See the article by Pfaff and Kessler et al, pp. 826–837
The German National Center for Tumor Diseases (NCT) has developed a noncomparative screening trial called Neuro Master Match (N2M2) for first-line unmethylated glioblastoma (GBM) patients.1 In the current issue of Neuro-Oncology, the NCT presents pilot data to evaluate feasibility and timeliness of performing complex multilayer molecular diagnostics in a clinical setting to support N2M2. These data are timely in our field, as many platform trials are either ongoing2 or planned3 where biomarker data are important for patient allocation. We will first provide a bit more depth on N2M2 and then compare and contrast this trial with 2 other platform trials: the INdividualized Screening trial of Innovative GBM Therapy (INSIGhT)2 and the GBM Adaptive Global Innovative Learning Environment (GBM AGILE).3
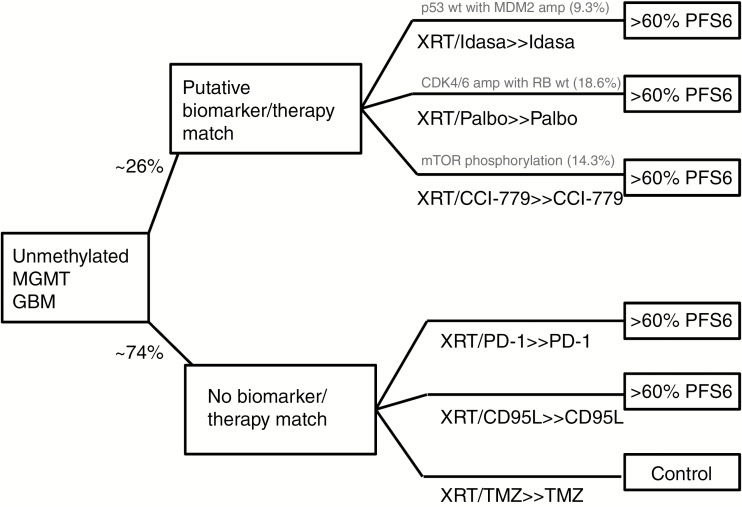
N2M2 leverages the master protocol/platform trial structure4 to create efficiency for multiplexed biomarker testing. Single-arm biomarker-selected trials have a large amount of screen failures, particularly when the targeted biomarker population is small. In contrast, N2M2 uses a combination of biomarker-selected arms and biomarker agnostic arms to allow every patient screened to be eligible. Currently, ~26% of N2M2 arms are biomarker specific, while 74% are biomarker negative (Fig. 1). The newly diagnosed unmethylated setting allows experimental arms to be studied without prior safety data in combination with temozolomide, which has potential to accelerate development for GBM.5,6 Forty patients will be enrolled in each arm and success is defined as >60% of the patients in any arm achieving 6-month progression-free survival (PFS6), the primary endpoint. N2M2 is a noncomparative study where all arms are evaluated independently with the control in biomarker-negative patients providing “reassurance for the validity of the assumptions of PFS.”
Fig. 1.
Schema for N2M2.
Ten different assays on formalin-fixed paraffin-embedded and/or fresh frozen tissue were used to acquire biomarkers for eligibility and arm assignment. One concern is the time to complete these assays—the median time to completion was 4–5 weeks but the range was well outside of the necessary timeframe to start therapy (4–6 wk). Furthermore, most processing and analysis took place at the same institution where the clinical tissues were obtained. In the planned multi-institutional trial, time for transfer/shipping can easily be an additional 7–14 days. Moving the biomarker identification timeframe closer to 2–3 weeks can likely be achieved by prioritizing select assays to provide enrollment biomarker information. Other assays can be processed but as a lower priority. Undoubtedly the totality of these biomarker data will provide important exploratory information to correlate with patient outcomes.
N2M2 uses a prespecified algorithm to assign patients to various experimental arms, but there are other possibilities as well.7 Biomarker-based clinical trial design choices are driven by 3 key factors: assignment of biomarker-positive patients, specificity of biomarker-specific arms, and the need for randomization. The choice of strategies depends on the frequency of the biomarker, the confidence in a biomarker-specific drug effect, and the desired endpoint. Design choices about biomarker-positive patient assignment depends on biomarker frequency. For example, targeting a small biomarker subpopulation would have accrual difficulties if biomarker-positive patients were allowed to be assigned to other arms. In such cases, assignment algorithms may be preferred over randomization. Dropping randomization has limitations in the interpretability of the outcome, however, particularly for endpoints such as PFS and overall survival (OS).8 Assignment algorithms may also become complicated based on the biology of the given cancer. Some cancers are characterized by mutually exclusive driver mutations and are amenable to simple algorithms but GBM is more complicated, with frequent overlapping biomarker groups.9 In such cases, more complex decision rules are needed, as patients are frequently eligible for more than one arm. Algorithms like N2M2 are attractive because they prioritize based on the relative likelihood of treatment effect. A downside of this approach is that there are generally limited data to support the prioritization. Such algorithms also don’t solve the overlapping biomarker problem. For example, in N2M2, all 4 murine double minute 2 (MDM2) amp/p53 wild-type (WT) patients also had cyclin-dependent kinase 4 amplification (one also had positive staining for mammalian target of rapamycin). If this relationship holds, the palbociclib arm will have to complete accrual before idasanutlin will accrue any patients. If this is not the desired outcome, consideration for arm accrual, relative biomarker frequency, or randomization elements could be added to avoid this result.
Decisions about biomarker-negative patients (biomarker specificity) depends on confidence in the biomarker. For therapeutic arms with well-established biomarkers, including biomarker-negative patients may be undesirable, particularly if there is significant risk or toxicity. The preclinical data supporting a p53 WT-specific effect for MDM2 antagonists (like idasanutlin) is a good example of strong pretrial biomarker evidence.10 But assigning only biomarker-positive patients to an arm means no biomarker evidence is generated. Other targeted arms may not be supported by similar strong pretrial biomarker data and would benefit from assigning biomarker-negative patients to targeted arms to test the biomarker hypothesis.
Finally, the need to randomize is based on the clinical trial endpoint. Endpoints that have significant variability related to nontherapy factors (PFS, OS) generally would benefit from randomization outside of very large signals.8 In N2M2, the primary endpoint is PFS6. The choice not to have randomized controls may be based on a desire to treat as many patients on drug as possible for biomarkers with small frequencies, but the tradeoff is interpretability of results. This is a major issue facing all trials of small biomarker subpopulations. One potential solution is to develop response-based endpoints associated with OS benefits so that randomization is less important. Another solution may be to leverage clinically annotated biomarker datasets to understand the natural history of subgroups for given endpoints.11 For example, if it were well established that MDM2 amp/p53 WT tumors have no different natural history than other tumors, perhaps comparison could be made to the biomarker-negative control arm for endpoints such as PFS and OS. Even the “noncomparative” nature of N2M2 makes the assumption of no different natural history between biomarker-defined groups and would be further supported by such knowledge. Certainly, if there was no evidence that patients with MDM2 amp/p53 WT tumors had better survival than those without, a doubling or tripling of survival in the idasanutlin arm would be compelling evidence for the drug. If patients were enrolled on N2M2 before their biomarker profile (and therefore arm assignment) was known, selection bias would be at least partially addressed as well. More work is needed to truly understand the implications of potential associated design innovations.
The primary endpoint of both INSIGhT2 and GBM AGILE3 is OS, so there is randomization against a common control arm (Table 1). Both trials are designed to randomize all patients equally among arms, regardless of biomarker status, and become biomarker restricted only over time if data generated during the course of the trial support a biomarker-specific effect. These designs have the opposite challenges of assignment algorithms such as N2M2. Biomarker-positive patients from low-frequency populations may be randomized to control or an alternative experiential arm. Furthermore, if pretrial evidence is strongly supportive of a biomarker-specific effect, biomarker-negative patients are needlessly randomized (at least initially) to a targeted arm. One potential compromise for this second issue is to allow both randomize-all and biomarker-only arms on the same trial, driven by explicit analysis of the strength of evidence for a biomarkers-specific effect.12 An arm could even start biomarker specific and open to biomarker-negative patients if big effects were seen during the trial.12 But the benefits of the randomization in INSIGhT and GBM AGILE are that complex and arbitrary assignment algorithms are avoided, biomarker development is maximized, and the results are more interpretable using a relevant endpoint (OS).
Table 1.
Current and planned later stage clinical development platform trials for glioblastoma
| Trial | ID | Primary Endpoint | Randomized Control | Indication | Biomarker Assignment | Phase Equivalent |
|---|---|---|---|---|---|---|
| INSIGhT | NCT02977780 | OS | Yes | NDU | RAR | II |
| N2M2 | NCT03158389 | PFS6 | No | NDU | Algorithm | II |
| GBM AGILE | Pending | OS | Yes | All | RAR | II/III |
RAR = response adaptive randomization; NDU = newly diagnosed unmethylated.
To summarize, designing clinical trials with both biomarker and therapeutic questions is challenging, particularly with rare biomarker subgroups. Different trials may make different choices depending on the relative biomarker frequencies, strength of evidence supporting a biomarker-specific drug effect, and endpoint choices. Additional work on trial design in the era of precision medicine is critical to address the challenges related to current design limitations and create new paradigms for evidence generation.
Funding
None.
Acknowledgment
The text is the sole product of the authors and no third party had input or gave support to its writing.
Conflict of interest statement. B.M.A. has consulted for AbbVie, Schlesinger Associates, Bristol-Myers Squibb, and Precision Health Economics; B.M.A. is Principal Investigator for INSIGhT which is funded by Puma, Eli Lilly and Celegene. B.M.A. is the President and CEO of Global Coalition for Adaptive Research. During the last year, T.F.C. consulted for Roche/Genentech, VBL, GW Pharma, Celgene, Merck, BMS, Abbvie, Pfizer, Agios, ProNai, MedQia, Tocagen, Cortice Biosciences, Cytrx, Novocure, NewGen, Oxigene, Novogen, and Wellcome Trust. T.F.C. has stock options with Notable Labs. T.F.C. is CMO for Global Coalition for Adaptive Research.
References
- 1. Pfaff E, Kessler T, Balasubramanian GP et al. Feasibility of real-time molecular profiling for patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation—the NCT Neuro Master Match (N2M2) pilot study. Neuro Oncol. 2018;20(6):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander BM, Trippa L, Gaffey SC et al. Individualized screening trial of innovative glioblastoma therapy (INSIGhT). J Clin Oncol. 2017;35(15 suppl):TPS2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander BM, Ba S, Berger MS et al. ; GBM AGILE Network Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2018;24(4):737–743. [DOI] [PubMed] [Google Scholar]
- 4. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. [DOI] [PubMed] [Google Scholar]
- 5. Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. [DOI] [PubMed] [Google Scholar]
- 6. Vanderbeek A, Rahman R, Fell G et al. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments?Neuro Oncol. 2018; doi:10.1093/neuonc/noy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tajik P, Zwinderman AH, Mol BW, Bossuyt PM. Trial designs for personalizing cancer care: a systematic review and classification. Clin Cancer Res. 2013;19(17):4578–4588. [DOI] [PubMed] [Google Scholar]
- 8. Grossman SA, Schreck KC, Ballman K, Alexander B. Point/counterpoint: randomized versus single-arm phase II clinical trials for patients with newly diagnosed glioblastoma. Neuro Oncol. 2017;19(4):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brennan CW, Verhaak RG, McKenna A et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verreault M, Schmitt C, Goldwirt L et al. Preclinical efficacy of the MDM2 inhibitor RG7112 in MDM2-amplified and TP53 wild-type glioblastomas. Clin Cancer Res. 2016;22(5):1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanguturi SK, Trippa L, Ramkissoon SH et al. Leveraging molecular datasets for biomarker-based clinical trial design in glioblastoma. Neuro Oncol. 2017;19(7):908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trippa L, Alexander BM. Bayesian baskets: a novel design for biomarker-based clinical trials. J Clin Oncol. 2017;35(6):681–687. [DOI] [PubMed] [Google Scholar]