], consistent actionability at the therapeutic level [6, 8], and favorable comparison with other precision medicine approaches support consideration for conditional approval of HER2-targeted agents for clinical use by regulatory agencies.
Supplementary Material
Acknowledgements
Authors are grateful to their dedicated clinical and research staff, and to the patients and their families who participated in the studies reviewed in this article. Third-party writing assistance for this review article, under the direction of the authors, was provided by Alison Whyteside of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche Ltd. Authors at Niguarda Cancer Center (SS, ASB, SM) and Candiolo Cancer Institute (AB, LT) are supported by Associazione Italiana Ricerca Cancro (AIRC), grants Targeting Resistances to Molecular Therapies in Metastatic Colorectal Carcinomas – Special Program Molecular Clinical Oncology 5 × 1000 (2015–2017) and AIRC Investigator Grant IG 20685 (2017–2023); CORDIS Community Research and Development Information Service, Horizon 2020 Project ID 635342, grant Molecularly Guided Trials with Specific Treatment Strategies in Patients with Advanced Newly Molecular Defined Subtypes of Colorectal Cancer (MoTriColor); Ministero della Salute, Codice Progetto NET 02352137, grant Genomic-Based Triage for Target Therapy in Colorectal Cancer; project 9970; and Fondazione Oncologia Niguarda Onlus, grant Terapia Molecolare dei Tumori. LT is also supported by AIRC Investigator Grant 18532, Fondazione Piemontese per la Ricerca sul Cancro-ONLUS, 5 × 1000 Ministero della Salute 2011 and 2014, and Transcan (project TACTIC).
Funding
F. Hoffmann-La Roche Ltd, Project 9970 (no grant number applies).
Disclosure
SS has acted in a consulting or advisory role to Amgen, Bayer, BMS, Daiichi-Sankyo, Ignyta, Lilly, Merck, Merus, Novartis, Roche, and Sanofi, received travel, accommodation, or expenses from Bayer, Roche, and Sanofi, participated in a speakers’ bureau for Amgen and Bayer, and received research funding from Amgen, Bayer, and Roche. AS-B has acted in a consulting or advisory role to Amgen, Bayer, Lilly, and Sanofi, participated in a speakers’ bureau for Amgen and Bayer, and received travel, accommodation, or expenses from Amgen, Bayer, and Sanofi. SM has no conflicts of interest to disclose. HIH has previously received honoraria from Roche, acted in a consulting or advisory role to Roche-Genentech and Merck, received research funding from Roche-Genentech and Novartis, and travel, accommodation, or expenses from Roche, and is currently an employee of Roche. SJM owns stock in LabCorp. FP-L has received honoraria from Roche, acted in a consulting or advisory role to Roche, and received research funding and travel, accommodation, or expenses from Roche. SS is an employee of Roche, owns stock in Roche, and has received travel, accommodation, or expenses from Roche. AB has received honoraria from Novartis, Illumina, and Roche, acted in a consulting or advisory role to Horizon Discovery and Biocartis, participated in a speakers’ bureau for Illumina and Biocartis, and received travel, accommodation, or expenses from Roche. LT has received research funding from Merus and Symphogen.
References
- 1. Bertotti A, Papp E, Jones S. et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 2015; 526(7572): 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sartore-Bianchi A, Loupakis F, Argilés G. et al. Challenging chemoresistant metastatic colorectal cancer: therapeutic strategies from the clinic and from the laboratory. Ann Oncol 2016; 27(8): 1456–1466. [DOI] [PubMed] [Google Scholar]
- 3. Siravegna G, Marsoni S, Siena S. et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017; 14(9): 531–548. [DOI] [PubMed] [Google Scholar]
- 4. Bertotti A, Migliardi G, Galimi F. et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011; 1(6): 508–523. [DOI] [PubMed] [Google Scholar]
- 5. The Cancer Genome Atlas Network (TCGAN). Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sartore-Bianchi A, Trusolino L, Martino C. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016; 17(6): 738–746. [DOI] [PubMed] [Google Scholar]
- 7. Hurwitz H, Raghav KPS, Burris HA. et al. Pertuzumab + trastuzumab for HER2-amplified/overexpressed metastatic colorectal cancer (mCRC): Interim data from MyPathway. J Clin Oncol 2017; 35: (suppl 4S; abstr 676). [Google Scholar]
- 8. Hainsworth JD, Meric-Bernstam F, Swanton C. et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol 2018; 36(6): 536–542. [DOI] [PubMed] [Google Scholar]
- 9. Laurent-Puig P, Balogoun R, Cayre A. et al. ERBB2 alterations a new prognostic biomarker in stage III colon cancer from a FOLFOX based adjuvant trial (PETACC8). Ann Oncol 2016; 27: (suppl 6; abstr 459O). [Google Scholar]
- 10. Hynes NE, Lane HA.. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005; 5(5): 341–354. [DOI] [PubMed] [Google Scholar]
- 11. Tzahar E, Waterman H, Chen X. et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996; 16(10): 5276–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rakha EA, Pinder SE, Bartlett JM. et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol 2015; 68(2): 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rüschoff J, Hanna W, Bilous M. et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol 2012; 25(5): 637–650. [DOI] [PubMed] [Google Scholar]
- 14. Kim EK, Kim KA, Lee CY. et al. The frequency and clinical impact of HER2 alterations in lung adenocarcinoma. PLoS One 2017; 12(2): e0171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ni S, Peng J, Huang D. et al. HER2 overexpression and amplification in patients with colorectal cancer: a large-scale retrospective study in Chinese population. J Clin Oncol 2017; 35: (suppl; abstr e15099). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richman SD, Southward K, Chambers P. et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol 2016; 238(4): 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valtorta E, Martino C, Sartore-Bianchi A. et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol 2015; 28(11): 1481–1491. [DOI] [PubMed] [Google Scholar]
- 18. Ingold Heppner B, Behrens HM, Balschun K. et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer 2014; 111(10): 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song Z, Deng Y, Zhuang K. et al. Immunohistochemical results of HER2/neu protein expression assessed by rabbit monoclonal antibodies SP3 and 4B5 in colorectal carcinomas. Int J Clin Exp Pathol 2014; 7(7): 4454–4460. [PMC free article] [PubMed] [Google Scholar]
- 20. Conradi LC, Styczen H, Sprenger T. et al. Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol 2013; 37(4): 522–531. [DOI] [PubMed] [Google Scholar]
- 21. Kruszewski WJ, Rzepko R, Ciesielski M. et al. Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers 2010; 29(5): 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kountourakis P, Pavlakis K, Psyrri A. et al. Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J 2006; 12(3): 229–236. [DOI] [PubMed] [Google Scholar]
- 23. Schuell B, Gruenberger T, Scheithauer W. et al. HER 2/neu protein expression in colorectal cancer. BMC Cancer 2006; 6: 123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Essapen S, Thomas H, Green M. et al. The expression and prognostic significance of HER-2 in colorectal cancer and its relationship with clinicopathological parameters. Int J Oncol 2004; 24(2): 241–248. [PubMed] [Google Scholar]
- 25. McKay JA, Loane JF, Ross VG. et al. c-erbB-2 is not a major factor in the development of colorectal cancer. Br J Cancer 2002; 86(4): 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi HA, Liu Q, Banner B. et al. The prognostic value of invariant chain (Ii) and Her-2/neu expression in curatively resected colorectal cancer. Cancer J 2002; 8(3): 268–275. [DOI] [PubMed] [Google Scholar]
- 27. Osako T, Miyahara M, Uchino S. et al. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology 1998; 55(6): 548–555. [DOI] [PubMed] [Google Scholar]
- 28. Kapitanović S, Radosević S, Kapitanović M. et al. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology 1997; 112(4): 1103–1113. [DOI] [PubMed] [Google Scholar]
- 29. Ross JS, Ali SM, Elvin JA. et al. Targeted therapy for HER2 driven colorectal cancer. J Clin Oncol 2017; 35: (suppl 15; abstr 3583). [Google Scholar]
- 30. Shimada Y, Yagi R, Kameyama H. et al. Utility of comprehensive genomic sequencing for detecting HER2-positive colorectal cancer. Hum Pathol 2017; 66: 1–9. [DOI] [PubMed] [Google Scholar]
- 31. Gong J, Cho M, Sy M. et al. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: a single-institution experience. Oncotarget 2017; 8(26): 42198–42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schrock AB, Young L, Klempner SJ. et al. Genomic profiling of circulating tumor DNA (ctDNA) from patients (pts) with advanced cancers of the GI tract and anus. J Clin Oncol 2017; 35: (suppl 4S; abstr 618). [Google Scholar]
- 33. Takegawa N, Yonesaka K, Sakai K. et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget 2016; 7(3): 3453–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edenfield WJ, Chung KY, Gatalica Z. et al. Molecular profiling of HER2-positive colorectal cancer for identification of multiple potential drug targets. J Clin Oncol 2014; 32: (suppl 15; abstr e14508). [Google Scholar]
- 35. Weinberg BA, Poorman K, Arguello D. et al. Impact of patient age on molecular alterations in left-sided colorectal tumors. J Clin Oncol 2017; 35: (suppl 15; abstr 3592). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marshall J, Lenz H-J, Xiu J. et al. Molecular variances between rectal and left-sided colon cancers. J Clin Oncol 2017; 35: (suppl 4S; abstr 522). [Google Scholar]
- 37. Siena S, Sartore-Bianchi A, Trusolino L. et al. Final results of the HERACLES trial in HER2 amplified colorectal cancer. Anti-HER2 treatment in HER2+ mCRC. Oral presentation at the AACR Annual Meeting 2017, Washington, DC. Abstract CT005.
- 38. Raghav KPS, Overman MJ, Yu R. et al. HER2 amplification as a negative predictor biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. J Clin Oncol 2016; 34: (suppl 15; abstr 3517). [Google Scholar]
- 39. Wolff AC, Hammond ME, Hicks DG. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J clin Oncol 2013; 31(31): 3997–4013. [DOI] [PubMed] [Google Scholar]
- 40. Bartley AN, Washington MK, Colasacco C. et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017; 35(4): 446–464. [DOI] [PubMed] [Google Scholar]
- 41. Seo AN, Kwak Y, Kim DW. et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One 2014; 9(5): e98528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Witzel I, Loibl S, von Minckwitz G. et al. Monitoring serum HER2 levels during neoadjuvant trastuzumab treatment within the GeparQuattro trial. Breast Cancer Res Treat 2010; 123(2): 437–445. [DOI] [PubMed] [Google Scholar]
- 43. Gevensleben H, Garcia-Murillas I, Graeser MK. et al. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin Cancer Res 2013; 19(12): 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 1990; 113(10): 779–788. [DOI] [PubMed] [Google Scholar]
- 45. Mik M, Berut M, Dziki L. et al. Right- and left-sided colon cancer – clinical and pathological differences of the disease entity in one organ. Arch Med Sci 2017; 13(1): 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arnold D, Lueza B, Douillard JY. et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol 2017; 28(8): 1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petrelli F, Tomasello G, Borgonovo K. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol 2016. [Epub ahead of print], doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 48. Holch JW, Ricard I, Stintzing S. et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017; 70: 87–98. [DOI] [PubMed] [Google Scholar]
- 49. Missiaglia E, Jacobs B, D'Ario G. et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014; 25(10): 1995–2001. [DOI] [PubMed] [Google Scholar]
- 50. Nam SK, Yun S, Koh J. et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS One 2016; 11(3): e0151865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sclafani F, Roy A, Cunningham D. et al. HER2 in high-risk rectal cancer patients treated in EXPERT-C, a randomized phase II trial of neoadjuvant capecitabine and oxaliplatin (CAPOX) and chemoradiotherapy (CRT) with or without cetuximab. Ann Oncol 2013; 24(12): 3123–3128. [DOI] [PubMed] [Google Scholar]
- 52. Sun S-J, Lin Q, Sun Q. et al. High HER-2 protein levels correlate with clinicopathological features in colorectal cancer. J Can Res Ther 2016; 12(1): 323–333. [DOI] [PubMed] [Google Scholar]
- 53. Leto SM, Trusolino L.. Primary and acquired resistance to EGFR-targeted therapies in colorectal cancer: impact on future treatment strategies. J Mol Med 2014; 92(7): 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siena S, Sartore-Bianchi A, Garcia-Carbonero R. et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann Oncol 2018; 29(1): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sepulveda AR, Hamilton SR, Allegra CJ. et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Clin Oncol 2017; 35(13): 1453–1486. [DOI] [PubMed] [Google Scholar]
- 56. Van Cutsem E, Cervantes A, Adam R. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27(8): 1386–1422. [DOI] [PubMed] [Google Scholar]
- 57. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer v2.2017. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (5 October 2017, date last accessed).
- 58. Yonesaka K, Zejnullahu K, Okamoto I. et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011; 3(99): 99ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martin V, Landi L, Molinari F. et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer 2013; 108(3): 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramanathan RK, Hwang JJ, Zamboni WC. et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest 2004; 22(6): 858–865. [DOI] [PubMed] [Google Scholar]
- 61. Clark JW, Niedzwiecki D, Hollis D. et al. Phase-II trial of 5-fluororuacil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzumab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy. Onkologie 2003; 26: 13–46.14605451 [Google Scholar]
- 62. Mohammed TA, Dennie T, Holen KD.. Activity of oxaliplatin plus capecitabine (CapeOx) with lapatinib for metastatic colorectal cancer: results from two patients treated on a clinical study. Clin Adv Hematol Oncol 2011; 9(6): 492–500. [PubMed] [Google Scholar]
- 63. Kavuri SM, Jain N, Galimi F. et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov 2015; 5(8): 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martinelli E, Troiani T, Sforza V. et al. Sequential HER2 blockade as effective therapy in chemorefractory, HER2 gene-amplified, RAS wild-type, metastatic colorectal cancer: learning from a clinical case. ESMO Open 2018; 3(1): e000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haslem DS, Ji HP, Ford JM, Nadauld LD.. Precision oncology strategy in trastuzumab-resistant human epidermal growth factor receptor 2-positive colon cancer: case report of durable response to ado-trastuxumab emtansine. JCO Precis Oncol 2017; 10.1200/PO.16.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Parikh A, Atreya C, Korn WM, Venook AP.. Prolonged response to HER2-directed therapy in a patient with HER2-amplified, rapidly progressive metastatic colorectal cancer. J Natl Compr Canc Netw 2017; 15(1): 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hyman DM, Piha-Paul SA, Won H. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018; 554(7691): 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sartore-Bianchi A, Marsoni S, Siena S.. Human epidermal growth factor receptor 2 as a molecular biomarker for metastatic colorectal cancer. JAMA Oncol 2018; 4(1): 19–20. [DOI] [PubMed] [Google Scholar]
- 69. André F. Developing anticancer drugs in orphan molecular entities – a paradigm under construction. N Engl J Med 2018; 378(8): 763–765. [DOI] [PubMed] [Google Scholar]
- 70. Peeters M, Oliner KS, Price TJ. et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res 2015; 21(24): 5469–5479. [DOI] [PubMed] [Google Scholar]
- 71. Loupakis F, Cremolini C, Masi G. et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371(17): 1609–1618. [DOI] [PubMed] [Google Scholar]
- 72. Douillard JY, Oliner KS, Siena S. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369(11): 1023–1034. [DOI] [PubMed] [Google Scholar]
- 73. Gelsomino F, Barbolini M, Spallanzani A. et al. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev 2016; 51: 19–26. [DOI] [PubMed] [Google Scholar]
- 74. Corcoran RB, André T, Atreya CE. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov 2018; 8(4): 428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Overman MJ, McDermott R, Leach JL. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18(9): 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.