Abstract
Bed bugs are one the most troublesome household pests that feed primarily on human blood. RNA interference (RNAi) is currently being pursued as a potential tool for insect population management and has shown efficacy against some phytophagous insects. We evaluated the different techniques to deliver dsRNA specific to bed bug muscle actin (dsactin) into bed bugs. Initially, stability of dsRNA in human blood was studied to evaluate the feasibility of feeding method. Adult bed bugs were injected with dsRNA between last thoracic segment and first abdominal segment on the ventral side, with a dose of 0.2 µg dsactin per insect. In addition to injection, dsactin was mixed in acetone and treated topically in the abdomens of fifth stage nymphs. We found the quick degradation of dsRNA in blood. Injection of dsactin caused significant depletion of actin transcripts and substantial reduction in oviposition and lethality in female adults. Topically treated dsRNA in fifth stage nymphs had no effect on actin mRNA expression and survival. Our results demonstrated that injection is a reliable method of dsRNA delivery into bed bugs while topical treatment was not successful. This research provides an understanding on effective delivery methods of dsRNA into bed bugs for functional genomics research and feasibility of the RNAi based molecules for pest management purposes.
Keywords: RNAi, bed bug, injection, topical application, gene knockdown
The discovery of RNA interference (RNAi) mechanism by Fire et al. (1998) in the nematode Caenorhabditis elegans Maupas provided a promising tool to control insects and diseases. RNAi-mediated suppression of pest populations through transgenic plants have been successful in some phytophagous insects, such as Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae), and Diaphorina citri Kuwayama (Hemiptera: Liviidae) (Baum et al. 2007, Zhu et al. 2011, Hajeri et al. 2014). RNAi-based insect control strategy is the future of pest management because of their specificity and relatively easy degradation in the environment (Whyard et al. 2009, Murphy et al. 2016). Feeding and injection are two common techniques used for dsRNA delivery in insects (Zhu et al. 2010, Rangasamy and Siegfried 2012, Zhu et al. 2012, Fishilevich et al. 2016). Injection has been a reliable method of dsRNA delivery into blood-feeding insects, such as bed bugs, ticks, triatomine bugs, but this technique is not practical for the field application (Araujo et al. 2006, Kocan et al. 2007, Zhu et al. 2012). Other dsRNA delivery approaches explored for pest management include topical application, soaking, feeding of transgenic microorganism expressing dsRNA, nanoparticles and plants expressing dsRNA (Baum et al. 2007, Pridgeon et al. 2008, Ghormade et al. 2011, Wang et al. 2011, Zhu et al. 2011, Das et al. 2015, Kim et al. 2015, Whitten et al. 2016).
Bed bugs, Cimex lectularius L. (Hemiptera: Cimicidae) is a hematophagous household insect pest that causes significant human health and economic consequences (Doggett et al. 2004, Anderson and Leffler 2008). Interest in genomic research of bed bugs has increased in recent years due to the resurgence of bed bugs around the globe and their potential to transmit human diseases (Adelman et al. 2013). A laboratory study showed that bed bugs can potentially transmit Chagas disease in mice (Salazar et al. 2015), but there is no factual data in humans. Bed bugs are principally controlled with insecticides application however indoor use of chemical insecticides, raises health risk and not always recommended. Heat treatment is another strategy commonly used but it is not economical.
The muscle actin is a highly-conserved gene abundant in eukaryotic cells including insects. Actin interacts with myosin during muscle contraction and this interaction is also involved in diverse cellular functions, such as cell division and movements of non-muscle cells (Cooper 2000, Dominguez and Holmes 2011). Previous studies have demonstrated robust RNAi in bed bugs (Zhu et al. 2012, 2013; Gujar and Palli 2016a,b; Basnet and Kamble 2017, 2018). In this study, we assessed the phenotypic responses from injection and topical application of dsRNA specific to the muscle actin gene. Injection of the dsRNA showed significant knockdown of target for 30 d as well as significant reduction in oviposition and survival. While the topical application of dsRNA had no effect in actin knockdown and the lifespan of treated insects. This research provides an insight on potential dsRNA delivery techniques in bed bugs.
Materials and Methods
Insects
A Harlan strain of bed bugs was reared in glass jars as described by Basnet and Kamble (2017). Bed bugs were maintained in a growth chamber (Incubator I-35LL, Percival Scientific, Perry, Iowa) adjusted to a photoperiod of 14:10 (L: D) h, 25 ± 1°C and 55 ± 5% RH (Montes et al. 2002).
Synthesis of dsRNA
The bed bug muscle actin gene is available in the NCBI (GenBank ID: 106674067). Sense and antisense primers were designed with a T7 promoter (TAATACGACTCACTATAGGG) at 5′end to initiate transcription from both strands of cDNA. In addition to the target gene, dsRNA specific to non-target Green Florescence Protein (GFP) was also amplified and used as negative control. GFP was prepared from pIZT/V5-His expression vector using gene-specific primers (Invitrogen, Waltham, MA). The polymerase chain reaction (PCR) products were purified with QIAquick PCR Purification Kit (Qiagen, Valencia, CA) following manufacturer’s protocol. Furthermore, PCR products were sequenced at the DNA Sequencing Core facility at the University of Nebraska, Omaha, NE and confirmed by BLASTN. A 1.5 µg of purified cDNA was used as a template for in vitro dsRNA synthesis using the Megascript High Yield Transcription Kit (Ambion, Waltham, MA). The dsRNA was purified using the RNeasy Mini Kit (Qiagen) and eluted with ultra-pure water. The dsRNA quality was evaluated by gel electrophoresis and quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
RNA Isolation, cDNA Synthesis and Real-Time Quantitative PCR (RT-qPCR)
Total RNA was extracted from an individual adult bed bug using RNeasy mini kit (Qiagen) and treated with RNase DNase Free (Qiagen) to avoid DNA contamination. 0.5 µg of total RNA was used to synthesize cDNA using QuantiTect Reverse Transcription Kit (Qiagen). The RT-qPCR primers were designed outside the dsRNA sequence of the target gene, and the efficiencies were evaluated using a fivefold serial dilution. Primers sequences and specifications are provided in Table 1. RT-qPCR reaction volume of 10 µl/well consisted of 5 µl Fast SYBR Green Master Mix (Applied Biosystems, Grand Island, NY), 2 µl of cDNA, 0.4 µl each of forward and reverse primers and 2.2 µl of water. The ribosomal protein L8 (rpL8) was used as the internal control gene to normalize expression level of the target gene (Zhu et al. 2012), but primers sequences were different (Table 1). The reaction was carried in triplicate per template in a 7500 Fast System real-time PCR detection system (Applied Biosystems, Grand Island, NY). The cycling parameters were 40 cycles each consisting of 95°C for 5 s, and 58°C for 30 s, as described in supplier’s protocol. A single melting curve in the melt curve analysis ruled out primer dimer formation and non-specific product formation. In addition, the amplified products were visualized after running in a 1.7% agarose gel, and a single product of expected size confirmed the amplification of the target gene. Relative quantification (RQ) of the transcript was calculated using comparative 2−ΔΔCT method (Livak and Schmittgen 2001) and the relative mRNA levels were analyzed.
Table 1.
Primers for dsRNA synthesis and RT-qPCR
| Gene | Primer sequences for dsRNA synthesis | bp | |||
|---|---|---|---|---|---|
| Actin | F: TAATACGACTCACTATAGGGCAGGGAAAAGATGACCCAGA | 410 | |||
| R: TAATACGACTCACTATAGGGTACCGATGGTGATGACCTGA | |||||
| GFP | F: TAATACGACTCACTATAGGGGGTGATGCTACATACGGAAAG | 370 | |||
| R: TAATACGACTCACTATAGGGTTGTTTGTCTGCCGTGAT | |||||
| Gene | Primer sequences for RT-qPCR | bp | Slope | R2 | Eff. |
| Actin | F: ATGAGATGGGTCTCGGAAAG | 124 | −3.47 | 0.99 | 94.09 |
| R: TCGATAGCGGAACAATGATG | |||||
| rpL8 | F: AGGCACGGTTACATCAAAGG | 131 | −3.4 | 0.99 | 98.6 |
| R: TCGGGAGCAATGAAGAGTTC |
bp: base pair, F: forward, R: reverse, R2: regression value, Eff: primer efficiency.
Stability of dsRNA in Blood and Acetone
Initially, feasibility of dsRNA delivery by feeding was evaluated by mixing dsRNA in human blood in vitro and assessing for degradation in agarose gel. 1 µg of dsactin or dsGFP was mixed in 8 µl of blood or water (control) and kept at room temperature. Samples were collected at 0, 5, and 10 min and run in 1% agarose to evaluate degradation. Topical application was evaluated by administrating intact dsRNA eluted in water and dsRNA mixed in acetone. Acetone was mixed in 1 µg of dsRNA, kept at room temperature for 30 min and assessed for degradation. dsRNA degradation of the mixtures was evaluated by observing bands in an agarose gel and observed in the spectrophotometer.
Injection Bioassay in Adults
Adult females and males 0–2 wk-old were segregated and placed in glass jars for 2 d to allow mating. After mating, males were removed and females were injected with 0.2 µg of dsRNAs or water. The treatment groups were injected with dsactin while the control groups were treated with dsGFP or water. The bed bugs were softly glued on an adhesive tape upside down and injected with a fine capillary tube fitted with a nanoinjector on the ventral side behind hind legs (Nanoject II Auto-Nanoliter Injector, Drummond Scientific Company, Broomall, PA). After injection, the bed bugs were kept in a growth chamber for 8 h to recover from injury and then provided with a bloodmeal. Only those bed bugs that fed to repletion were transferred to small Petri dishes (50 × 11 mm) with a sterile pad for laying eggs (Advantec MFS, Inc., Dublin, CA). All treatments were replicated eight times, each consisting of six bed bugs. Bed bugs were fed every 10 d and monitored for mean number of eggs laid per female, egg hatching percentage per replicate and survival.
Topical Application in Nymphs
Unlike the injection bioassay, fifth stage nymphs were used in this bioassay. The cuticle in nymphs is less sclerotized compared to adults; therefore, we performed topical application in fifth stage nymphs. The nymphs were topically applied with 1 µg dsRNA per insect. The controls included bed bugs topically treated with 1 µl of acetone, 1 µg dsGFP diluted in acetone (3:1) and the treatments included 1 µg dsactin eluted in water and the dsactin mixed in acetone (3:1). All treatments were replicated eight times, each consisting of six nymphs. The nymphs were placed in a Petri dish, frozen at −20°C for 5 min and topically treated with 1 µl of the solution in the abdomen using a pipette (VWR 0–2 µl pipette, Radnor, PA). The bed bugs were kept in the growth chamber and fed every 10 d. The average number of eggs laid per female and mortality of bed bugs were monitored.
Statistics
The injection bioassay data on egg laying was analyzed as repeated measures analysis of variance (ANOVA). Repeated measures ANOVA can be used when the same parameters are measured at different time frames in the same experimental unit (Ott and Longnecker 2015). In addition, when a significant effect of the treatment is observed, simple effect analysis provides significant differences among treatments at each feeding interval. The cumulative percent survival for 30 d with injection and topical application were analyzed with one-way ANOVA. RQ values on gene expression for both bioassays were analyzed separately with a one-way ANOVA and means were compared using Tukey Kramer’s adjustment. All analyses were performed with PROC GLIMMIX in SAS 9.4 (SAS Institute Inc 2013).
Results
Stability of dsRNA in Blood and Acetone
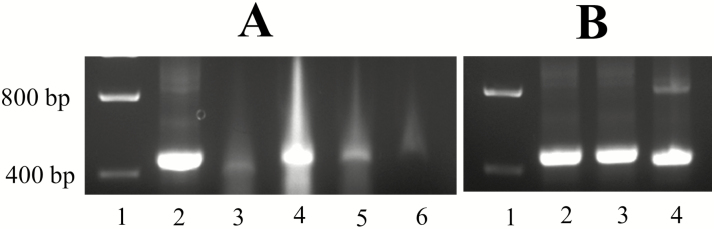
We observed a quick degradation of dsRNA in blood. The band from dsactin mixed in water showed intense band, while bands produced from dsRNA mixed in blood at different time frames produced less intense bands (Fig. 1A). The dsactin and dsGFP mixed in acetone were intact along with that mixed in water which is shown in the gel image (Fig. 1B).
Fig. 1.
Degradation of dsRNA in (A) blood and (B) acetone. (A) Fate of dsRNA on blood, lane (ln) 1: ladder, ln 2: dsactin + water, ln 3: dsactin + blood at 0 min, ln 4: dsGFP + blood at 0 min, ln 5: dsactin + blood at 5 min, ln 6: dsactin + blood at 10 min. (B) Fate of dsRNA in acetone after 30 min. ln 1: ladder, ln 2: water + dsactin, ln 3: acetone + dsactin, ln 4: acetone + dsGFP. The size of dsactin is 410 bp and dsGFP is 370 bp.
Injection Bioassay Data
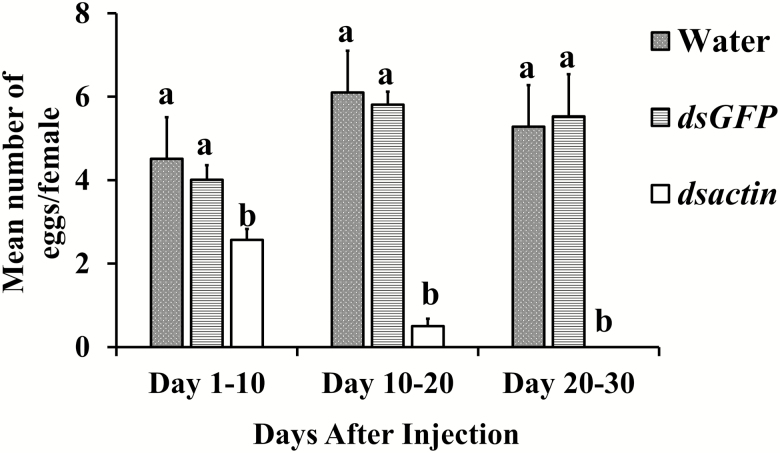
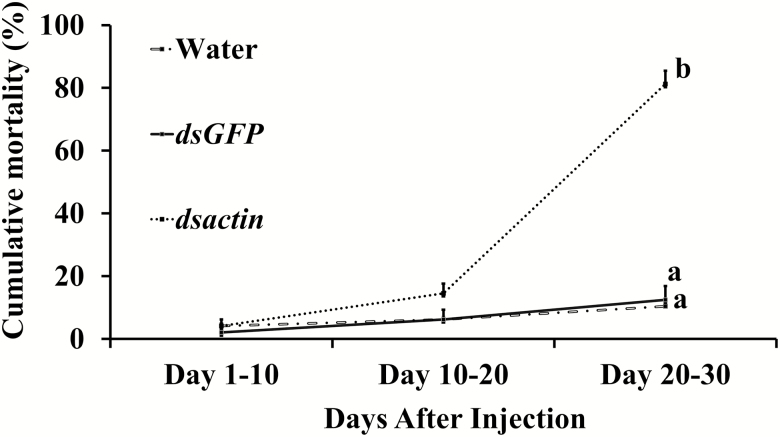
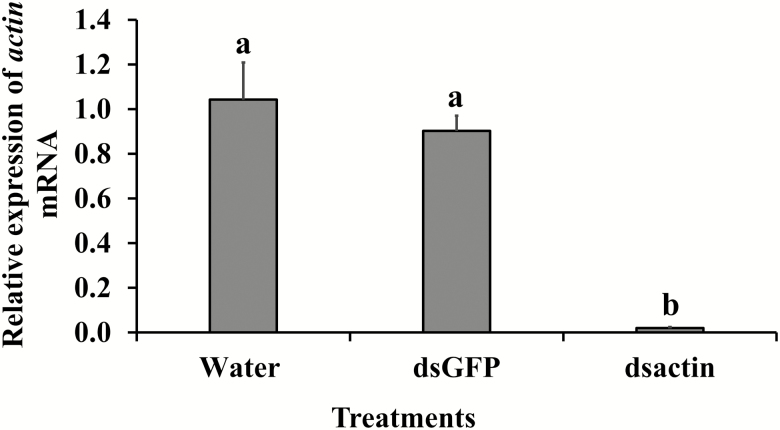
We observed a significant negative effect of treatment on oviposition (F = 52.12; df = 23.73, 4; P < 0.0001). A simple effects analysis revealed that there was a significantly lower number of eggs laid by bed bug females exposed to dsactin when compared to controls in all feeding intervals. No significant differences were observed between the two control groups, water and GFP (Fig. 2). Oviposition was ceased after 20 d, which was correlated with knockdown of the actin gene in bed bug at 30 d post injection. In addition. we observed significant mortality of bed bugs injected with dsactin (F = 69.05; df = 21, 2; P < 0.0001, Fig. 3) as compared to bed bugs injected with water or dsGFP. In the first 10 d, the mortality was <15%, but increased abruptly to >80% after 20 d. The bed bugs that survived after 30 d in the treatment group were moribund and died within next 10 d.
Fig. 2.
Effect of muscle actin RNAi in bed bug oviposition. Data analyzed as repeated measures ANOVA. Control groups: water, and dsGFP (0.2 μg), Treatment groups: dsactin (0.2 μg). Means with different letters at each feeding interval are significantly different (P < 0.005).
Fig. 3.
Survival of female bed bugs (n = 48 per treatment) injected with dsactin, monitored after 30 d and analyzed as one-way ANOVA. Means with different letters (a, b) are significantly different (P < 0.05).
Topical Application Bioassay Data
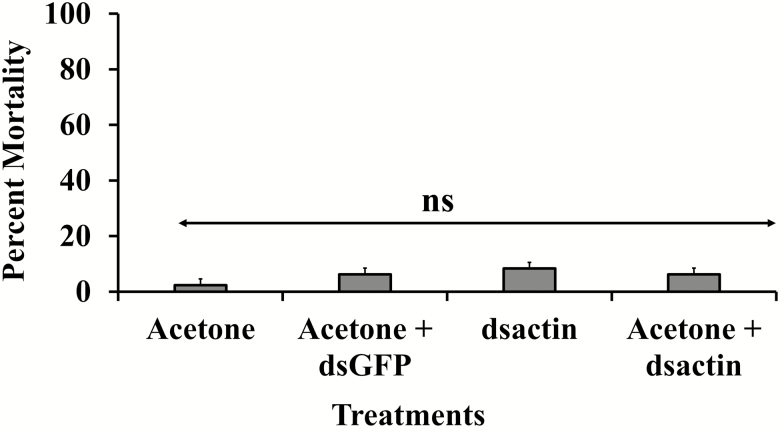
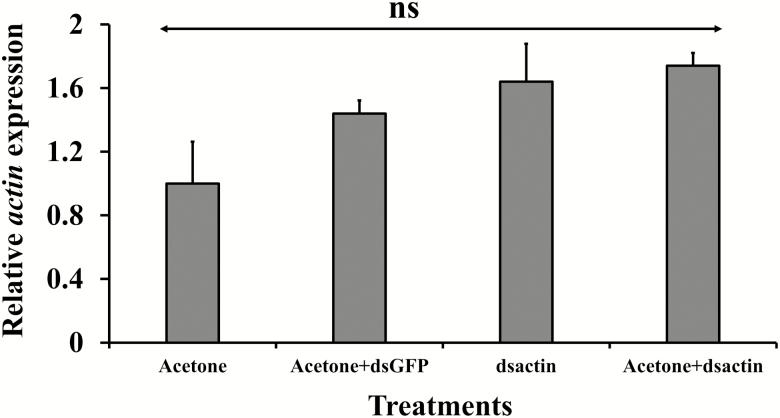
No significant difference was observed among the treatments and controls on survival of bed bug nymphs (F = 0.24; df = 28, 3; P > 0.05; Fig. 4) when we performed the topical application. As dsRNA was stable for at least 30 min in acetone, the mixture was topically applied to nymphs, but no significant effect was observed in the survival of nymphs.
Fig. 4.
Mortality of bed bugs (n = 48 per treatment) with topical application of dsactin, monitored after 30 d. Data analyzed as one-way ANOVA and not significant (ns) represent no significant differences between treatments (P > 0.05).
RT-qPCR Analysis
The transcript levels of actin were assessed at 30 d after dsRNA injection and topical application. Gene expression data analyzed from bed bugs injected with dsactin disclosed significant reduction of targeted mRNA levels (F = 28.73; df = 9, 2; P < 0.0001; Fig. 5). In contrast to injection bioassays, data collected from bed bugs topically treated with dsactin had no significant effect in the expression of actin mRNA (F = 1.38; df = 12, 3; P > 0.05; Fig. 6).
Fig. 5.
Relative actin transcript abundance at 30 d in the bed bugs injected with dsRNA. Relative actin transcript levels were normalized to rpL8 and compared to water (controls). RQ values were analyzed as one-way ANOVA. Means with different letters (a, b) are significantly different. Means comparisons were performed using Tukey Kramer method (four biological and three technical replicates, P < 0.05).
Fig. 6.
Relative actin transcript abundance at 30 d in bed bugs topically applied with dsRNA. Relative actin transcript levels were normalized to rpL8 and compared to acetone (control). RQ values were analyzed with one-way ANOVA, and not significant (ns) represent no significant difference (four biological and three technical replicates, P > 0.05).
Discussion
Our study demonstrated that injected dsactin significantly depleted actin mRNA levels and resulted high mortality (>90%) in bed bugs. In addition, there was complete inhibition of oviposition over time. However, topical application of dsactin mixed in acetone into nymphs had no effect in its expression and survival of nymphs or the adults. This study showed that injection is a reliable method to deliver dsRNA into bed bugs for RNAi studies. Although feeding and injection are commonly used for dsRNA delivery, topical application has been successfully demonstrated in several insects. Topical application of dsRNA generated RNAi response for five Cytochrome P450 genes family 4 (CYP4) in Diaphorina citri (Killiny 2014). Spraying dsRNA specific to DS10 and DS18 directly on the newly hatched larvae of Ostrinia furnacalis Guenée caused 40~50% mortality (Wang et al. 2011). In a study by Pridgeon et al. (2008) in Aedes aegypti, dsRNA specific to the apoptosis protein 1 gene was mixed in acetone and topically applied, which resulted in 42% mortality in 24 h. Foliar sprayed actin dsRNA controlled Leptinotarsa decemlineata Say for at least 28 d, but the mode of delivery was through ingestion of dsRNA dried on leaves (San Miguel and Scott 2016). The possible explanations for unsuccessful topical applications may be the difficulty in cuticular penetration of dsRNA in nymphs, or the mixture may have crossed the cuticle but not at the critical concentration to generate phenotypes. Insect cuticle is hydrophobic, and therefore the dsRNA eluted in water formed water bubble in the cuticle. The dsRNA was diluted in acetone to increase the absorption of dsRNA through the cuticle as demonstrated by Pridgeon et al. (2008) in Aedes aegypti.
In this study, significant mortality with dsactin was observed 20 d after injection. Mortality was below 20% in the dsactin injected females until 20 d but increased sharply above 80% thereafter. These results are consistent with previous results on RNAi induced mortality in bed bugs with vATPase-A, brahma, and vitellogenin (Moriyama et al. 2016; Basnet and Kamble 2017, 2018). In other insects, such as Frankliniella occidentalis Pergande (Badillo-Vargas et al. 2015), significant mortality was observed as early as 6 d post injection. Rangasamy and Siegfried (2012) observed 100% mortality in 14 d after the knockdown of vATPase in D. v. virgifera. Gene knockdown and other phenotypes, such as reduced fecundity and survival, were observed within 1 wk, but it took a longer time to show the lethality in bed bugs (Basnet and Kamble 2017, 2018).
Our data revealed that dsRNA is readily degraded in blood. The degradation is possibly due to the presence of RNase in blood. Delivery of dsRNA by mixing in blood does not seem feasible, unless is coated in nanoparticles. The dsRNA eluted in water and applied in the abdomen formed bubbles and was not absorbed by the cuticle as it was expected. While dsRNA mixed in acetone appeared to be absorbed by the cuticle. Failure to initiate RNAi by topical treatment of the actin dsRNA mixed in acetone suggests acetone mediated dsRNA delivery in bed bug nymphs may not be feasible. Further screening of additional chemicals that do not degrade dsRNA but can be easily absorbed through the insect cuticle can be explored.
References Cited
- Adelman Z. N., D. M. Miller, and Myles K. M.. 2013. Bed bugs and infectious disease: a case for the arboviruses. PLoS Pathog. 9: e1003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. L., and Leffler K.. 2008. Bed bug infestations in the news: a picture of an emerging public health problem in the United States. J. Environ. Health. 70: 24. [PubMed] [Google Scholar]
- Araujo R. N., A. Santos F. S. Pinto N. F. Gontijo M. J. Lehane, and Pereira M. H.. 2006. RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem. Mol. Biol. 36: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badillo-Vargas I. E., D. Rotenberg B. A. Schneweis, and Whitfield A. E.. 2015. RNA interference tools for the western flower thrips, Frankliniella occidentalis. J. Insect Physiol. 76: 36–46. [DOI] [PubMed] [Google Scholar]
- Basnet S., and Kamble S. T.. 2017. Knockdown of the chromatin remodeling gene brahma by RNA interference reduces reproductive fitness and lifespan in common Bed Bug (Hemiptera: Cimicidae). J. Med. Entomol. 55: 534–539. [DOI] [PubMed] [Google Scholar]
- Basnet S., and Kamble S. T.. 2018. RNAi-mediated knockdown of vATPase subunits affects survival and reproduction in bed bugs (Hemiptera: Cimicidae). J. Med. Entomol. 55: 540–546. [DOI] [PubMed] [Google Scholar]
- Baum J. A., Bogaert T., Clinton W., Heck G. R., Feldmann P., Ilagan O., and Roberts J.. 2007. Control of coleopteran insect pests through RNA interference. Nat. Biotech. 25: 1322–1326. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. 2000. The cell: a molecular approach. 2nd ed Sinauer Associates, Sunderland, MA. [Google Scholar]
- Das S., N. Debnath Y. Cui J. Unrine, and Palli S. R.. 2015. Chitosan, carbon quantum dot, and silica nanoparticle mediated dsrna delivery for gene silencing in aedes aegypti: a comparative analysis. ACS Appl. Mater. Interfaces. 7: 19530–19535. [DOI] [PubMed] [Google Scholar]
- Doggett S. L., Geary M. J., and Russell R. C.. 2004. The resurgence of bed bugs in Australia: with notes on their ecology and control. Environ. Health. 4: 30. [Google Scholar]
- Dominguez R. and Holmes K. C.. 2011. Actin structure and function. Annu. Rev. Biophys. 40: 169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., S. Xu M. K. Montgomery S. A. Kostas S. E. Driver, and Mello C. C.. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391: 806–811. [DOI] [PubMed] [Google Scholar]
- Fishilevich E., A. M., Vélez C., Khajuria M. L., Frey R. L., Hamm H., Wang G. A., Schulenberg A. J., Bowling H. E., Pence P., Gandra et al. 2016. Use of chromatin remodeling ATPases as RNAi targets for parental control of western corn rootworm (Diabrotica virgifera virgifera) and Neotropical brown stink bug (Euschistus heros). Insect Biochem. Mol. Biol. 71: 58–71. [DOI] [PubMed] [Google Scholar]
- Ghormade V., M. V. Deshpande, and Paknikar K. M.. 2011. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 29: 792–803. [DOI] [PubMed] [Google Scholar]
- Gujar H. and Palli S. R.. 2016a. Krüppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 6: 26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar H. and Palli S. R.. 2016b. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 6: 35546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajeri S., N. Killiny C. El-Mohtar W. O. Dawson, and Gowda S.. 2014. Citrus tristeza virus-based RNAi in citrus plants induces gene silencing in Diaphorina citri, a phloem-sap sucking insect vector of citrus greening disease (Huanglongbing). J. Biotechnol. 176: 42–49. [DOI] [PubMed] [Google Scholar]
- Killiny N., S. Hajeri S. Tiwari S. Gowda, and Stelinski L. L.. 2014. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS One. 9: e110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Y. Park, and Kim Y.. 2015. A transformed bacterium expressing double-stranded RNA specific to integrin β1 enhances Bt toxin efficacy against a polyphagous insect pest, Spodoptera exigua. PLoS One. 10: e0132631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K. M., R. Manzano-Roman, and de la Fuente J.. 2007. Transovarial silencing of the subolesin gene in three-host ixodid tick species after injection of replete females with subolesin dsRNA. Parasitol. Res. 100: 1411–1415. [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Montes C., C. Cuadrillero, and Vilella D.. 2002. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J. Med. Entomol. 39: 675–679. [DOI] [PubMed] [Google Scholar]
- Moriyama M., T. Hosokawa M. Tanahashi N. Nikoh, and Fukatsu T.. 2016. Suppression of bedbug’s reproduction by RNA interference of vitellogenin. PLoS One. 11: e0153984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. A., C. A. Tabuloc K. R. Cervantes, and Chiu J. C.. 2016. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 6: 22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott R. L., and Longnecker M. T.. 2015. An introduction to statistical methods and data analysis, 6th ed. Brooks/Cole, Belmont, CA. [Google Scholar]
- Pridgeon J. W., L. Zhao J. J. Becnel D. A. Strickman G. G. Clark, and Linthicum K. J.. 2008. Topically applied AaeIAP1 double-stranded RNA kills female adults of Aedes aegypti. J. Med. Entomol. 45: 414–420. [DOI] [PubMed] [Google Scholar]
- Rangasamy M. and Siegfried B. D.. 2012. Validation of RNA interference in western corn rootworm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) adults. Pest Manag. Sci. 68: 587–591. [DOI] [PubMed] [Google Scholar]
- Salazar R., Castillo-Neyra R., Tustin A. W., Borrini-Mayorí K., Náquira C., and Levy M. Z.. 2015. Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 92: 31–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel K. and Scott J. G.. 2016. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 72: 801–809. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc 2013. Base SAS® 9.4 procedures guide: statistical procedures, 2nd ed SAS Institute Inc, Cary, NC. [Google Scholar]
- Wang Y., H. Zhang H. Li, and Miao X.. 2011. Second-generation sequencing supply an effective way to screen RNAi targets in large scale for potential application in pest insect control. PLoS One. 6: e18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten M. M., P. D. Facey R. Del Sol L. T. Fernández-Martínez M. C. Evans J. J. Mitchell O. G. Bodger, and Dyson P. J.. 2016. Symbiont-mediated RNA interference in insects. Proc. Biol. Sci. 283: 20160042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyard S., A. D. Singh, and Wong S.. 2009. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 39: 824–832. [DOI] [PubMed] [Google Scholar]
- Zhu F., J. Wigginton A. Romero A. Moore K. Ferguson R. Palli M. F. Potter K. F. Haynes, and Palli S. R.. 2010. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch. Insect Biochem. Physiol. 73: 245–257. [DOI] [PubMed] [Google Scholar]
- Zhu F., J. Xu R. Palli J. Ferguson, and Palli S. R.. 2011. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 67: 175–182. [DOI] [PubMed] [Google Scholar]
- Zhu F., Sams S., Moural T., Haynes K. F., Potter M. F., and Palli S. R.. 2012. RNA interference of NADPH-cytochrome P450 reductase results in reduced insecticide resistance in the bed bug, Cimex lectularius. PLoS One. 7: 31037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Gujar H., Gordon J. R., Haynes K. F., Potter M. F., and Palli S. R.. 2013. Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci. Rep. 3: 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]