Abstract
Background
The 21-gene recurrence score (RS) (Oncotype DX®; Genomic Health, Redwood City, CA) partitions hormone receptor positive, node negative breast cancers into three risk groups for recurrence. The Anne Arundel Medical Center (AAMC) model has previously been shown to accurately predict RS risk categories using standard pathology data. A pathologic‐genomic (P-G) algorithm then is presented using the AAMC model and reserving the RS assay only for AAMC intermediate-risk patients.
Patients and methods
A survival analysis was done using a prospectively collected institutional database of newly diagnosed invasive breast cancers that underwent RS assay testing from February 2005 to May 2015. Patients were assigned to risk categories based on the AAMC model. Using Kaplan–Meier methods, 5-year distant recurrence rates (DRR) were evaluated within each risk group and compared between AAMC and RS-defined risk groups. Five-year DRR were calculated for the P-G algorithm and compared with DRR for RS risk groups and the AAMC model’s risk groups.
Results
A total of 1268 cases were included. Five-year DRR were similar between the AAMC low-risk group (2.7%, n = 322) and the RS < 18 low-risk group (3.4%, n = 703), as well as between the AAMC high-risk group (22.8%, n = 230) and the RS > 30 high-risk group (23.0%, n = 141). Using the P-G algorithm, more patients were categorized as either low or high risk and the distant metastasis rate was 3.3% for the low-risk group (n = 739) and 24.2% for the high-risk group (n = 272). Using the P-G algorithm, 44% (552/1268) of patients would have avoided RS testing.
Conclusions
AAMC model is capable of predicting 5-year recurrences in high- and low-risk groups similar to RS. Further, using the P-G algorithm, reserving RS for AAMC intermediate cases, results in larger low- and high-risk groups with similar prognostic accuracy. Thus, the P-G algorithm reliably identifies a significant portion of patients unlikely to benefit from RS assay and with improved ability to categorize risk.
Keywords: early breast cancer, 21-gene assay, recurrence, prognosis, pathological assessment
Key Message
A pathologic–genomic (P-G) algorithm, applying the pathology model first and reserving the 21-gene assay’s recurrence score (RS) for pathology-intermediate cases, predicts recurrence more effectively than the RS assay alone. Both approaches show similar 5-year distant recurrence rates within matched risk groups. The P-G algorithm decreases unnecessary RS testing and increases actionable results.
Introduction
Adjuvant therapy decisions for breast cancer patients can be challenging, as prognosis and risk of recurrence are often difficult to assess. The 21-gene recurrence score (RS) assay (Oncotype DX®; Genomic Health, Redwood City, CA) is a 21-gene reverse transcriptase-PCR assay first introduced in 2004 to provide prognostic information regarding the risk of recurrence in estrogen receptor (ER)-positive, human epidermal growth factor receptor type 2 (HER2)-negative, node-negative breast cancers [1]. In this test, tumor expression of 21 genes is analyzed and, using a weighted formula, calculated into a single RS, which is classified into one of three risk categories: low, RS ≤ 17; intermediate, RS 18–30; or high, RS ≥ 31. On retrospective analysis of hormone receptor-positive, axillary lymph node-negative breast cancer patients from two trials (NSABP B-14 and B-20), Paik et al. [1, 2] found that the RS assay predicted recurrence risk as a continuous variable.
NCCN Guidelines recommend that the RS assay be considered in ER-positive, HER2-negative breast cancers >0.5 cm, and at some institutions, the assay is ordered routinely for all such cases [3]. At approximately $4000 per test [4], it is prudent to identify patients for whom the RS assay will provide additional information to predict recurrence more accurately. The Anne Arundel Medical Center (AAMC) group previously published a risk model, the AAMC model, that identified patients unlikely to benefit from the RS assay testing based on overlap of the AAMC low-risk and high-risk definitions with low-risk and high-risk RS, as defined in the TAILORx trial [5] (low risk, RS < 11; high risk, RS≥ 26) [6]. The AAMC model uses standard pathology data, so there is no additional cost to the patient. AAMC low risk is defined as a tumor that is both low grade (grade 1, Scarff–Blom–Richardson (Nottingham) grading system) and progesterone receptor (PR) positive. AAMC high risk is defined as a tumor that is either high grade (grade 3) or ER <20%. The model was externally validated and shown to have a two-step discordance (for predicting RS risk group) of <3% [6].
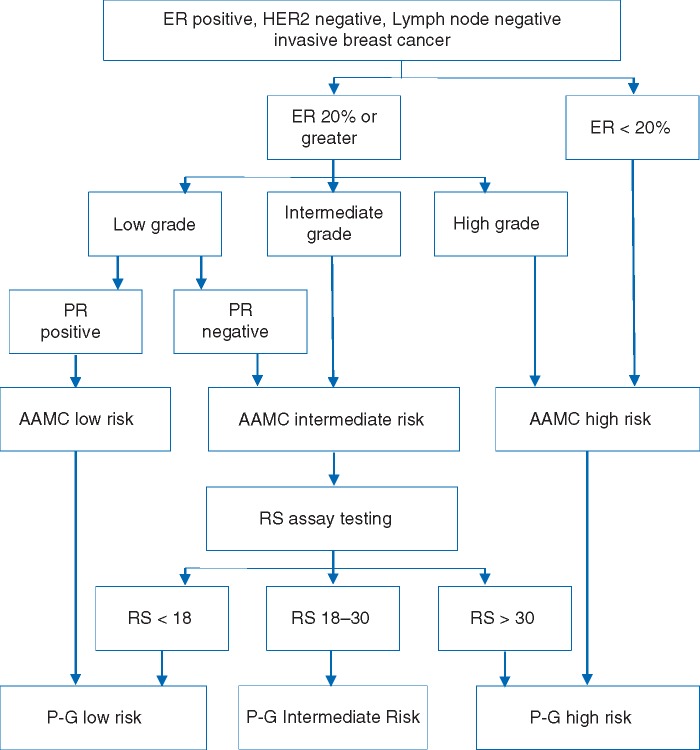
Whereas the AAMC model was shown to strongly correlate with RS risk groups, prognostic value was not previously assessed. The present study addressed this by comparing the distant metastatic recurrence rate in AAMC risk groups to those in RS-based risk groups, as defined by the TAILORx trial and RS assay. Furthermore, we evaluated the performance of a two-step pathologic–genomic (P-G) algorithm (Figure 1) . In the P-G algorithm, the AAMC model is applied first. The RS assay is reserved for patients categorized as AAMC intermediate risk, and risk category is re-assigned based on RS assay results. We examined recurrence outcomes by P-G algorithm-defined risk groups.
Figure 1.
The P-G Algorithm: Guidelines for when the RS assay is most likely to provide useful information for adjuvant chemotherapy decision-making for a breast cancer patient. The original AAMC Model ends at AAMC risk assessment of low, intermediate, or high. AAMC, The Anne Arundel Medical Center; ER, estrogen receptor; P-G, pathologic‐genomic; PR, progesterone receptor.
Material and methods
With IRB approval from both participating institutions, we identified from a prospective registry of all breast cancer patients at the University of Texas MD Anderson Cancer Center all cases tested with the RS assay between February 2005 and May 2015. Cases with the following criteria were excluded: other cancer within 5 years before diagnosis, T4 category, node positive, missing grade, missing ER%, ER < 1%, or HER2 positive. Patient characteristics are shown in supplementary Table S6, available at Annals of Oncology online. Included cases were categorized for metastatic risk using three methods: (i) RS Recurrence Score (RS) risk groups, (ii) TAILORx RS risk groups, and (iii) AAMC risk groups.
For each risk categorization method, the 5-year distant recurrence rates (DRR) in low-, intermediate-, and high-risk groups were determined using Kaplan–Meier methods with JMP 9.0.3 software (SAS Institute Inc., Cary, NC). Contingency tables were examined for RS categories and pathologic variables. Mean times to events of interest were calculated. The 5-year DRR were compared between risk groups as defined using the RS assay, TAILORx, and AAMC risk group criteria.
Cases defined as low risk that experienced distant recurrence were individually assessed for potential predictors of metastasis. Low PR and high-grade cases were also individually evaluated for recurrence risk. Potential outcomes of the P-G algorithm, a two-step algorithm in which the AAMC model would be applied first, and RS assay would be carried out only on AAMC intermediate-risk tumors, also were evaluated.
Results
Study population
From the prospective registry, 1268 cases were included in the analysis. Mean follow-up was 3.5 years, with 25% having ≥ 5 years of follow-up. The median follow-up time for cases without distant metastases was 3.4 years, with a range of 0.01–9.6 years. The median follow-up time for cases with distant metastases was 2.6 years, with a range of 0.36–7.5 years. Ninety percent of the distant metastases observed occurred before 4.9 years. Eighty-two cases (6.5%, 95% CI 5.1% to 7.8%) experienced distant metastasis.
Supplementary Figure S1, available at Annals of Oncology online, shows the Kaplan–Meier curves for freedom from distant metastasis for the four models presented in this article.
Cases defined as low risk
The 5-year DRR in the AAMC low-risk group (2.7%) was not significantly different than the TAILORx (4.0%) or the RS assay (3.4%) low-risk groups. The AAMC low-risk group (n = 322) was less than half the size of the RS assay low-risk group (n = 703), but larger than the TAILORx low-risk group (n = 250) (Table 1).
Table 1.
Distant metastatic recurrence rates calculated by Kaplan-Meier estimates for each risk model
| Oncotype DX | TAILORx | AAMC model | P-G algorithm: with RS assay for AAMC Intermediates | |
|---|---|---|---|---|
| Low risk | RS < 18 (n = 703) | RS < 11 (n = 250) | Grade 1 and PR ≥ 1% (n = 322) | AAMC Low plus Intermediates that are RS<18 (n = 739) |
| 3.4% (95% CI 1.6% to 5.1%, nf = 17) | 4.0% (95% CI 0.8% to 7.2%, nf = 8) | 2.7% (95% CI 0.0% to 5.4%, nf = 5) | 3.3% (95% CI 1.4% to 5.2%, nf = 16) | |
| Intermediate risk | RS 18–30 (n = 424) | RS 11–25 (n = 787) | Not meeting AAMC definition for low or high risk (n = 716) | AAMC Intermediates with RS 18-30 (n = 257) |
| 15.2% (95% CI 10.3% to 20.1%, nf = 38) | 7.3% (95% CI 4.7% to 9.9%, nf = 35) | 8.3% (95% CI 5.4% to 11.3%, nf = 36) | 12.0% (95% CI 5.8% to 18.1%, nf = 15) | |
| High risk | RS > 30 (n = 141) | RS > 25 (n = 231) | Grade 3 or ER < 20% (n = 230) | AAMC High plus Intermediates that are RS>30 (n = 272) |
| 23.0% (95% CI 14.7% to 31.3%, nf = 27) | 22.9% (95% CI 15.9% to 29.9%, nf = 39) | 22.8% (95% CI 16.1% to 29.5%, nf = 41) | 24.2% (95% CI 17.9% to 30.5%, nf = 51) |
AAMC, The Anne Arundel Medical Center; CI, confidence interval; nf, number of recurrences; P-G, pathologic‐genomic; RS, Recurrence Score.
Recurrences in each low-risk group were examined. In the AAMC low-risk group, there were five recurrences. Of these five, one was RS assay low-risk with a PR value only recorded as negative (<10%) and four were RS assay intermediate risk with two of the four having a PR value of 1%. In the RS assay low-risk group, there were 17 recurrences. Of these 17, 5 were AAMC high risk and these 5 were all high grade. Three of the RS assay low-risk cases that recurred had PR staining <10% (Table 2).
Table 2.
Characteristics of all cases defined as Low Risk by RS assay criteria which experienced distant metastasis
| RS | AAMC risk | ER% | PR% | Grade | T stage | Years to metastasis |
|---|---|---|---|---|---|---|
| 1 | Unknown | 95 | Negative (<10) | 1 | T1c | 1.5 |
| 6 | High | 100 | 100 | 3 | T1c | 1.7 |
| 9 | Intermediate | 96 | 96 | 2 | T3 | 1.7 |
| 9 | Intermediate | 96 | 40 | 2 | T1b | 0.7 |
| 9 | High | 85 | 75 | 3 | T2 | 1.9 |
| 9 | Intermediate | 98 | 50 | 2 | T2 | 7.5 |
| 10 | Intermediate | 95 | 60 | 2 | T1c | 4.1 |
| 10 | High | 100 | 70 | 3 | T2 | 1.1 |
| 11 | Intermediate | 90 | 90 | 2 | T1c | 2.5 |
| 13 | Intermediate | 100 | 50 | 2 | T1b | 1.3 |
| 13 | Intermediate | 95 | 95 | 2 | T1c | 2.8 |
| 14 | Intermediate | 75 | 75 | 2 | T2 | 5.6 |
| 14 | Intermediate | 90 | 60 | 2 | T1c | 3.6 |
| 15 | Intermediate | 90 | 2 | 2 | T1b | 3.1 |
| 15 | Intermediate | 100 | 30 | 2 | T1c | 4.0 |
| 17 | High | 80 | 80 | 3 | T1c | 2.2 |
| 17 | High | 100 | 5 | 3 | T1c | 3.3 |
AAMC, The Anne Arundel Medical Center; ER, estrogen receptor; PR, progesterone receptor.
The TAILORx low-risk cases are RS <11. Low PR and high grade cases are highlighted.
Cases defined as high risk
The 5-year DRR were not significantly different between the high-risk groups defined using AAMC (22.8%), TAILORx (22.9%), and RS assay (23.0%) definitions. The number of patients in the AAMC high-risk group (n = 230) was greater than the RS assay high-risk group (n = 141) and similar to TAILORx (n = 231).
Recurrences in each high-risk group were assessed. In the AAMC high-risk group, there were 41 recurrences. Of the 41, 5 were RS assay low risk, 19 were RS assay intermediate risk, and 17 were RS assay high risk. In the RS assay high-risk group, there were 27 recurrences. Of these, none were AAMC low risk, 10 were AAMC intermediate risk and 17 were AAMC high risk. Of the 10 AAMC intermediate-risk cases, 6 had a PR staining < 3%. Eleven of the 27 RS assay high-risk cases that recurred had a PR ≤ 10%. Five of the 10 AAMC intermediate-risk cases likewise had a PR ≤ 10%. Seventeen (63%) of the 27 cases that recurred in the RS assay high-risk group had high grade tumors.
Assessment of high-grade cases
From the 1268 cases, 225 were high grade (17.7%). By definition, these cases were all AAMC high risk. Eighteen of these cases (8%) were TAILORx low risk, while 51 (23%) were RS assay low risk. One hundred twenty-eight of these cases (57%) were TAILORx high risk, while 98 cases (44%) were RS assay high risk. Forty-eight of the high-grade cases were also ER ≤ 20% or PR ≤ 1%.
Of these 41 high-grade cases which metastasized, 5/41 (12%) were RS assay low risk. Six of the high-grade cases that metastasized also were PR < 3% or ER ≤ 20%. The average time to distant recurrence of all high-grade cases which recurred was 2.4 years. Furthermore, half (41/82) of all recurrences were in high-grade tumors and over 25% of the RS assay low-risk cases that experienced distant metastasis were high grade (5/17).
Assessment of tumor size
Recurrences by tumor size were evaluated for evidence of size as a prognostic variable. The proportion of distant metastasis cases were 11% (3/28) in T1a tumors, 3% (8/275) in the T1b cases, 8% (48/640) in the T1c cases, 7% (22/304) in the T2 tumors, and 5% (1/20) in the T3 tumors.
P-G algorithm results
In the P-G algorithm (Figure 1), the AAMC model was applied first, and RS assay results were considered only for AAMC intermediate-risk cases. Of the studied group of 1268 patients, 552 were classified as AAMC high risk (n = 230) or AAMC low risk (n = 322). Using the P-G algorithm, these patients require no further testing. In the remaining 716/1268 (56.5%) AAMC intermediate-risk cases, 417/716 (58.2%) were RS assay low risk and 42/716 (5.9%) were RS assay high risk (Table 3).
Table 3.
Incidence of distant metastasis of the AAMC Intermediate cases by RS
| RS < 11 | RS 11–17 | RS 18–25 | RS 26-30 | RS > 30 | Total | |
|---|---|---|---|---|---|---|
| No Metastasis | 148 | 258 | 196 | 46 | 32 | 680 |
| 97.4% | 97.4% | 94.2% | 93.9% | 76.2% | ||
| Metastasis | 4 | 7 | 12 | 3 | 10 | 36 |
| 2.6% | 2.6% | 5.8% | 6.1% | 23.8% | ||
| Total | 152 | 265 | 208 | 49 | 42 | 716 |
RS assay high and low risk shaded in grey.
Table 1 also shows the proportion of patients who recurred in each risk group of the P-G algorithm; DRR were 3.3% in the low-risk group and 24.2% in the high-risk group. This is very similar to the performance of each of the models (RS assay, TAILORx, and AAMC) alone.
Discussion
The AAMC model previously demonstrated reliable classification of many patients into a group for which the RS assay will not provide additional actionable information [6]. The present study also demonstrates that AAMC low- and high-risk groups were prognostic for the likelihood of distant metastasis with similar DRR to TAILORx and RS assay low- and high-risk groups.
The prognostic significance of grade
Numerous studies, including the original RS validation study [1, 7, 8], have shown that grade is an independent prognostic indicator. The West German Plan B Trial likewise found grade, both locally and centrally determined, to be independently prognostic for disease-free survival by multivariate analysis; in their study, RS, nodal status, and grade were independent predictors of disease-free survival [9]. Our analysis suggests that RS assay underestimates recurrence risk in high-grade tumors. In the present study, half of all recurrences were in high-grade tumors and 29% (5/17) of the RS assay low-risk cases that experienced distant recurrence were high grade. These findings differ from the TAILORx trial [5] and those reported in a recent study by Stemmer et al. [10], in which recurrence rates of low-risk cases were very low regardless of histologic grade.
Revising the use of PR in the AAMC model
The AAMC model uses negative PR as a risk criterion, eliminating such cases from the low-risk category. A post hoc analysis suggests that not only negative PR but also very low PR predicts recurrence. The supplementary Material, available at Annals of Oncology online presents a discussion of the findings of very low PR. We suggest that in the future, using the rule that grade 1 tumors with a PR percent <3%–5% not be considered low risk in our algorithms.
Using clinical/pathologic data with genomic data to predict recurrence
Numerous studies have questioned the superiority of RS assay over routine histopathologic analysis and sought ways to determine recurrence risk with information from routine tests carried out for breast cancer [7, 8, 11–18]. The value of RS assay testing over known clinical prognostic factors in stage I breast cancer patients has been shown to be questionable [19]. This is likely due to the importance of certain clinical prognostic factors, such as grade, on recurrence. Notably, Dowsett et al. [20] compared clinical treatment score (CTS) and IHC4 to the RS assay and demonstrated superior predictive ability for CTS and IHC4 over RS in all patients. Predictive ability improved when RS was added to CTS and IHC4. Similar to the present study, this suggests that clinical data used along with RS assay may be the best approach for predicting distant recurrence.
P-G algorithm
Our study found that by utilizing the P-G algorithm, the proportion of patients categorized as intermediate risk is greatly decreased, therefore resulting in more actionable clinical information (Figure 1). This is accomplished with preservation of model accuracy similar to that of the RS assay alone. Using the P-G algorithm, 80% of all patients were categorized into actionable low- or high-risk groups, whereas when the RS assay is used alone in this population, only 67% of patients were categorized into these actionable groups. The majority of patients were categorized as low or high risk by the P-G algorithm, therefore providing more prognostic information than either a 21-gene model or the AAMC pathologic model alone.
Limitations
There are limitations to the present study. The results demonstrate accurate prediction of prognosis but cannot demonstrate whether the AAMC model or P-G algorithm can predict chemotherapy benefit. The impact of adjuvant treatment regimens, including endocrine therapy, was not recorded fully, and thus was not evaluated in this study. The supplementary Material, available at Annals of Oncology online does report on the available data regarding adjuvant treatment in the studied population.
Our study was also limited by patients being lost to follow-up in the first 5 years causing higher recurrence rates. However, this should not have biased the analysis of one model relative to another.
Conclusion
The histology-based AAMC model’s low- and high-risk groups showed similar recurrence rates to the RS-based low- and high-risk groups (Table 1). Using the P-G algorithm, 43.5% (552/1268) of patients would have avoided RS testing, as the AAMC model classified them as low or high risk. Furthermore, the P-G algorithm categorized more patients into an actionable low or high-risk group, compared with RS assay alone. These compelling data call for further analysis of the P-G algorithm as an approach to recommending adjuvant treatment in patients with ER-positive, HER2-negative, node-negative breast cancer. Specifically, a retrospective analysis of prospective data with reliable information on adjuvant treatment, such as from a clinical trial, could confirm the validity of the P-G algorithm as an approach to selecting adjuvant treatment. Studies evaluating the role of RS testing, such as the TAILORx trial, need to demonstrate not only the predictive and prognostic value of RS testing but also its superiority over prognostic models such as the P-G algorithm. In the interim, clinicians in low-resource settings requiring more selective use of the RS assay may integrate the P-G algorithm into current clinical practice.
Funding
The MD Anderson Cancer Center Support Grant from the National Cancer Institute (P30CA016672) for all MD Anderson investigators.
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Paik S, Shak S, Tang G. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351(27): 2817–2826. [DOI] [PubMed] [Google Scholar]
- 2. Paik S, Tang G, Shak S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor–positive breast cancer. J Clin Oncol 2006; 24(23): 3726–3734. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network. Breast Cancer (Version 2.2017). https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (2 March 2018, date last accessed).
- 4.BreastCancer.org. Oncotype DX Test, http://www.breastcancer.org/symptoms/testing/types/oncotype_dx (5 March 2018, date last accessed).
- 5. Sparano JA, Gray RJ, Makower DF. et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015; 373(21): 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gage MM, Rosman M, Mylander WC. et al. A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Cancer 2015; 15(6): 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rakha EA, Reis-Filho JS, Baehner F. et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 2010; 12(4): 207.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orucevic A, Bell JL, McNabb AP, Heidel RE.. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat 2017; 163(1): 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gluz O, Nitz UA, Christgen M. et al. West German Study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 2016; 34(20): 2341–2349. [DOI] [PubMed] [Google Scholar]
- 10. Stemmer S, Steiner M, Rizel S. et al. Clinical outcomes in ER+ HER2-node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer 2017; 3(1): 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ono M, Tsuda H, Yoshida M. et al. Prognostic significance of progesterone receptor expression in estrogen-receptor positive, HER2-negative, node-negative invasive breast cancer with a low Ki-67 Labeling Index. Clin Breast Cancer 2017; 17(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 12. Cuzick J, Dowsett M, Pineda S. et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011; 29(32): 4273–4278. [DOI] [PubMed] [Google Scholar]
- 13. Milburn M, Rosman M, Mylander C, Tafra L.. Is Oncotype DX recurrence score (RS) of prognostic value once HER2-positive and low-ER expression patients are removed? Breast J 2013; 19(4): 357–364. [DOI] [PubMed] [Google Scholar]
- 14. Ingoldsby H, Webber M, Wall D. et al. Prediction of Oncotype DX and TAILORx risk categories using histopathological and immunohistochemical markers by classification and regression tree (CART) analysis. Breast 2013; 22(5): 879–886. [DOI] [PubMed] [Google Scholar]
- 15. Klein ME, Dabbs DJ, Shuai Y. et al. Prediction of the oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Modern Pathol 2013; 26: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang G, Cuzick J, Costantino JP. et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor–positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol 2011; 29(33): 4365–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flanagan MB, Dabbs DJ, Brufsky AM. et al. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol 2008; 21(10): 1255–1261. [DOI] [PubMed] [Google Scholar]
- 18. Farrugia DJ, Landmann A, Zhu L. et al. Magee Equation 3 predicts pathologic response to neoadjuvant systemic chemotherapy in estrogen receptor positive, HER2 negative/equivocal breast tumors. Mod Pathol 2017; 30: 1078–1085. [DOI] [PubMed] [Google Scholar]
- 19. Le Du F, Gonzalez-Angulo AM, Park M. et al. Effect of 21-gene RT-PCR assay on adjuvant therapy and outcomes in patients with stage I breast cancer. Clin Breast Cancer 2015; 15(6): 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dowsett M, Sestak I, Lopez-Knowles E. et al. Comparison of PAM50 risk of recurrence score with Oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013; 31(22): 2783–2790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.