Abstract
Despite diagnostic performance limitations, urine lipoarabinomannan (LAM) predicts death in human immunodeficiency virus (HIV)–infected adults with tuberculosis. Pediatric data are limited. Among 137 hospitalized HIV-infected children, mortality was 4.9-fold higher among those with positive LAM (127 vs 31 per 100 person-years; adjusted hazard ratio, 4.92; 95% confidence interval [CI], 1.79–13.49; P = .002). Lipoarabinomannan identifies HIV-infected children at risk for death potentially missed by respiratory sampling.
Clinical Trials Registration
Keywords: children, mortality, tuberculosis; human immunodeficiency virus (HIV), lipoarabinomannan, urine
Human immunodeficiency virus (HIV)–infected children are at high risk of tuberculosis-related morbidity and mortality, potentially due to disseminated or extrapulmonary disease often missed by respiratory sampling [1].
Lipoarabinomannan (LAM) is a lipopolysaccharide component of the Mycobacterium tuberculosis cell wall, detectable in urine of individuals with tuberculosis, including pulmonary, extrapulmonary, and disseminated forms of tuberculosis [2]. The commercially available LAM point-of-care test uses a lateral flow dipstick platform to detect LAM antigen in urine. The World Health Organization (WHO) recommends LAM testing in HIV-infected individuals with low CD4 or who are seriously ill, extending this recommendation to children based primarily on adult data [3]. Despite modest diagnostic performance [2], LAM positivity is associated with a >2-fold increased risk of death among HIV-infected adults with tuberculosis, suggesting potential prognostic utility for mortality [4].
Data are limited regarding LAM’s prognostic performance for mortality in seriously ill HIV-infected children. We prospectively evaluated mortality associated with LAM positivity among HIV-infected hospitalized children in Kenya.
METHODS
Hospitalized HIV-infected, antiretroviral therapy (ART)–naive children aged ≤12 years were enrolled in the parent randomized controlled trial of urgent (<48 hours of enrollment) versus poststabilization (7–14 days) ART initiation, with ART regimen selection per Kenyan and WHO guidelines [5]. Children underwent tuberculosis evaluation irrespective of randomization arm or clinical suspicion of tuberculosis. Two sputa or gastric aspirates were collected for liquid culture, including 1 for GeneXpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA). A trained laboratory technician performed LAM testing (Determine TB LAM Antigen test, Alere Inc, Waltham, MA) on fresh urine. Color change corresponding to manufacturer reference card grade ≥1 was considered positive at the time of the study. Tuberculosis diagnostic results (including LAM) were made available to study clinicians, with treatment decisions at their discretion. Details regarding clinical procedures, sample processing, parent trial results, and diagnostic performance of LAM compared with Xpert and culture are described elsewhere [5, 6].
Outcomes and Statistical Analysis
The primary outcome of 6-month cumulative mortality incidence by LAM result was compared using a multivariate Cox proportional hazard regression model adjusted for CD4%, age, and parent trial arm. Secondary outcomes included proportion of children who died by LAM result compared by χ2 test, and 6-month cumulative mortality incidence by LAM result stratified by tuberculosis status (defined post hoc as confirmed and unconfirmed tuberculosis vs unlikely tuberculosis per updated international consensus clinical case definitions) [7], HIV-associated immunosuppression (severe vs not severe by WHO-recommended age-appropriate CD4% or count cutoffs) [8], and malnutrition status (wasted with weight-for-height z score <−2 or mid–upper arm circumference <12.5 cm vs not wasted). Data were entered in a Research Electronic Data Capture (REDCap, Vanderbilt University, Nashville, TN) database and analyzed with STATA version 12 (StataCorp, College Station, TX).
Ethics Approval
Written informed consent was obtained from primary caregivers. Study procedures were approved by the University of Washington Institutional Review Board, Kenyatta National Hospital/University of Nairobi Ethics and Research Committee, and Kenya Pharmacy and Poisons Board. The trial is registered at ClinicalTrials.gov (NCT02063880).
RESULTS
Between April 2013 and May 2015, 181 HIV-infected children enrolled in the parent trial, 137 of whom had valid LAM results (Supplementary Figure 1). The median age was 26 months (interquartile range, 14–73), and 74 (54.0%) were male (Supplementary Table 1). Ninety-six (70.1%) were severely immunosuppressed, and 43 (44.3%) were malnourished with wasting. Nine (6.6%) children had confirmed tuberculosis (positive Xpert or culture on sputa/gastric aspirate), 59 (43.1%) had unconfirmed tuberculosis, and 69 (50.4%) had unlikely tuberculosis. Fifty-one (37.5%) initiated tuberculosis treatment. Fifteen (11.0%) were LAM-positive, including 5 of 15 (33.3%) with confirmed and 3 of 15 (20.0%) with unconfirmed tuberculosis. Seven (46.7%) LAM-positive children received tuberculosis treatment. Over 6 months of follow-up, 21 (15.3%) children died (cumulative mortality incidence, 40 per 100 person-years). In the parent trial, there was no significant mortality difference between arms (median ART initiation after 1 day for urgent and 8 days for poststabilization arms). Similar proportions received ritonavir-boosted lopinavir or NNRTI-based regimen in each arm.
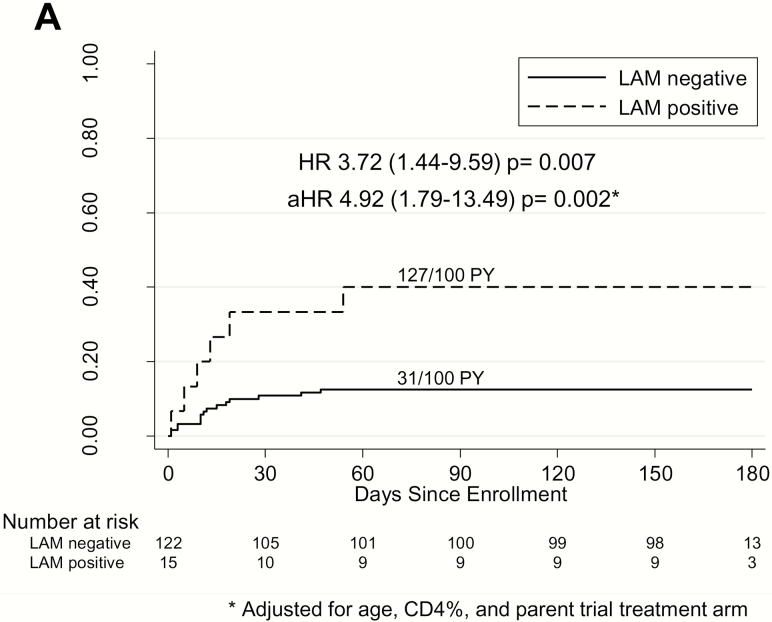
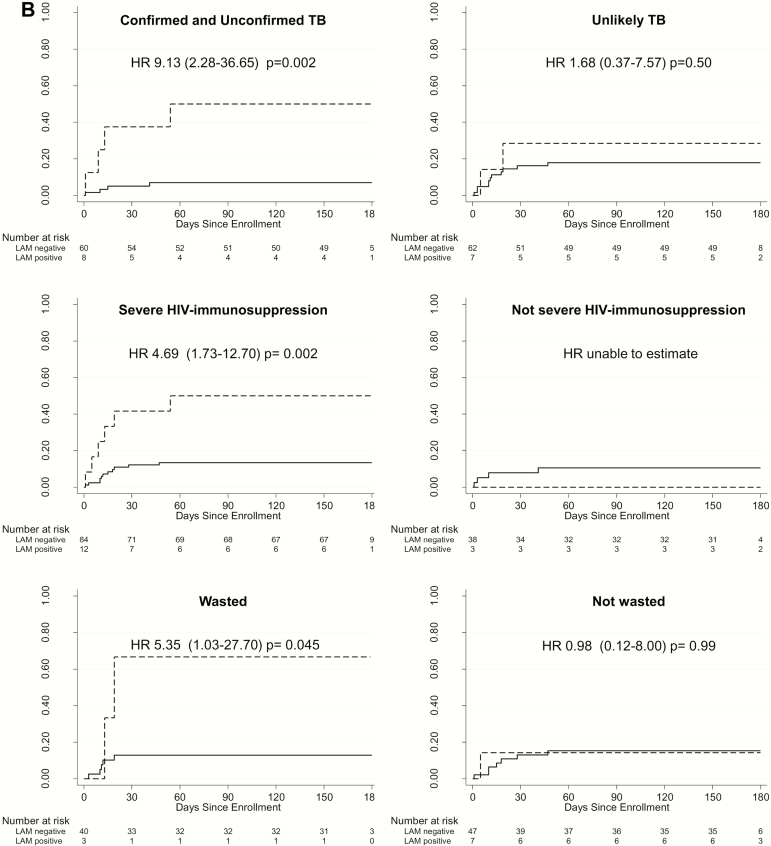
Six-month mortality was 3.7-fold higher among LAM-positive children compared with LAM-negative children (127/100 person-years vs 31/100 person-years; hazard ratio [HR], 3.72; 95% confidence interval [CI], 1.44–9.59; P = .007) (Figure 1A). This risk remained similarly high after adjusting for baseline CD4%, age, and parent trial arm (adjusted HR, 4.92; 95% CI, 1.79–13.49; P = .002). The overall proportion of children who died was higher among LAM-positive children than LAM-negative children (n = 6/15 [40%] vs n= 15/122 [12%]; P = .005). Stratified by tuberculosis status, mortality risk associated with LAM positivity remained elevated in children with confirmed and unconfirmed tuberculosis (HR, 9.13; 95% CI, 2.28–36.65; P = .002) but not unlikely tuberculosis (HR, 1.68; 95% CI, .37–7.57; P = .50) (Figure 1B). Risk was driven primarily by LAM-positive children with unconfirmed tuberculosis (HR, 10.4; 95% CI, .94–115.18; P = .06; data not shown). LAM positivity was also predictive of mortality in children with HIV-related severe immunosuppression (HR, 4.69; 95% CI, 1.73–12.70; P = .002) and malnutrition (as defined by wasting; HR, 5.35; 95% CI, 1.03–27.70; P = .045) (Figure 1B). Due to the small number of deaths in LAM-positive nonimmunosuppressed children, we could not compare mortality by LAM result in this subgroup.
Figure 1.
A, Cumulative probability of death by urine LAM result among hospitalized HIV–infected children. * indicates adjusted for age, CD4%, and parent trial antiretroviral therapy treatment arm. B, Cumulative probability of death by urine LAM result among hospitalized HIV-infected children stratified by TB, HIV-related immunosuppression, and malnutrition status. Confirmed TB was that for which a bacterial confirmation was obtained with positive respiratory sample Xpert or culture result. Unconfirmed TB was that for which bacterial confirmation was not obtained and at least 2 of the following were present: suggestive symptoms of TB, chest X-ray consistent with TB, close TB exposure or immunologic evidence of Mycobacterium tuberculosis infection, TB treatment response. Unlikely TB was that not fitting in any of the TB diagnosis criteria. (Graham et al [7]). Severe immunosuppression was based on World Health Organization (WHO) age-specified CD4 cutoffs—CD4%: aged <12 months, <25%; aged 12–35 months, <20%; aged >36 months, <15%—or, in absence of CD4% data, CD4 count: aged <12 months, <1500 cells/µL; aged 12–35 months, <750 cells/µL; aged >36 months, <350 cells/µL (WHO 2007). Malnourished was based on WHO definitions of wasting with either weight-to-height z score <−2 or mid–upper arm circumference <12.5 cm. Abbreviations: aHR, adjusted hazard ratio; HIV, human immunodeficiency virus; HR, hazard ratio; LAM, lipoarabinomannan; PY, person-years; TB, tuberculosis.
DISCUSSION
Among HIV-infected hospitalized children initiating ART, 6-month mortality was 3.7-fold higher among LAM-positive children. This association remained after adjusting for factors potentially linked with mortality, including age, HIV immunosuppression, and ART initiation timing (per parent trial arm). Stratified by tuberculosis status, the probability of mortality among children with tuberculosis (confirmed and unconfirmed tuberculosis) with positive LAM remained elevated. Notably, risk of death was especially high (>10-fold) for LAM-positive children with unconfirmed tuberculosis, potentially missed by standard respiratory sampling. Similarly, risk of death among LAM-positive children with severe HIV immunosuppression or malnutrition was greater than among those with negative LAM.
Multiple studies in HIV-infected adults demonstrated increased mortality among LAM-positive tuberculosis cases [2]. In a recent meta-analysis, LAM positivity was associated with a 2.3-fold higher risk of mortality, with similar estimates after adjustment for additional mortality risk factors [4]. Our study confirms these findings, now among LAM-positive HIV-infected children. In children, mycobacterial culture is positive in 30%–62% of clinically diagnosed tuberculosis cases [9]. We intentionally evaluated LAM as a predictor of mortality in the overall cohort rather than in the subset with microbiologically confirmed tuberculosis. An important finding is LAM predicts mortality among children with unconfirmed tuberculosis. These children may benefit from treatment even in absence of microbiologically confirmed tuberculosis, and urine LAM could reduce time to treatment in children at highest risk for death. Results of a multicountry trial indicate bedside LAM-guided tuberculosis treatment in HIV-infected hospitalized adults reduced mortality [10].
The underlying pathway between LAM and tuberculosis mortality is not completely clear. Lipoarabinomannan may be a marker of disseminated tuberculosis or higher mycobacterial burden, both associated with worse prognosis. Additionally, LAM may function as a virulence factor, inhibiting macrophage function and proinflammatory cytokines and enhancing anti-inflammatory cytokines [4].
Strengths of our study include that it is one of the largest to date evaluating LAM in HIV-infected children and the only, to our knowledge, to assess mortality as primary endpoint. Well-characterized presentations and standardized tuberculosis investigations allowed us to provide mortality estimates by LAM result in important subgroups. In this cohort, LAM positivity was associated with a higher risk of mortality among children most likely to have tuberculosis (including confirmed and unconfirmed tuberculosis), children with severe immunosuppression, and children with malnutrition. Previous pediatric LAM studies focused on diagnostic performance instead of patient outcomes. Nicol et al reported poor diagnostic performance in a South African cohort of less–severely ill HIV-infected and -uninfected children, of which none died [11]. In Tanzania, LAM performance improved among HIV-infected children, and mortality was associated with urine LAM detected by enzyme-linked immunosorbent assay among confirmed and probable tuberculosis cases [12]. Sample size restricted their ability to evaluate performance by HIV immunosuppression, and mortality estimates among LAM-positive HIV-infected children were not reported. Interestingly, death only occurred in children with probable or possible tuberculosis (considered unconfirmed tuberculosis in current guidelines). In our recent tuberculosis diagnostic accuracy study conducted in this same population, urine LAM performance (compared with sputum/gastric aspirate Xpert or culture) improved among children with severe immunosuppression and those aged <24 months [6]. This current study extends our findings by focusing on the clinically relevant patient outcome—mortality—and evaluating mortality risk by LAM result in the entire cohort, not just those with confirmed pulmonary disease. This strategy likely identified children with tuberculosis missed by respiratory sampling at risk of death.
Limitations include lack of confirmatory testing for disseminated or extrapulmonary tuberculosis. Urine was not collected from all children; it was not collected primarily among children who died ≤24 hours after enrollment and during LAM assay stock-out. Although tuberculosis was common (50% of children met criteria for confirmed or unconfirmed tuberculosis), there were likely additional causes of death for which LAM is unlikely to predict mortality. A comprehensive evaluation of all-cause mortality correlates is currently underway.
In conclusion, we found an increased risk of mortality among LAM-positive HIV-infected hospitalized children initiating ART—a group in which tuberculosis diagnosis is often missed and for whom early tuberculosis treatment is lifesaving. These data provide evidence to support WHO’s recommendation that urine LAM is helpful for tuberculosis diagnosis in severely ill HIV-infected children, including those at greatest mortality risk. Benefits of urine LAM include its rapid point-of-care platform, low cost, and readily accessible sample type. Future studies are needed to confirm whether incorporation of urine LAM testing can prevent mortality in HIV-infected children.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors wish to thank parent study participants and their caregivers, as well as the research administrative, clinical, and data teams for their dedication and support. We thank members of the University of Washington Global Center for Integrated Health of Women, Adolescents and Children, and Dr Grace John-Stewart’s pre/postdoctoral mentorship group for their support during the preparation of this article.
Disclaimer. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health (NIH). Funding sources were not involved in analyses or interpretation of data. None of the authors was paid to write this article by a pharmaceutical company or other agency.
Funding. This work was supported by the National Institute of Child Health and Human Development (NICHD), the National Institute of Allergy and Infectious Diseases (NIAID), the Fogarty International Center, and the National Center For Advancing Translational Sciences at the NIH (R01 HD023412 and K24 HD054314-06 to G. J. S., K23 AI 120793-01 to S. M. L., T32 AI007140 to P. B. P., K12 HD000850 to L. M. C., D43TW009783 to I. N., and UL1TR000423 for REDCap), the Firland Foundation (pilot award to J. L. W.), the University of Washington Center for AIDS Research (P30 AI027757), and the Pediatric Scientist Development Program through grants from the American Pediatric Society and the American Academy of Pediatrics (to L. M. C.). Determine TB LAM Ag kits were donated by Alere.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 48th Union World Conference on Lung Health, Guadalajara, Mexico, 11–14 October 2017.
References
- 1. Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah M, Hanrahan C, Wang ZY, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev 2016; CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV Available at: http://www.who.int/tb/publications/use-of-lf-lam-tb-hiv/en/. Accessed 21 September 2017.
- 4. Gupta-Wright A, Peters JA, Flach C, Lawn SD. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Med 2016; 14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Njuguna IN, Cranmer LM, Otieno VO, et al. Urgent versus post-stabilisation antiretroviral treatment in hospitalised HIV-infected children in Kenya (PUSH): a randomised controlled trial. Lancet HIV 2018; 5:e12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LaCourse SM, Pavlinac PB, Cranmer LM, et al. Stool Xpert MTB/RIF and urine lipoarabinomannan for the diagnosis of tuberculosis in hospitalized HIV-infected children. AIDS 2018; 32:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61(suppl 3):S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. Accessed 5 October 2017.
- 9. Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis 2006; 42:e69–71. [DOI] [PubMed] [Google Scholar]
- 10. Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387:1187–97. [DOI] [PubMed] [Google Scholar]
- 11. Nicol MP, Allen V, Workman L, et al. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Glob Health 2014; 2:e278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kroidl I, Clowes P, Reither K, et al. Performance of urine lipoarabinomannan assays for paediatric tuberculosis in Tanzania. Eur Respir J 2015; 46:761–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.