Abstract
The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE), a phase 2/3 trial of investigational rVSV∆G-ZEBOV-GP vaccine, was conducted during an unprecedented Ebola epidemic. More than 8600 eligible healthcare and frontline response workers were individually randomized to immediate (within 7 days) or deferred (within 18–24 weeks) vaccination and followed for 6 months after vaccination for serious adverse events and Ebola virus infection. Key challenges included limited infrastructure to support trial activities, unreliable electricity, and staff with limited clinical trial experience. Study staff made substantial infrastructure investments, including renovation of enrollment sites, laboratories, and government cold chain facilities, and imported equipment to store and transport vaccine at ≤−60oC. STRIVE built capacity by providing didactic and practical research training to >350 staff, which was reinforced with daily review and feedback meetings. The operational challenges of safety follow-up were addressed by issuing mobile telephones to participants, making home visits, and establishing a nurse triage hotline. Before the Ebola outbreak, Sierra Leone had limited infrastructure and staff to conduct clinical trials. Without interfering with the outbreak response, STRIVE responded to an urgent need and helped build this capacity.
Clinical Trials Registration. ClinicalTrials.gov [NCT02378753] and Pan African Clinical Trials Registry [PACTR201502001037220].
Keywords: Africa, clinical trial, Ebola virus, implementation, outbreak, vaccine
The evaluation of a new candidate vaccine during the response to the Ebola epidemic in Sierra Leone [1]—a country with limited resources, infrastructure, and clinical trial experience—posed special challenges. Sponsored by the US Centers for Disease Control and Prevention (CDC), the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) began enrollment in April 2015 in partnership with the College of Medicine and Allied Health Sciences of the University of Sierra Leone (COMAHS) and the Sierra Leone Ministry of Health and Sanitation (MOHS) [2].
Several factors and considerations shaped the design, planning, and implementation of the study. First, providing the vaccine to as many high-risk individuals as was safe, ethical, and practical was essential. Second, the need for urgent implementation required making and refining key decisions on the study design and sample size quickly, as information from the epidemic was evolving. Third, the study had to be carried out in a country with substantial logistical and staffing challenges and shortages of healthcare workers that intensified during the ongoing epidemic. Finally, the trial could not impede the national Ebola response.
This paper describes the implementation challenges related to infrastructure, staffing, participant communication, and technology integration during the planning (October 2014–March 2015), enrollment (April–August 2015), and follow-up (April 2015–November 2016) stages of the trial; successful strategies used; and lessons learned.
STRIVE OVERVIEW
The STRIVE was a randomized, unblinded phase 2/3 trial with no placebo that was designed to evaluate the safety and efficacy of the investigational Ebola virus vaccine, rVSV∆G-ZEBOV GP (Merck and Company, Inc). Enrolled participants were randomized to either immediate (within 7 days) or deferred (18–24 weeks after enrollment) vaccination and followed for 6 months after vaccination to monitor for serious adverse events and Ebola virus disease (Ebola). The operational challenges of safety follow-up for a trial with thousands of participants spread over 5 districts were addressed by issuing mobile phones to participants, conducting home visits if participants were unreachable by telephone, establishing a nurse triage hotline, and providing access to free medical care. Details of study design, methods, and results are reported elsewhere [2, 3].
PLANNING, ENROLLMENT, AND FOLLOW-UP
Establishing Study Sites and Core Trial Infrastructure
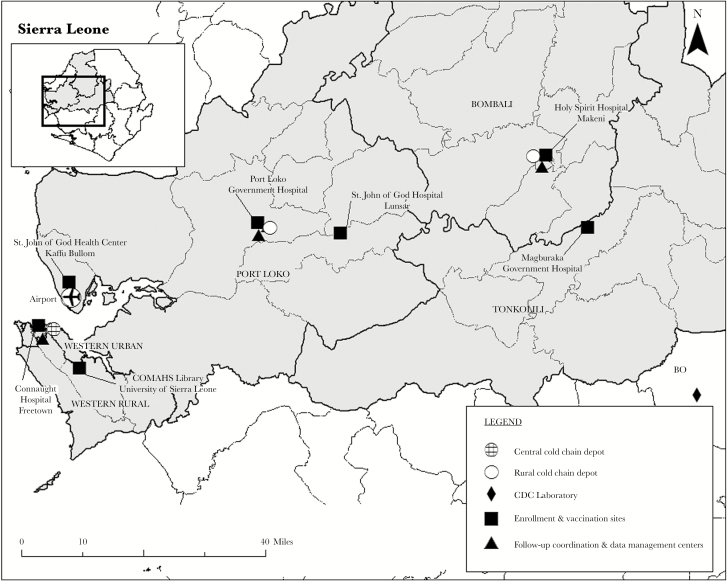
The study focused on healthcare workers, who had been shown to have a 100-fold greater risk of Ebola than the general population [4], and frontline Ebola responders. To achieve the targeted number of outcomes for the event-driven design, initial incidence assumptions projected enrollment of approximately 6000 persons [2]. At the time of study planning, no reliable roster of healthcare workers existed because many had changed positions or transferred to other facilities to support the response. Therefore, we enumerated healthcare and Ebola frontline workers in the districts with high Ebola incidence by reviewing human resource records at each healthcare facility. The 5 trial districts (Western Area Urban [Freetown], Western Area Rural, Port Loko, Bombali, and Tonkolili) were selected because they each had (1) enough high-risk healthcare and frontline workers to ensure sufficient target population sample size and (2) high levels of disease, so ongoing risk of exposure to Ebola was likely. Within these districts, 7 study sites and 3 cold chain depot locations were chosen based on infrastructure and logistics factors such as accessibility for participants and ease of vaccine transport (Figure 1), which resulted in a decentralized operations model. Staff conducted enrollment, screening, and vaccination at all study sites, including 3 government district hospitals, 3 faith-based health facilities, and the COMAHS campus (Figure 2). The main coordination, follow-up, and training center in Freetown (COMAHS Coordinating Center [CCC]) and 2 participant follow-up centers in the districts distant from Freetown were open throughout the study for conducting follow-up telephone calls, home visits, and electronic entry of data collected on paper forms.
Figure 1.
Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) site map, Sierra Leone.
Figure 2.
STRIVE staff member discussing vaccination with a participant. Reprinted with permission.
At each of the enrollment and vaccination sites, we used a common standardized set of study protocols but developed detailed site microplans and work throughput models that were critical for determining equipment and supply needs, maximizing patient flow, estimating the appropriate number of staff required for each task to eliminate bottlenecks during the most time-consuming activities (administering informed consent and checkout), and minimizing vaccine wastage. Given the urgency of the epidemic, we did not use fixed targets or maximums for daily enrollment, but we gave sites the flexibility to enroll based on turnout of eligible participants; this resulted in very rapid enrollment in the first weeks of the trial and put stress on study sites and especially participant follow-up systems.
Building and Providing Water and Electricity for Study Sites, Cold Store Facilities, and Laboratories
Because of the lack of appropriate infrastructure in Sierra Leone, the CDC Foundation provided funds to extensively renovate study facilities, especially the CCC. As many as 60 staff members worked at the CCC, which had separate rooms for conducting study activities, including participant telephone follow-up and data entry, filing, and storage of supplies. Approximately 4000 participants were monitored from this site, which also served as the regulatory office headquarters, staff meeting area, and training center. At the study sites where participants were enrolled and vaccinated, toilet facilities had to be installed to allow the required urine testing for pregnancy among female participants, and walls had to be constructed to separate areas for screening, consent, vaccination, blood sample collection (for the immunogenicity substudy conducted at the Freetown enrollment site), and postvaccination observation. Portable hospital screens were used to provide privacy when permanent renovations were not possible.
Sierra Leone has frequent power shortages in the Freetown area, especially during the dry season, and limited access to electricity in rural districts [5]. Limited access to running water for handwashing hampered infection control practices in government health facilities [6]. To ensure the availability of electricity and water for handwashing and toilets at all study sites, we installed diesel generators and water storage containers and purchased fuel and water.
The need for consistent electricity was most acute at STRIVE’s 3 cold chain depots to ensure maintenance of rVSV∆G-ZEBOV GP vaccine at the required extremely low temperature (≤–60°C) [7]. The trial's central cold storage depot in Freetown required multiple back-up electrical systems, including a generator supporting the entire Expanded Program for Immunizations (EPI) cold chain compound (where the national supply of routine vaccines was also stored) and a generator and batteries to ensure power in the STRIVE vaccine storage cold room. This room, which held 4 –80°C freezers and a refrigerator-freezer, required continuous temperature monitoring and a staff member on call around the clock to respond when routine and back-up power systems failed. One time, a fire in the EPI compound generator room required use of the STRIVE generator as the sole electrical source for approximately 24 hours. Cold storage depots in Port Loko and Bombali districts had similar infrastructure upgrades, including the provision of back-up generators. Renovations included creating work space for preparing vaccine doses, using dedicated equipment and standard procedures to minimize contamination, according to manufacturer guidelines and trial requirements.
To test for Ebola (the trial efficacy outcome measure), we established a dedicated trial laboratory within an existing CDC Ebola testing laboratory in Bo District, which was staffed by rotating CDC laboratory experts. For Ebola surveillance, STRIVE participants referred to an Ebola facility had one blood specimen sent to a national Ebola laboratory and a second specimen sent to the STRIVE laboratory [8] for testing under Good Clinical Laboratory Practice conditions. For the STRIVE immunogenicity substudy, part of the Connaught National Hospital laboratory in Freetown was renovated to collect, process, and provide short-term storage for serum specimens for immunogenicity assay testing that was conducted in the United States at the end of the study.
Infrastructure investment included ensuring satellite and wireless Internet access—for entering data at each of 3 participant follow-up centers. Despite these provisions, Internet access was often slow and connectivity was unreliable, which led to the decision to use paper forms rather than real-time electronic data capture onsite.
Communication Activities to Build Awareness and Community Trust
Before the trial launched, the staff initiated communication efforts with the main objectives of recruiting participants, supporting human subjects’ protection, and building trust in the community [9]. Ongoing formative activities with the general public, public health leaders, and healthcare workers to identify their knowledge, attitudes, and beliefs about Ebola vaccines and the vaccine trial allowed us to adapt study messages and materials in response to newly identified issues. Trial staff coordinated with the National Ebola Response Committee Social Mobilization Pillar so that STRIVE messages were aligned with the overall Ebola response. Community engagement activities included parliamentary briefings, high-level government meetings, community stakeholder and international partner meetings, and health facility leadership briefings to continuously update partners, stakeholders, and the community on STRIVE activities.
To educate the target population about the trial, we conducted more than 175 sensitization and information sessions for potential study participants at hospitals, community health centers, Ebola Treatment Units, and Ebola Holding Centers. The informational materials were designed to maximize understanding of the trial procedures as well as risks and benefits among participants with widely varied educational backgrounds. After enrollment, participants had access to a 24-hour study hotline for any further questions.
Innovative Approaches to Staffing and Capacity Building
The trial was led by a Sierra Leonean principal investigator and coinvestigators, all of whom were senior physicians with research experience. Implementing the trial required hiring approximately 350 local staff members with medical, nursing, or pharmacy experience for a variety of roles (Table 1). The latest data available to trial planners (from 2012) indicated that Sierra Leone was a medically underserved country, with only 136 doctors and 1036 nurses and midwives serving a population of 6 million [10]. Most of these healthcare workers were actively engaged in the response to the ongoing Ebola epidemic. The trial employed physicians who worked part time after completing their clinical responsibilities at health facilities where study sites were located. Retired senior nurses were hired for management roles, and recent nursing graduates awaiting their first government posts were employed as participant follow-up monitors [11]. Because universities were closed during the Ebola outbreak, we had a unique opportunity to hire medical and pharmacy students from COMAHS who had very limited work experience but brought energy, enthusiasm, and a desire to contribute. However, sending students and recent graduates from Freetown to study sites in the rural districts was challenging because they were not always familiar with the local area and languages and did not have established relationships with the District Health Management teams, critical liaisons between the trial and the Ebola response.
Table 1.
STRIVE Staff Roles
| Title | Qualifications | Role in Trial |
|---|---|---|
| Study physician | Senior physician; often Medical Superintendent of hospital | • Provide medical care to participants |
| • Evaluate SAEs; review medical charts | ||
| • Complete SAE forms | ||
| • Conduct verbal autopsies with family of deceased participant | ||
| Medical Director/Assistant Medical Director | Senior physician | • Supervise study physicians |
| • Report SAEs, including deaths, to national regulatory authorities | ||
| • Determine whether SAEs are vaccine related | ||
| Study nurse | Senior clinical nurse | • Triage participant medical complaints |
| • Refer/schedule appointment with study physician | ||
| • Follow up with participant to assess whether SAEs have resolved | ||
| • Follow up with pregnant women for newborn assessment | ||
| • Complete SAE forms | ||
| Vaccinators | Nurse graduates with 2 years clinical experience | • Vaccinate participants |
| • Complete participant forms | ||
| • Complete vaccine accountability forms | ||
| Screeners | Recent nurse graduate | • Administer screening questions to potential participants |
| • Perform urine pregnancy test and provide results | ||
| • Complete enrollment forms to determine whether participant is eligible | ||
| Consenters | Medical student | • Administer informed consent to participant |
| • Determine need for consent witness | ||
| • Cosign consent forms | ||
| Enrollment site managers | Experienced staff, some retired hospital matrons or physicians | • Manage/supervise enrollment staff |
| • Manage crowd control for potential participants | ||
| • Maintain site and supply inventory | ||
| • Submit completed participant forms daily | ||
| • Track daily enrollment and vaccination | ||
| Vaccination site managers | Retired hospital matrons | • Observe participants post vaccination and supervise provision of medical care if needed |
| • Supervise vaccinators and checkout clerks | ||
| • Maintain inventory and replenish supplies | ||
| Surveillance monitors | Recent university graduate, recently graduated nurse | • Conduct monthly assessment phone calls to participants |
| • Conduct home visits | ||
| • Complete study forms; track completion of monthly calls and schedule home visits if participant was not reached |
Abbreviations: SAE, serious adverse events; STRIVE, Sierra Leone Trial to Introduce a Vaccine against Ebola.
The STRIVE used staff resources strategically, creating a mobile team for the smallest 2 sites that could complete enrollment in 1 month at the first site and then relocate to staff the second site. We rapidly trained 20 new staff members when we expanded the enrollment target from 6000 to 8600 participants because of declining Ebola incidence. After enrollment ended, we retrained 40 staff members for new roles to address the increased workload related to participant follow-up.
All staff members participated in a multiday training on the study protocol, standard operating procedures, Good Clinical Practice, and Human Subjects Protection. The staff also underwent role-specific trainings, supplemented by small-group, hands-on training at the sites the week before they opened. Periodic training sessions were then conducted to introduce new procedures or address errors related to staff inexperience with clinical research. The staff identified topics for these refresher training sesions during weekly site staff meetings, and local and international subject-matter experts conducted trainings as needed.
Staff members based in the United States also provided key trial support in Sierra Leone. Approximately 200 people, mainly from the CDC but also the from the US Food and Drug Administration (FDA) and the Department of Health and Human Services Biomedical and Advanced Research and Development Authority, deployed from the United States to Sierra Leone for 6-week or longer tours to support the trial’s vaccine safety monitoring, disease surveillance, regulatory, information technology, supply chain, and management and staff supervision activities. An Atlanta-based principal investigator and a team of clinical trial, regulatory, and management and operations experts supported the field teams. Because there were few local healthcare workers with the appropriate clinical trial experience, identifying and deploying qualified health personnel to oversee and train local staff was critical early in the trial. Officers from CDC’s 2-year applied epidemiology training program [12] (Epidemic Intelligence Service) and experienced CDC staff were effective coleaders in helping guide local managers at the district study sites.
Using international experts for a short term allowed us to quickly launch the trial, but this staffing model also had disadvantages—namely, it placed the burden of orientation and training on local and long-term trial staff and also resulted in frequent adjustments to existing systems when new staff arrived with different perspectives. In contrast, the small number of CDC long-term international residential staff hired at the beginning of the trial, each twinned with a local counterpart, provided essential continuity of functions and effective long-term mentorship and partnership with the Sierra Leonian staff. Pairing the CDC staff with local staff in key trial roles for project management, medical and safety monitoring, and regulatory affairs, literally sitting side by side, helped strengthen the technical skills of Sierra Leone’s staff while enhancing the United States team’s understanding of local context. This approach was successful in building not only the technical capacity to implement clinical trial procedures, but also mangagment capacity of local staff to oversee a complex trial with a large and diverse staff. Together, the “twins” introduced management structures such as (1) weekly action-oriented staff meetings facilitated by local managers and the Assistant Project Director and (2) training in providing positive feedback to local staff. The twins also worked together to design and deliver effective training. This cooperation proved useful in the identification of culturally appropriate solutions to challenges in participant recruitment and follow-up.
Integrating Technology Into Trial OperationsMany decisions had to be made about technology use for participant identification, reimbursement, and data capture early in the trial design process. To prevent double enrollment, we considered using biometrics to confirm participant identity, but ultimately we decided against it because the software was still being pilot-tested and therefore was not compliant with the FDA’s electronic records and signature regulations (21 Code of Federal Regulations [CFR] Part 11).
We used Internet-based procedures to prepare participant identification cards, although, as previously mentioned, we did not collect study forms electronically. Handheld tablets and portable printers with backup solar batteries were used to take photographs of participants, print identification cards with a photo and study number, and record their name and contact information, which formed the core online participant locator database used for participant follow-up activities. We had anticipated that the tablets could provide real-time enrollment and vaccination rates, but unreliable Internet connectivity made this impossible. However, the tablets’ offline capability was valuable in establishing a successful electronic participant follow-up system, using a paper-based system as back-up.
We initially reimbursed enrollees for their participation via electronic payment sent directly to their study-issued cell phone number. This eliminated the need for keeping large amounts of cash at the sites. However, participants’ inexperience with electronic financial transfers and a lack of outlets for receiving cash, especially outside of Freetown, made it difficult for them to receive their reimbursement, so the STRIVE staff ultimately switched to an immediate cash system with an onsite banker. Although the change allowed participants to receive their reimbursements immediately, it required management of large amounts of cash onsite and reconfiguration of space to establish a secure area for the banker and security guard.
One of the most successful technology adoptions was the modification and use of Arktek portable coolers capable of maintaining approximately −80°C temperature for up to 5 days without power. As described elsewhere [7], these modified “deep freeze” units, referred to as Arktek DFs, were used to transport vaccine vials weekly to district cold chain depots where staff prepared and delivered individual syringes to the vaccination sites daily. Arktek DFs were also used for short-term vaccine storage in the district depots and could serve as backup for –80°C freezers in case of electrical system failure [13].
Conducting Participant Follow-upFollow-up continued for 6 months after vaccination, with the deferred group followed 4–6 months before and then 6 months after vaccination. We conducted follow-up by telephone rather than requiring participants to return to a medical clinic. The study staff called participants monthly to document serious and nonserious adverse events, including deaths, new pregnancies that occurred after enrollment, and any changes in use of personal protective equipment. Participants who became acutely ill before a scheduled assessment called a toll-free study number and were connected to a study nurse for initial assessment and possible medical referral.
Data from a 2012 survey showed that approximately 60% of adults in Sierra Leone had access to a cell phone, but a much smaller percentage actually owned one [14]. Therefore, to facilitate postenrollment follow-up, each participant who enrolled was given a new Nokia cell phone and a SIM card that provided access to a “closed user group.” This allowed free calls for the study staff to contact participants each month, for participants to dial the study hotline if they had procedural questions or medical concerns, and for study nurses to follow up on illnesses.
However, using cell phones also presented challenges, especially with keeping them charged when electricity was in short supply. SIM cards and phones were occasionally lost or damaged and had to be replaced. Participants sometimes had trouble carrying and charging both their personal and study cell phones; in some instances, participants sold their study phones but kept the study SIM card and used it with their personal device. To overcome these technological challenges, we collected alternative telephone numbers, such as personal cell phone numbers and numbers of friends and family members, at enrollment, which was critical for reaching participants when they did not respond to calls on their study phones. When participants still could not be reached, staff conducted home visits in both densely populated urban Freetown and rural villages in the districts. The rural visits were resource intensive due to geographic spread and the difficulty of finding unnumbered dwellings, but successful visits resulted in participant reengagement and resolution of issues they may have been experiencing. Overall, more than 92% of participants were retained in the trial 6 months after enrollment and vaccination [15].
Impact of Ongoing Ebola Transmission During Sierra Leone Trial to Introduce a Vaccine against Ebola Trial Implementation
Although the number of Ebola cases peaked and then declined dramatically during the 6-month period between initiation and trial launch, the occurrence of new Ebola cases in the study districts, some involving healthcare workers, presented challenges. For example, when we commenced enrollment in the Kaffa Bullom Chiefdom of Port Loko District, the existence of a new Ebola cluster required staff and pharmacists delivering the vaccine to the study site to plan for extra travel time because new Ebola checkpoints had been established along the main road. There was also heightened awareness of routine procedures, such as temperature screening of potential participants before they entered the facility. Despite these complications, the local situation resulted in very high interest and turnout for educational sessions and trial enrollment; we even conducted educational sessions for nurses quarantined at the district hospital dormitory and successfully enrolled eligible nurses after they had completed their 21 days of quarantine.
In August 2015, the World Health Organization-sponsored ring vaccination trial in Guinea that used rVSVΔG-ZEBOV-GP reported interim vaccine efficacy results of 100% (95% confidence interval, 75%–100%) [16]. After these encouraging results, the ring trial expanded to Sierra Leone in September 2015. In response, we modified the STRIVE protocol to allow for early vaccination of deferred participants who were exposed or at risk for exposure to Ebola. When a new Ebola case was confirmed and ring vaccination implemented in one of the STRIVE study districts, Tonkolili, in September 2015, we vaccinated approximately 95 participants working at high-risk Ebola Treatment Units and hospitals in the outbreak-affected area.
LESSONS LEARNED
To implement the trial successfully, as with other vaccine trials in West Africa [17–19], basic challenges related to inadequate infrastructure and limited resources had to be addressed (see Table 2). These included allocating time to renovate sites and providing ongoing support to maintain newly installed water, electricity, and Internet services. Conducting the trial in 5 districts allowed us to enroll and vaccinate a large number of health and frontline workers in high-risk areas. Microplanning and enumerating the target population in each district before starting the trial were key to this success by allowing us to estimate the number of participants that could be screened and enrolled per day, given the logistics at each site, and then efficiently deploy equipment and staff.
Table 2.
Challenges, Solutions, and Lessons Learned During Implementation of the Sierra Leone Trial to Introduce a Vaccine against Ebola
| Area | Challenge | Solutions | Lessons Learned |
|---|---|---|---|
| Staffing | • Local hiring with limited pool of qualified applicants due to Ebola response | • Hired university students (universities were closed during the epidemic) and newly graduated nurses and retired matrons. • Hired parttime study physicians. • Deployed international skilled staff to train and support local staff. |
• Student workforce worked well—enthusiastic, built capacity for research and interest in public health. • Must consider burden on existing Human Resources system and need to supplement staff/ policies to support large number of short-term hires in short period of time. • Long-term international hires were more effective than short-term international staff for providing appropriate oversight and capacity-building functions in a country with skilled local staff shortages. |
| • Staff inexperienced in clinical trials | • Implemented large-scale protocol, SOP training, HSP and GCP training, ongoing training. • Built senior staff’s capacity for managing large staff. |
• Ongoing training is needed for new procedures, refreshers, protocol amendments. • Building training skills among senior staff is critical. • Twinning of local staff with international experts allowed hands-on supervision and “mentoring” that consolidated skills not learned in a class. |
|
| • Workforce comprising mainly students, recent nursing school graduates, and retired nurse managers (matrons) | • Provided basic work skills/professional training. | • More intensive supervision and training for students with no prior work experience was needed. • Retired nurse managers effective in a cultural context where age is respected, but managerial skills required strengthening. |
|
| Infrastructure | • Lack of infrastructure to support the first-ever large-scale clinical trial in Sierra Leone | • Partnered with CDC Foundation to renovate space for vaccination, cold chain depots, laboratory work, and data management. | • Renovations were a form of capacity building that now allows COMAHS and MOHS to undertake new research projects more easily. |
| • No reliable sources of electricity, water, and Internet at study sites and cold chain facilities | • Procured generators, water towers for buildings, and back-up generators. • Installed satellite-routed Internet and wireless capacity. |
• This was a critical challenge at all sites and must be planned for in trial preparation and budgeting, including for maintenance and fuel. | |
| Logistics | • Need to deliver and replenish daily vaccine and other supplies to decentralized sites | • Established cold chain depots in districts. | • Considering transportation needs is critical during planning, including for obtaining vehicles and fuel and for making time estimations, based upon road infrastructure, for travel. |
| Community relations | • Need for ongoing communications with community and religious leaders for trial updates, addressing misconceptions, and controlling rumors | • Held regular in-person meetings with political and community leaders and a final meeting to brief them on trial results. | • Engaging and periodically updating key leaders throughout the planning and implementation stages of a trial is vital to its success. |
| Technology | • Problems with electronic payments for participants and study cell phones, Internet, and mobile connectivity | • Used paper-based forms with centralized data entry, instead of entering real-time data using laptops and portable devices in all study sites. • Discontinued electronic payments in favor of direct cash payment at time of enrollment. • Recorded alternative contact numbers and made home visits to reach participants. |
• Using new technology requires existence or new building of basic infrastructure (ie, access to power) and understanding local population behavior to ensure that it is accepted and properly used. Pilot testing or flexibility to course-correct in the middle of the project is important. |
| • Need to maintain vaccine at −60°C | • Adopted new technology Arktek DF for temporary vaccine storage and transport. | • Although Arkteks were helpful, the trial still needed back-up generators and on-call staff for central vaccine storage. |
Abbreviations: CDC, US Centers for Disease Control and Prevention; COMAHS, College of Medicine and Allied Health Sciences of the University of Sierra Leone; DF, deep freeze; GCP, Good Clinical Practice; HSP, Human Subjects Protection; MOHS, Ministry of Health and Sanitation; SOP, standard operating procedure.
The trial's creative approach to selecting and training approximately 350 local staff in light of a severe national healthcare worker shortage, as well as the overarching commitment that trial activities could not interfere with the country’s response to the Ebola epidemic, is noteworthy. Their participation gave recent graduates and current students in the allied health sciences a unique opportunity, early in their careers, to receive research training that built capacity for future trials and raised interest in public health. The success of this approach can be measured by the number of local STRIVE staff working on new clinical trials in Sierra Leone or engaged in other clinical research. One year after the trial, staff report that they have applied useful management strategies such as action-oriented staff meetings and other lessons in professionalism and effective communication to new workplaces.
Integrating technology into trial design, where possible, resulted in both successes and challenges. Flexible approaches, such as building in offline capability for trial software, were necessary adaptations for sites in rural districts where cell phone and Internet coverage were less reliable. The burden of using paper-based systems (printing, storage, transporting files) was substantial for a trial of this size, and further work remains to improve the use of tablets with offline capability for real-time electronic entry and improving Internet coverage across the country.
Conducting a successful trial that vaccinated almost 8000 high-risk healthcare workers under a rigorous investigational new drug protocol in the midst of an Ebola epidemic, and in coordination with the national Ebola response, was a challenging but ultimately successful experience. During the trial, new Ebola clusters were identified in all 5 districts, although no cases occurred among STRIVE participants [3]. Yet even as the epidemic waned, concern about Ebola continued to motivate healthcare workers and frontline responders to enroll.
CONCLUSIONS
The strengthened infrastructure and cadre of trained staff has left Sierra Leone better equipped to conduct future clinical trials. Demonstrating the feasibility of enrolling healthcare workers in a vaccine trial using strategies to minimize absenteeism and impact on clinical services will help inform policies and procedures for prophylactic or outbreak-related vaccination of healthcare workers. This research capacity, along with investments in Emergency Response and Integrated Disease Surveillance through the Global Health Security Agenda [20], will put Sierra Leone in a stronger position to manage new Ebola cases and clusters. With experience in storing, transporting, and administering the rVSVΔG- ZEBOV-GP vaccine, the Sierra Leone MOHS is incorporating Ebola ring vaccination, still conducted under investigational new drug procedures, into national Ebola response planning and is planning preparedness exercises and trainings for local staff in vaccination and follow-up while awaiting licensure of the vaccine. Data to support use of vaccine during an Ebola response to prevent outbreaks from becoming humanitarian crises is surely the best legacy of STRIVE and the other Ebola vaccine trials.
Notes
Acknowledgments. We express our gratitude to the trial participants and thanks to the hundreds of dedicated Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) staff from the College of Medicine and Allied Health Sciences of the University of Sierra Leone, University of Sierra Leone, the Sierra Leone Ministry of Health and Sanitation, and the US Centers for Disease Control and Prevention (CDC) STRIVE teams working tirelessly in Atlanta and Sierra Leone. We recognize the Government of Sierra Leone, Oliver Morgan, and the CDC Sierra Leone Ebola Response and Country teams, and the staff of the United States Embassy for their support during the trial and especially for facilitating delivery of vaccine and trial equipment and supplies.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. The trial was funded by the Centers for Disease Control and Prevention, the Biomedical Advanced Research and Development Authority, and the National Institutes of Health, with additional support from the CDC Foundation.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interests. All authors: No reported conflicts of interest. All authors have submitted the ICJME Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society for Tropical Medicine and Hygiene 65th Annual Meeting, Atlanta, GA; November 13–17, 2016; Abstract 1891.
References
- 1.World Health Organization. Ebola situation report 16 March 2016. Geneva: World Health Organization, 2016. Available at: http://apps.who.int/ebola/current-situation/ebola-situation-report-16-march-2016. Accessed January 29, 2018. [Google Scholar]
- 2. Widdowson MA, Schrag SJ, Carter RJ, et al. Implementing an Ebola Vaccine Study - Sierra Leone. MMWR Suppl 2016; 65:98–106. [DOI] [PubMed] [Google Scholar]
- 3. Samai M, Seward JF, Goldstein ST, et al. The Sierra Leone Trial to Introduce a Vaccine against Ebola: an evaluation of rVSVΔG-ZEBOV vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis 2018; 217:s6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kilmarx PH, Clarke KR, Dietz PM, et al. Ebola virus disease in health care workers — Sierra Leone, 2014. MMWR 2014; 63:1167–71. [PMC free article] [PubMed] [Google Scholar]
- 5. Angelou N, Azuela GE, Banerjee SG, et al. Global Tracking Framework The World Bank, 2013. Available at: http://documents.worldbank.org/curated/en/603241469672143906/Global-tracking-framework. Accessed November 30, 2017. [Google Scholar]
- 6. A situational analysis of WASH in Sierra Leone World Health Organization, 2016. p. 1–3. Available at: https://www.washinhcf.org/documents/SierraLeone_WASH-Situational-Analysis_March2016.pdf. Accessed November 30, 2017. [Google Scholar]
- 7. Jusu MO, Glauser G, Seward JF, et al. Rapid establishment of ≤–60°C cold chain capacity for the STRIVE Ebola vaccine trial during the Ebola outbreak in Sierra Leone. J Infect Dis 2018; 217:s48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conteh MA, Goldstein ST, Wurie HR, et al. Clinical surveillance and evaluation of suspected Ebola cases in a vaccine trial during an Ebola epidemic: STRIVE. J Infect Dis 2018; 217:s33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Callis A, Carter VC, Albert AP. Lessons learned in clinical trial communication during an Ebola outbreak: the implementation of STRIVE. J Infect Dis 2018; 217:s40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Global Health Observatory (GHO) Data World Health Organization, 2017. Available at: http://www.who.int/gho/en/. Accessed Novemeber 30, 2017. [Google Scholar]
- 11. Edem-Hotah J, McDonald W, Abu PM, et al. Utilizing nurses to staff an Ebola vaccine clinical trial in Sierra Leone during the Ebola outbreak. J Infect Dis 2018; 217:s60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thacker SB, Dannenberg AL, Hamilton DH. Epidemic intelligence service of the Centers for Disease Control and Prevention: 50 years of training and service in applied epidemiology. Am J Epidemiol 2001; 154:985–92. [DOI] [PubMed] [Google Scholar]
- 13. Friend M, Stone S, editors. Challenging Requirements in Resource Challenged Environment on a Time Challenged Schedule: A Technical Solution to Support the Cold Chain for the VSV-Zebov (Merck) Ebola Vaccine in Sierra Leone Guinea. Proceedings of the 5th IEEE Global Humanitarian Technology Conference Seattle, WA: GHTC 2015, 2015. [Google Scholar]
- 14. Wittels A, Maybanks N. Research Report: Communication in Sierra Leone- an analysis of media and mobile audiences. BBC Media Action 2016; 1–56. [Google Scholar]
- 15. Carter RJ, Senesi RG, Dawson P. Participant retention in a randomized clinical trial in an outbreak setting: lessons from the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). J Infect Dis 2018; 217:s65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857–66. [DOI] [PubMed] [Google Scholar]
- 17. Cutts FT, Enwere G, Zaman SM, Yallop FG. Operational challenges in large clinical trials: examples and lessons learned from the gambia pneumococcal vaccine trial. PLoS Clin Trials 2006; 1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larson GS, Baseler BR, Hoover ML, et al. Conventional wisdom versus actual outcomes: challenges in the conduct of an Ebola vaccine trial in Liberia during the International Public Health Emergency. Am J Trop Med Hyg 2017; 97:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marchetti E, Mazarin-Diop V, Chaumont J, et al. Conducting vaccine clinical trials in sub-Saharan Africa: operational challenges and lessons learned from the Meningitis Vaccine Project. Vaccine 2012; 30:6859–63. [DOI] [PubMed] [Google Scholar]
- 20. Bali S, Taaffe J. The sustainable development goals and the Global Health Security Agenda: exploring synergies for a sustainable and resilient world. J Public Health Policy 2017; 38:257–68. [DOI] [PubMed] [Google Scholar]