Abstract
Background
The value of induction chemotherapy (ICT) remains under investigation despite decades of research. New advancements in the field, specifically regarding the induction regimen of choice, have reignited interest in this approach for patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). Sufficient evidence has accumulated regarding the benefits and superiority of TPF (docetaxel, cisplatin, and fluorouracil) over the chemotherapy doublet cisplatin and fluorouracil. We therefore sought to collate and interpret the available data and further discuss the considerations for delivering ICT safely and optimally selecting suitable post-ICT regimens.
Design
We nonsystematically reviewed published phase III clinical trials on TPF ICT in a variety of LA SCCHN patient populations conducted between 1990 and 2017.
Results
TPF may confer survival and organ preservation benefits in a subgroup of patients with functionally inoperable or poor-prognosis LA SCCHN. Additionally, patients with operable disease or good prognosis (who are not candidates for organ preservation) may benefit from TPF induction in terms of reducing local and distant failure rates and facilitating treatment deintensification in selected populations. The safe administration of TPF requires treatment by a multidisciplinary team at an experienced institution. The management of adverse events associated with TPF and post-ICT radiotherapy-based treatment is crucial. Finally, post-ICT chemotherapy alternatives to cisplatin concurrent with radiotherapy (i.e. cetuximab or carboplatin plus radiotherapy) appear promising and must be investigated further.
Conclusions
TPF is an evidence-based ICT regimen of choice in LA SCCHN and confers benefits in suitable patients when it is administered safely by an experienced multidisciplinary team and paired with the optimal post-ICT regimen, for which, however, no consensus currently exists.
Keywords: ICT, TPF, LA SCCHN, organ preservation, functional inoperability, cetuximab
Key Message
Induction chemotherapy remains a relevant treatment alternative for selected patients with locally advanced squamous cell carcinoma of the head and neck and is suitable for promoting a variety of treatment goals when administered correctly. TPF (docetaxel, cisplatin, and fluorouracil) is the current evidence-based induction regimen of choice.
Introduction
The role of induction chemotherapy (ICT) in locally advanced squamous cell carcinoma of the head and neck (LA SCCHN) has been heavily investigated, yet clear guidelines for the optimal use of ICT outside of cases where organ preservation is a primary goal have yet to be defined. Available data have been primarily inconclusive regarding whether ICT confers overall superior benefits versus the standard of care (concurrent chemoradiotherapy), except in the larynx preservation setting [1–3], because a definitive phase III trial has yet to be completed in other settings. Moreover, it has taken >2 decades to arrive at a consensus, evidence-based ICT regimen of choice: TPF [docetaxel, cisplatin, and fluorouracil (5-FU)]. TPF is now accepted to be superior to PF (cisplatin plus 5-FU) in multiple phase III trials and a meta-analysis (Table 1 and supplementary Table S1, available at Annals of Oncology online) [4–10].
Table 1.
Summary of phase III trials involving ICT in LA SCCHN between 1990 and 2017
| Study | # Patients | Regimen | Resectability Criteria | Primary End Point: Outcome | Toxicity |
|---|---|---|---|---|---|
| Spain 1998 [7] | 382 | PF → cisplatin-RT/surgery versus TPF → cisplatin-RT/surgery | Stages III–IV resectable and unresectable disease | Complete response rate: higher in TPF arm | Patients in the PF arm had significantly more grades 2–4 mucositis than patients in TPF arm |
| TAX 324 [5] | 501 | PF → carboplatin-RT versus TPF → carboplatin-RT | Unresectable or resectable (suitable for organ preservation) | OS: higher OS in TPF arm |
|
| TAX 323/EORTC 24971 [4] | 358 | PF → RT versus TPF → RT | Unresectable disease | PFS: higher PFS and OS in TPF arm |
|
| TTCC 2002 [8] | 439 | PF → cisplatin-RT versus TPF → cisplatin-RT versus cisplatin-RT | Unresectable disease | PFS and TTF: no difference in either | Toxicity in ICT arms was manageable |
| GORTEC 2000-01 [9, 10] | 213 | PF → RT/surgery versus TPF → RT/surgery | Disease suitable for total laryngectomy | Larynx preservation: higher 3-, 5-, and 10-year larynx preservation rates (and ORR) in TPF arm |
|
| DeCIDE [28] | 285 | TPF → chemo-RTa versus chemo-RT | N2 or N3 disease | OS: no difference | Serious AEs were significantly more common in the ICT arm |
| PARADIGM [29] | 145 | TPF → chemo-RTb versus cisplatin-RT | Unresectable disease | OS: no difference | Febrile neutropenia was numerically more common in the ICT → chemo-RT arm than in the chemo-RT arm |
| RTOG 91-11 [49, 76] | 517-520 | PF → RT/surgery + RT versus cisplatin-RT versus RT | Glottic/supraglottic stages III–IV LA SCC | LFS: similar efficacy between PF → RT and cisplatin-RT | Higher rate of non–treatment-/disease-related death occurred with cisplatin-RT versus PF → RT and RT alone |
| EORTC 24954 [48] | 450 | A: PF → RT/surgery versus B: (PF → RT) × 3 → PF | Resectable laryngeal/hypopharyngeal disease | Larynx preservation: OS with functional larynx was numerically improved in arm B versus A | Grade 3/4 mucositis was numerically lower in arm B versus A |
| Italian trial [27] | 414 | TPF → cisplatin-RT or cetuximab-RT versus cisplatin-RT or cetuximab-RT | Stages III–IV disease of the oral cavity, oropharynx, hypopharynx |
|
Induction TPF did not affect compliance to cetuximab-RT and cisplatin-RT |
Docetaxel, 5-FU, hydroxyurea.
Docetaxel or carboplatin.
5-FU, fluorouracil; AE, adverse event; CT, chemotherapy; EORTC, European Organisation for Research and Treatment of Cancer; ICT, induction chemotherapy; LA SCCHN, locally advanced squamous cell carcinoma of the head and neck; LFS, laryngectomy-free survival; LRC, locoregional control; ORR, overall response rate; OS, overall survival; PF, cisplatin plus 5-FU; PFS, progression-free survival; RT, radiotherapy; RTOG, Radiation Therapy Oncology Group; TTF, time to treatment failure; TPF, docetaxel, cisplatin, and 5-FU.
Although radiotherapy is the only post-ICT regimen currently supported by level IA evidence in the European Society for Medical Oncology guidelines [11], a strong interest in adding a sensitizing agent to radiotherapy post-ICT has become evident in recently-initiated clinical trials. Accordingly, we consider both radiotherapy with/without carboplatin post-ICT as standard-of-care options (the former being commonly used in the United States). We have, therefore, pooled our knowledge and experience regarding the indications for TPF and other ICT regimens to review and interpret the available phase III data concerning the utility of ICT in LA SCCHN. We review the phase III evidence, published in 1990–2017, for TPF as the new gold-standard, evidence-based ICT regimen of choice and discuss the settings where induction TPF may confer benefits in patients with LA SCCHN over the current standard of care. The goal of this communication is to provide a future perspective on its use and role for the treatment of LA SCCHN in clinical practice.
Available treatment options in LA SCCHN
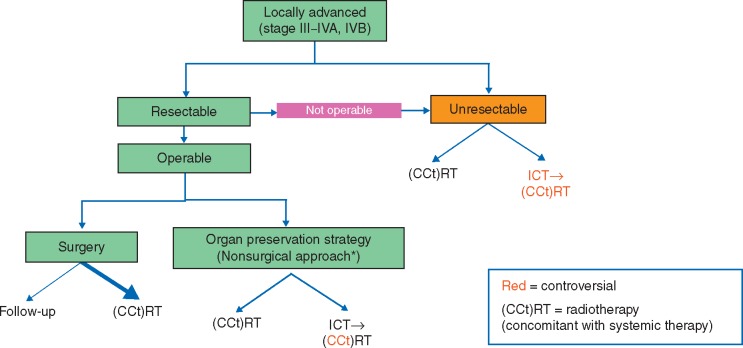
The current available treatment options for patients with LA SCCHN are mapped out in Figure 1 [11]. For patients with operable disease where organ preservation is a key therapy goal, the available options are concurrent chemoradiotherapy or sequential treatment with induction TPF → radiotherapy (although radiotherapy and carboplatin/cisplatin/cetuximab are being investigated as post-ICT options as well) [12, 13]. The treatment options available to patients with unresectable LA SCCHN include radiotherapy and cisplatin, carboplatin and 5-FU (French regimen), or cetuximab (for cisplatin-unsuitable patients). More controversial is the use of ICT followed by a radiotherapy or chemoradiotherapy as a routine treatment in patients with inoperable disease.
Figure 1.
Current standard-of-care paradigm in LA SCCHN. *ICT→RT is only an accepted standard approach for larynx preservation in locoregionally advanced larynx and hypopharynx cancer. ICT, induction chemotherapy; LA SCCHN, locally advanced squamous cell carcinoma of the head and neck.
TPF as a breakthrough for ICT
TPF has now been established as an ICT regimen that yields better response rates and a milder toxicity profile than earlier induction regimens, including PF. The superiority of TPF over PF has been confirmed via meta-analysis of the pooled data from 5 phase III studies (Spain 1998, TAX 323/EORTC 24971, TAX 324, GORTEC 2000-01, and TTCC 2002, representing a total of 1772 patients; Table 1) conducted by Blanchard et al. [6], which concluded that patients who receive TPF versus PF experience benefits in progression-free survival (PFS), overall survival (OS), and in locoregional failure rate (LFR) and distant failure rate (DFR) [6]. Consistent with these findings, Blanchard et al. [6] found that the calculated hazard ratio (HR) for death (0.79) strongly favored the TPF regimen. Of note, however, limitations of this meta-analysis included the use of pooling methodology on five rather heterogeneous studies (especially regarding the selection of and administration practices for post-ICT regimens) and a general concern regarding partially or completely missing treatment failure data for the Spain 1998, TAX 323/EORTC 24971, and TTCC 2002 trials [14]. Finally, ICT responders and nonresponders commonly received different follow-up treatments, further complicating any meta-analysis of the benefits of sequential chemotherapy.
Nevertheless, improved safety with the addition of docetaxel to the PF doublet ICT regimen has been observed in clinical trials. In the TAX 323/EORTC 24971 trial, more patients completed treatment in the TPF arm than did patients in the PF arm (75.7% versus 65.7%, respectively), and fewer deaths from toxicity were encountered (2.3% versus 5.5%), and in the TAX 324 trial, fewer patients had treatment delays in the TPF arm versus in the PF arm (29% versus 65%, respectively) [4, 5]. Across all five studies, patients who received TPF experienced fewer grade 3/4 mucositis events and had lower frequencies of nausea, vomiting, stomatitis, and hearing loss (likely owed to the reduction in dosage of cisplatin and 5-FU). Furthermore, the rate of toxicity-related deaths tended to be lower among patients in the TPF arms, and the only adverse events that appeared to be more prevalent with TPF versus PF were neutropenia, febrile neutropenia, and leukopenia (Table 1) [4, 5, 7–10].
Further analysis of TAX 323/EORTC 24971 also demonstrated improved quality of life (QoL) in patients in the TPF versus PF arms, including a decrease in swallowing problems and coughing. Measured using the European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire C30, global QoL was statistically significantly higher in the TPF versus the PF arm following completion of radiotherapy (at 6 months after treatment initiation); the numerical difference in global health-related QoL scores of 9.5 points almost reached the accepted 10-point margin for clinically meaningful change in QoL [15]. Finally, economic analyses of TAX 323/EORTC 24971 and TAX 324 showed TPF is more cost-effective than PF, with a gain of 0.33–0.41 quality-adjusted life-years in the TPF arms of the two trials [16]. The cost–utility ratio of TPF induction was also determined to be comparable to that observed for other widely accepted treatment options [16].
Additional studies also investigated whether another triplet regimen, TPE (taxane, platinum, and cetuximab), can be effective as ICT. Although no randomized phase III trials comparing TPE versus TPF induction have been completed, the available early results of smaller studies using TPE have been encouraging [17–21]. For example, response rates and OS rates of >80% at 2–5 years have been reported for patients who complete a TPE induction regimen [18–20]. Notably, however, adding a fourth agent (such as cetuximab) to TPF has proven difficult, necessitating either a reduction in the TPF dose or the removal of the 5-FU component to prevent unacceptable toxicity [17, 22, 23].
TPF regimens and what it takes to administer them
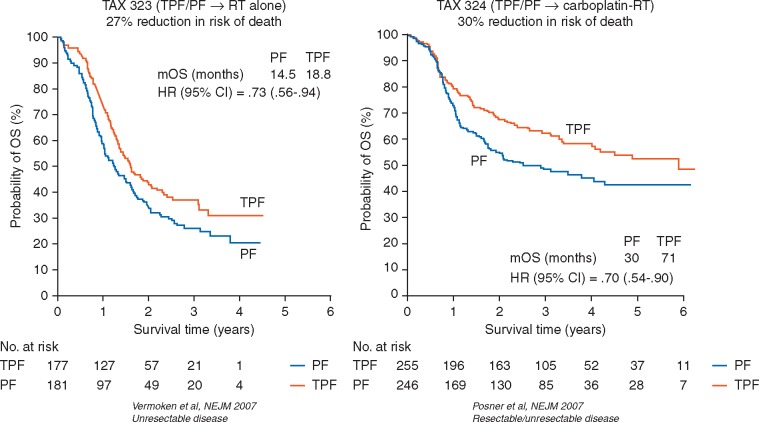
Two key randomized phase III trials have established standard practices for the safe administration of TPF: the European trial TAX 323/EORTC 24971 and the American trial TAX 324. The TAX 323/EORTC 24971 study delivered four cycles of TPF [75 mg/m2 docetaxel, 75 mg/m2 cisplatin, both on day 1 and 750 mg/m2/day 5-FU by continuous intravenous (i.v.) infusion for five consecutive days] followed by radiotherapy and conferred significant survival benefits in a population of patients with previously untreated, unresectable LA SCCHN. Results indicated that TPF → radiotherapy prolonged PFS (median, 11.0 versus 8.2 months in the TPF versus PF arms of TAX 323, respectively), reduced risk of death by 27% (Figure 2), and prolonged OS (median, 18.8 months versus 14.5 months in the TPF versus PF arms of TAX 323, respectively) over PF ICT [4]. Furthermore, this TPF regimen, when applied in larynx preservation trials such as GORTEC 2000-01, increased the rates of 3-, 5-, and 10-year larynx preservation by 12.8%, 15.9%, and 23.8%, respectively, over PF, and significantly improved laryngeal dysfunction-free survival [9, 10]. Similar findings were observed with the TPF regimen used in the TAX 324 study (three cycles of 75 mg/m2 docetaxel, 100 mg/m2 cisplatin, both on day 1 and 1000 mg/m2/day 5-FU by continuous i.v. infusion for four consecutive days), with carboplatin (area under the curve of 1.5) plus radiotherapy as the post-ICT therapy, where median OS more than doubled in the TPF arm versus the PF arm (71 versus 30 months, respectively). Notably, the patient population of TAX 324 included patients with LA SCCHN that was either unresectable or of low surgical curability, as well as patients with LA SCCHN who were candidates for organ preservation strategy [5]. Both trials concluded that the overall response rate (ORR) with TPF was significantly (TAX 323/EORTC 24971) or numerically (TAX 324) higher than with PF [4, 5]. Both the TAX 323/EORTC 24971 and TAX 324 TPF regimens demonstrated clear survival benefits over PF ICT in patients with unresectable LA SCCHN. Although previous studies have demonstrated benefit with ICT → radiotherapy versus radiotherapy alone in unresectable disease [24, 25], the role of ICT versus concurrent chemoradiotherapy in unresectable/inoperable disease remains controversial, due to difficulties in trial design, execution or insufficient patient accrual [26–29]. However, in most of these studies, the sequential design induced more toxicity than the concurrent design. A recent report of GORTEC 2007-02, comparing TPF followed by cetuximab/radiotherapy versus concurrent chemoradiation with carboplatin/5-FU in patients with inoperable LA SCCHN showed no improvement in PFS, OS or LRC, but a significant delay in distant metastases in the TPF arm, with more toxicity and 7% TPF-related deaths [30, 31].
Figure 2.
TPF versus PF in two different patient cohorts (TAX 323/EORC 24971 and TAX 324). 5-FU, fluorouracil; HR, hazard ratio; mOS, median overall survival; PF, cisplatin plus 5-FU; RT, radiotherapy; TPF, docetaxel, cisplatin, and 5-FU. From Refs [4, 5]. Copyright © 2007 Massachusetts Medical Society. Reprinted with permission.
Notably, the TAX 323/EORTC 24971 regimen was associated with a more favorable safety profile than the previously standard PF regimen, likely owing to the lower overall doses of cisplatin (75 mg/m2 instead of 100 mg/m2 on day 1) and 5-FU (750 mg/m2/day ×5 instead of 1000 mg/m2/day ×4) (Table 2). Consequently, patients in TAX 323/EORTC 24971 received four cycles of ICT versus three cycles in TAX 324, while experiencing a lower frequency of grade 3/4 stomatitis, nausea/vomiting, dysphagia, and neutropenia [4, 5]. Accordingly, we suggest that the less dose-dense TPF regimen administered in TAX 323/EORTC 24971 may come with milder toxicities than the regimen used in TAX 324 [4, 5], but both regimens are suitable for ICT and subject to the institution’s preference. Crucially, however, any TPF regimen should be administered by experienced oncologists familiar with the necessary protocols and supportive care requirements to ensure patient safety and maximize adherence throughout the treatment. Investigators of the GSTTC Italian Collaborative Group study [27] showed that a modified dose of TPF (docetaxel 75 mg/m2, cisplatin 80 mg/m2, and 5-FU 800 mg/m2/day × 96 hours) given in three cycles did not compromise subsequent chemoradiotherapy in a phase II study, although no randomized phase III trials have been completed to compare TPF versus modified TPF. Additionally, adaptation of ICT doses may be needed regionally, particularly in Asia, to maximize patient safety [32].
Table 2.
Comparison of the TPF regimens and associated toxicities used in TAX 323/EORTC 24971 and TAX 324 (European versus American TPF regimens)
| Study | TPF Regimen | Detailed Toxicities |
|---|---|---|
| TAX 323/EORTC 24971 [4] (four cycles of TPF) | Docetaxel (75 mg/m2) as a 1-h infusion on day 1 |
|
| Cisplatin (75 mg/m2) as a 1-h infusion on day 1 | ||
| 5-FU (750 mg/m2/day) by continuous infusion on days 1–5 | ||
| TAX 324 [5] (three cycles TPF) | Docetaxel (75 mg/m2) as a 1-h intravenous infusion |
|
| Intravenous cisplatin (100 mg/m2) over a period of 0.5–3 h | ||
| 5-FU (1000 mg/m2/day) as a continuous 24-h infusion for 4 days |
5-FU, fluorouracil; ICT, induction chemotherapy; RT, radiotherapy; TPF, docetaxel, cisplatin, and 5-FU.
Sequential chemotherapy poses unique challenges, partially due to the lack of completed phase III trials comparing potential post-ICT therapy options. The PARADIGM trial closed prematurely [27] and no definitive conclusions could be drawn from the results (Table 1). Furthermore, due to decreased adherence in patients receiving cisplatin during TPF and during subsequent chemoradiotherapy (e.g. in the DeCIDE trial [28]), TPF → cisplatin/radiotherapy may be suitable only for highly selected patients with good to excellent performance status and no contraindications to cisplatin or for those who received a protocol-driven reduced total dose of cisplatin during induction [3, 33, 34]. Alternative post-ICT therapy options, which may result in improved patient adherence (versus cisplatin 100 mg/m2 q3w), include radiotherapy alone or radiotherapy/cetuximab or radiotherapy/carboplatin. However, in that setting, neither radiotherapy plus carboplatin nor radiotherapy plus cetuximab have been compared with radiotherapy plus cisplatin (q3w or qw) in a phase III trial, and no definitive conclusions can be drawn from the TREMPLIN phase II study, partly due to the high rate of patient dropout before radiotherapy [12, 35].
Additionally, discussion of the best practices for prophylaxis and management of severe hematologic toxicity during ICT and mucositis during radiotherapy is ongoing. Oral dexamethasone during induction may prevent docetaxel-related hypersensitivity/toxicity (fluid retention, skin toxicity) [4, 5]. Indeed, fluid management through i.v. means (especially on days 1–2 during TPF administration) is crucial in preventing renal toxicity, hypovolemia, and severe fatigue [4, 5]. Current evidence also supports the use of prophylactic treatment with antibiotics (e.g. ciprofloxacin) or granulocyte colony-stimulating factor (though with a lower amount of evidence), which may decrease incidence of hematologic toxicities and infection associated with TPF [36, 37].
National Institute for Health and Care Excellence guidelines specify that all patients with SCCHN be treated within the context of a multidisciplinary team (MDT), considering aspects such as patients’ psychological and nutritional state and the potential for palliative care, addiction services, and speech therapy [38]. Furthermore, discussing patient selection within an MDT is particularly important when the planned treatment involves sequential chemotherapy because appropriate selection can prevent subjecting patients to unsuitable treatments. Interestingly, institutional experience and familiarity with the patient population and treatments impacts outcomes significantly. In a retrospective analysis of the Radiation Therapy Oncology Group (RTOG) 0129 study, Wuthrick et al. [33] showed that centers with historically low clinical trial accrual rates (calculated based on a total of 21 RTOG studies in SCCHN) tended to enroll patients with an overall better performance status at the start of a study and yet saw threefold as many radiotherapy protocol deviations and a lower 5-year survival (51% versus 69%) than institutions with a historically high patient accrual rate [39]. Indeed, low-accrual centers appeared to correlate with an HR for death 91% higher than observed in the high-accrual centers (adjusted for prognostic factors) [39]. It must be noted that the RTOG 0129 study was conducted in patients with LA SCCHN but did not include an ICT arm. However, given the additional complexities of care during the administration of triplet chemotherapy induction, it is likely that the experience of the institution will also correlate with outcomes in patients receiving sequential chemotherapy.
The role of ICT in patients with high-risk SCCHN
Patients with a high risk of distant failure (DF) and LA SCCHN with multiple involved nodes, large-volume nodal disease, and low nodes appear to gain certain benefits from the sequential chemotherapy approach. In DeCIDE, patients with N2–N3 disease experienced a trend in improved recurrence-free survival and lower cumulative incidence of SCCHN-related death with TPF → chemoradiotherapy (versus chemoradiotherapy) [28]. Additionally, patients with N2c/N3 disease experienced an improvement in OS with ICT [28]. Furthermore, the location of lymph node involvement may suggest which patients stand to gain a longer distant metastasis-free survival, as shown in a retrospective study by Kim et al., who determined that patients with present versus absent lower neck nodal involvement had a significantly lower 5-year distant metastasis-free survival rate (34.3% versus 55.2%, P = 0.008) [40]. Additionally, the same study determined that patients with hypopharyngeal SCC may be at higher risk of DF than those with laryngeal tumors (the risk for patients with oropharyngeal and oral cavity disease being somewhere in the middle) [40]. A meta-analysis by Zhang et al. [41] indicated that the ICT approach significantly reduced the DFR in patients with unresectable disease. Yet, this was not sufficient to yield a survival benefit.
The role of ICT in patients by operability status
Resectability in LA SCCHN is generally determined by the extent of disease, invasion and attainability of clear margins. Improved outcomes with sequential chemotherapy in patients with unresectable disease have been reported in several patient groups. For example, Izawa et al. [42], based on the data from the DeCIDE study [28], investigated whether induction TPF followed by concurrent chemoradiotherapy could contribute to a reduction in metastases, leading to improved survival outcomes in comparison with platinum-based concurrent chemoradiotherapy alone for patients with LA SCCHN with clinical stage N2c or N3 nodal disease, or N2b disease with supraclavicular lymph node metastases. In the DeCIDE study, a trend in better survival with TPF → chemoradiotherapy versus concurrent chemoradiotherapy was observed for patients with N2c or N3 disease (P = 0.19). Izawa et al. [42] reported that median survival in the concurrent chemoradiotherapy arm was 14 months, while not reached in the TPF arm at the time of publication. Although of interest, such a retrospective analysis can have major bias. Nevertheless, we consider it certainly a reason for further study of ICT in this patient population.
An MDT can find consensus to classify tumors as resectable or of a borderline category of resectable disease with a poor prognosis or poor resulting functionality (e.g. multinodal involvement or requiring total laryngectomy) [11, 43]. This ‘functional inoperability’ scenario, where surgery will lead to unacceptable loss of function, has an entire subset of guidelines for organ preservation, with the understanding that patients whose tumors have low surgical curability could benefit from an aggressive, nonsurgical approach instead of initial surgery [3, 11]. Adding to the complexity of this classification is the fact that criteria for staging and functional inoperability differ based on primary tumor site [44]. Kreeft et al. [43] found that certain procedures, such as total glossectomy, are more universally recognized by surgeons as leading to unacceptable loss of function than other procedures, e.g. total soft palate resection or resection at the base of the tongue [43]. If there were better evidence that ICT is an efficacious systemic therapeutic alternative for cases of functional inoperability, then the decision whether to operate could be made more easily.
The recently published phase II/III study by Ghi et al. [27] suggests that TPF → chemoradiotherapy or cetuximab/radiotherapy may be effective for a mixed population of patients with low surgical curability or functionally inoperable stages III–IV LA SCCHN of the oral cavity, oropharynx and hypopharynx [27]. The chemotherapy given during the chemoradiotherapy part of the study consisted of a relatively low cumulative dose of cisplatin (160 mg/m2) in combination with 5-FU. Although interpretation may be confounded by the addition of cetuximab/radiotherapy only in the phase III portion of the study, the reported results suggested an improvement in PFS, OS, and locoregional control (LRC) in patients receiving TPF → radiotherapy and cetuximab or PF [27]. Due to lack of statistical power, a possible interaction between ICT and the post-ICT regimens (cetuximab/radiotherapy or PF/radiotherapy) could not be excluded, thereby precluding a definite answer as to what post-ICT regimen should be preferred [45].
Larynx preservation is currently the only widely accepted setting for patients with resectable LA SCCHN in which ICT has consensus value. Patients with untreated laryngeal or hypopharyngeal LA SCCHN and who require total laryngectomy can opt to receive (sequential or concurrent) chemoradiotherapy, with surgery as a secondary plan if still needed. PF ICT → radiotherapy was originally reported to lead to a 31% complete response rate and 54% partial response rate in a phase III trial of 332 patients with previously untreated laryngeal LA SCCHN [2]. At 3 years, 53% of patients were still alive [46]. Merlano et al. [47] (and later, Lefebvre et al. [48]) used ICT (vinblastine sulfate + bleomycin + methotrexate + leucovorin and PF, respectively) → radiotherapy either sequentially or on an alternating schedule [47, 48]. In Merlano et al. [47], the alternating approach appeared to yield a better response rate and was deemed less toxic. In Lefebvre et al. [48], survival with a functional larynx was 45% and ≈ 30%–36% at 3 and 5 years, respectively (Table 1) [47, 48]. However, the alternating approach is difficult to perform in clinical practice and requires an extremely close collaboration between the different disciplines. Nevertheless, TPF ICT has shown even more efficacy in larynx preservation than PF, with larynx preservation rates > 70% at 3, 5, and 10 years [9, 10]. In the only study comparing sequential chemotherapy (PF) and radiation with concomitant chemoradiotherapy and radiotherapy alone (RTOG 91-11) in patients with laryngeal SCC, the 10-year update indicated a significant improvement in laryngectomy-free survival and a trend in improved OS with sequential chemotherapy over concurrent chemoradiotherapy as well as a significantly greater number of non–treatment-related and non–disease-related deaths in the concurrent chemoradiotherapy arm [49]. Thus, the data appear to support ICT as the better long-term treatment option in this patient population. However, the ultimate conclusion on best practices for larynx preservation will come from the phase III SALTORL trial (NCT03340896; TPF → radiotherapy versus concurrent high-dose cisplatin/radiotherapy for patients with T2-3, N0-2c laryngeal/hypopharyngeal disease) currently running in France, comparing the best available concurrent chemoradiation with the best available sequential approach.
Studies enrolling exclusively patients with operable LA SCCHN have thus far failed to show a survival benefit with induction versus locoregional treatment [25, 50–52], suggesting that ICT treatment may not be suitable in patients with resectable disease who are not candidates for organ preservation. Additionally, a meta-analysis of 14 trials (n = 2099) also determined no significant OS benefit from ICT versus locoregional treatment in patients with operable disease [1]. However, three studies of mixed populations of patients with resectable and unresectable disease did suggest a survival benefit with PF (versus locoregional treatment including surgery and/or radiotherapy) [24, 25] or TPF induction (versus PF → chemoradiotherapy) [5]. The latter, TAX 324, compared PF and TPF regimens in a mixed population (resectable disease of low surgical curability, for organ preservation or expected poor functional outcome, partly with unresectable disease) and showed that TPF ICT reduced risk of death by 30% and improved loco-regional control over a PF regimen (Figure 2) [5]. Furthermore, patients with operable disease who received sequential therapy treatment tended to experience a marked reduction in DFR. For example, in the phase III trial reported by Paccagnella et al. [25] testing the role of PF ICT, the subgroup of 66 patients with operable disease showed a 3-year DF rate of 3% versus 31% in patients in the ICT versus no-ICT arms [25]. Also, in the mixed population of the GETTEC trial, comprising only oropharyngeal cancer patients, the overall risk of DF was 36% higher in patients who did not receive ICT, although this difference was not statistically significant [24]. The effect of ICT on reducing the risk of DF is an important observation because metastatic SCCHN is usually associated with poor prognosis and low OS (< 1 year) [53]. Therefore, the prevention of future distant metastasis may be an important outcome to be considered also during treatment decisions for patients with resectable LA SCCHN. Although data are premature, identification and validation of potential biomarkers for benefit from ICT will also be crucial tools for clinicians making these treatment decisions. Some such biomarkers currently under investigation include, but are not limited to, annexin A1, acetylated tubulin, GDF15, cancer stem cell markers, p53 functional status, and low neck nodes [30, 54–59].
Finally, it is notable that ICT may have a role in the preoperative setting for cancers of the oral cavity. A meta-analysis of phase III studies comparing ICT → surgery (with or without postoperative radiotherapy) versus surgery (with or without postoperative radiotherapy) in resectable oral cavity SCC found a potential survival benefit of ICT in patients with N2 disease [60]. A small randomized trial identified no survival benefit of preoperative ICT (before surgery and optional radiotherapy versus upfront surgery and optional radiotherapy) in patients with T2–T4, N0–N2 oral cavity SCC, but did note lowered fibrosis and dysphagia in the ICT arm at long-term follow-up, which the investigators ascribed to the fact that, with the use of ICT, less extensive surgery had to be carried out and fewer patients needed to receive postoperative radiation [61].
The role of ICT in human papillomavirus-associated LA SCCHN
Patients with human papillomavirus (HPV)–associated LA SCCHN generally have more favorable prognoses, better responses to therapy, and longer OS than do patients with HPV-negative disease [62–65]. The prognostic value of HPV and p16 positivity has been demonstrated in the ICT (paclitaxel/carboplatin or TPF) setting, where patients with HPV-positive disease had a higher ORR and more than double the 5-year survival rates versus patients with HPV-negative disease [66, 67]. In the phase II Eastern Cooperative Oncology Group 1308 study, Marur et al. [68] suggested that ICT with cisplatin/paclitaxel/cetuximab may allow patients with HPV-positive oropharyngeal carcinoma and an otherwise favorable prognosis to undergo reduced-dose intensity-modulated radiation therapy (IMRT) without reducing efficacy. This chemoradiotherapy de-escalation plan led to a significant decrease in radiotherapy-associated toxicities, such as difficulty swallowing or impaired nutrition, compared with patients who received regular-dose IMRT (40% versus 89% difficulty swallowing and 10% versus 44% impaired nutrition in the reduced-dose versus regular-dose IMRT arms, respectively) [69]. Although the data concerning the utility of ICT in patients with HPV-associated LA SCCHN are still incomplete, these early investigations suggest a role for ICT in treatment deintensification in patients with favorable prognoses [68].
Selecting a post-TPF regimen
No consensus exists yet regarding the optimal post-TPF regimens, although evidence suggests that some options confer high toxicity without additional benefit. Radiotherapy alone has the largest body of evidence in cases where organ preservation is the primary objective [9, 10], yet other regimens (radiotherapy plus either carboplatin, PF or cetuximab) have shown encouraging results in the post-ICT setting in smaller studies [12, 69]. However, phase III randomized trials are imperative to further establish these regimens’ role in sequential chemotherapy treatment.
Cisplatin’s inclusion in both TPF (75 mg/m2 q3w) and the follow-up chemoradiotherapy (100 mg/m2 q3w) regimen have been generally associated with low adherence and unacceptably high rates of toxicity [33, 70]. A study of 65 patients randomized to receive either high-dose (100 mg/m2 q3w) or weekly (40 mg/m2) cisplatin plus radiotherapy following 4 cycles of TPF was terminated early because only 32% of all patients were able to receive the full planned cisplatin dose due to toxicity [70]. Although patients receiving weekly cisplatin were twice as likely to receive the full planned dose (22% versus 41% in the high-dose versus weekly cisplatin arms, respectively), no difference in OS rate was observed at 2 years between the two arms [70]. Although the vast majority of patients included in this study were still able to receive > 90% of the planned dose of radiotherapy [70], this study suggested that it is inadvisable to administer high cumulative cisplatin doses (300 mg/m2) post-TPF due to associated toxicity issues and low adherence to the systemic component of the treatment. Furthermore, the same study suggested also that the weekly cisplatin schedule failed to provide a milder alternative to high-dose cisplatin. Notably, while chemoradiotherapy (with cisplatin 100 mg/m2 q3w) plus cetuximab resulted in significantly higher toxicity (with no improvement in efficacy) than chemoradiotherapy alone, even without prior induction treatment [71], certain subgroups of patients may benefit from this regimen. In a retrospective analysis of the RTOG 0522 study, the presence of genetic variants appeared to correlate with improved survival with the addition of cetuximab to cisplatin and radiotherapy [72]. The randomized, phase II TREMPLIN study in previously untreated patients with stages III to IV laryngeal/hypopharyngeal SCC administered three cycles of the TAX 323/EORTC 24971 TPF regimen [12]. Poor responders (<50% tumor shrinkage) underwent salvage surgery. Responders (≥50% tumor shrinkage) were randomly assigned to conventional radiotherapy (70 Gy) with concurrent cisplatin (100 mg/m2/day) on days 1, 22, and 43 of radiotherapy or concurrent cetuximab (400-mg/m2 loading dose and 250 mg/m2/week) during radiotherapy. Many patients ended participation in the trial before receiving the radiotherapy-based portion of the treatment. However, those who did and were randomized to the cetuximab and radiotherapy arm had fewer treatment interruptions and a higher rate of treatment completion than those randomized to the cisplatin and radiotherapy arm [12, 35]. Despite that, more relapses occurred in the cetuximab/radiotherapy arm than in the cisplatin/radiotherapy arm (the majority of which could be salvaged by surgery) with no difference in OS [12, 35]. This finding needs to be confirmed in a phase III setting with an amended trial design which hopefully will allow a final conclusion on this topic. Finally, carboplatin or PF can also be paired with radiotherapy as post-ICT regimens, as used in TAX 324 and in the Italian trial, respectively. Both combination treatments demonstrated favorable safety results, and the majority of patients completed those regimens [3, 5, 27]. However, no results are available directly comparing the combination of radiotherapy with cisplatin versus radiotherapy plus carboplatin or PF. From the meta-analyses performed until now, comparing carboplatin/radiotherapy and cetuximab/radiotherapy to cisplatin/radiotherapy in the LA SCCHN setting [73–75], it can be concluded that the standard cisplatin-based chemoradiation should remain the standard of care until equivalence with carboplatin or cetuximab has been prospectively demonstrated. Moreover, none of these regimens have been fully investigated in the post-ICT setting. Therefore, as carboplatin and cetuximab both may offer more tolerable alternatives to cisplatin in chemoradiation after cisplatin-based ICT, their further investigation in that setting is warranted. Also of note, as immune checkpoint inhibitors gain approval and are increasingly tested in combinations with other therapies, their potential role in follow-up therapy will require examination. Immunotherapies’ toxicity profiles are encouraging and may become a key factor in determining their place within the treatment paradigm.
Discussion
Conclusion
By examining the available data from phase III, randomized clinical trials investigating TPF regimens, we have concluded that TPF is the current evidence-based gold standard for ICT. The favorable efficacy and safety profiles with TPF over PF have been clearly demonstrated, particularly in the TAX 323/EORTC 24971 and TAX 324 studies. Currently, the only guideline-mandated indication for TPF is as an induction regimen before radiotherapy in patients requiring total laryngectomy, with organ preservation as the main objective [11]. We maintain, however, that while the exact patient populations who stand to gain the most benefit from induction remain to be fully defined, TPF has an important role in various situations. In operable disease, TPF ICT can reduce the rates of local and DF and enhance organ preservation and function. In patients with unresectable disease, TPF ICT improves survival over PF. While no definitive conclusions can be made about whether TPF ICT is an overall superior treatment to concurrent chemoradiotherapy, certain patient subgroups (those with high-risk disease or for whom organ preservation and reducing the probability of distant relapse are key end goals) could gain important benefits with sequential over concurrent therapy. Additionally, TPF ICT could provide a new avenue of radiotherapy de-intensification in patients with favorable prognoses. Although ICT → radiotherapy alone has the largest available body of evidence, other options warrant further investigation. Cisplatin/radiotherapy appears to come with serious toxicity and adherence concerns for most patients, especially after ICT with high cumulative cisplatin dosages. Although cetuximab and carboplatin with radiotherapy could represent safer options, more data need to be collected for these regimens. Further investigation in phase III trials is warranted. Future investigations must determine the optimal post-ICT TPF regimens and further characterize those patients who stand to gain significant benefits in terms of survival and organ function with sequential over concurrent chemoradiotherapy regimens.
Supplementary Material
Acknowledgements
Medical writing assistance was provided by ClinicalThinking, Inc., Hamilton, NJ, USA, and funded by Merck KGaA, Darmstadt, Germany.
Funding
Merck KGaA, Darmstadt, Germany (no grant number applies).
Disclosure
JBV participated in advisory boards of Amgen, AstraZeneca, Boerhinger-Ingelheim, Innate Pharma, Merck KGaA, Merck Sharp & Dohme Corp., PCI Biotech, Synthon Biopharmaceuticals and received lecture fees from Merck KGaA and Sanofi. MP serves on IDMCs for Merck Sharp & Dohme Corp. and Cel Sci. All remaining authors have declared no conflicts of interest.
References
- 1. Ma J, Liu Y, Yang X. et al. Induction chemotherapy in patients with resectable head and neck squamous cell carcinoma: a meta-analysis. World J Surg Oncol 2013; 11: 67. 67-7819-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf GT, Fisher SG. et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med 1991; 324: 1685–1690. [DOI] [PubMed] [Google Scholar]
- 3. Paccagnella A, Ghi MG, Loreggian L. et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol 2010; 21(7): 1515–1522. [DOI] [PubMed] [Google Scholar]
- 4. Vermorken JB, Remenar E, van Herpen C. et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007; 357(17): 1695–1704. [DOI] [PubMed] [Google Scholar]
- 5. Posner MR, Hershock DM, Blajman CR. et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007; 357(17): 1705–1715. [DOI] [PubMed] [Google Scholar]
- 6. Blanchard P, Bourhis J, Lacas B. et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 2013; 31(23): 2854. [DOI] [PubMed] [Google Scholar]
- 7. Hitt R, Lopez-Pousa A, Martinez-Trufero J. et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 2005; 23(34): 8636–8645. [DOI] [PubMed] [Google Scholar]
- 8. Hitt R, Grau JJ, Lopez-Pousa A. et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 2014; 25(1): 216–225. [DOI] [PubMed] [Google Scholar]
- 9. Janoray G, Pointreau Y, Garaud P. et al. Long-term results of GORTEC 2000-01: a multicentric randomized phase III trial of induction chemotherapy with cisplatin plus 5-fluorouracil, with or without docetaxel, for larynx preservation. ASCO 2015: abstr 6002.
- 10. Pointreau Y, Garaud P, Chapet S. et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 2009; 101(7): 498–506. [DOI] [PubMed] [Google Scholar]
- 11. Gregoire V, Lefebvre JL, Licitra L. et al. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21(Suppl 5): v184–v186. [DOI] [PubMed] [Google Scholar]
- 12. Lefebvre JL, Pointreau Y, Rolland F. et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol 2013; 31(7): 853–859. [DOI] [PubMed] [Google Scholar]
- 13. Posner MR, Norris CM, Wirth LJ. et al. Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324: survival, surgery, and organ preservation. Ann Oncol 2009; 20(5): 921–927. [DOI] [PubMed] [Google Scholar]
- 14. Forastiere AA, Adelstein DJ, Manola J.. Induction chemotherapy meta-analysis in head and neck cancer: right answer, wrong question. J clin Oncol 2013; 31(23): 2844–2846. [DOI] [PubMed] [Google Scholar]
- 15. van Herpen CM, Mauer ME, Mesia R. et al. Short-term health-related quality of life and symptom control with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF), 5-fluorouracil (PF) for induction in unresectable locoregionally advanced head and neck cancer patients (EORTC 24971/TAX 323). Br J Cancer 2010; 103(8): 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberato NL, Rognoni C, Rubrichi S. et al. Adding docetaxel to cisplatin and fluorouracil in patients with unresectable head and neck cancer: a cost-utility analysis. Ann Oncol 2012; 23(7): 1825–1832. [DOI] [PubMed] [Google Scholar]
- 17. Dietz A, Wichmann G, Flentje M. et al. Final results of the randomized phase II DeLOS-II trial: induction chemotherapy (IC) followed by radiotherapy (R) vs. cetuximab (E) plus IC and R for functional larynx preservation in resectable laryngeal and hypopharyngeal cancer (LHSCC). J Clin Oncol 2016; 34: (suppl; abstr 6025). [Google Scholar]
- 18. Lee KW, Koh Y, Kim SB. et al. A randomized, multicenter, phase II study of cetuximab with docetaxel and cisplatin as induction chemotherapy in unresectable, locally advanced head and neck cancer. Oncologist 2015; 20(10): 1119–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seiwert TY, Melotek JM, Blair EA. et al. Final results of a randomized phase 2 trial investigating the addition of cetuximab to induction chemotherapy and accelerated or hyperfractionated chemoradiation for locoregionally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2016; 96(1): 21–29. [DOI] [PubMed] [Google Scholar]
- 20. Argiris A, Heron DE, Smith RP. et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol 2010; 28(36): 5294–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmaltz H, Borel C, Ciftci S. et al. Induction chemotherapy before surgery for unresectable head and neck cancer. B-ENT 2016; 12(1): 29–32. [PubMed] [Google Scholar]
- 22. Chibaudel B, Lacave R, Lefevre M. et al. Induction therapy with cetuximab plus docetaxel, cisplatin, and 5-fluorouracil (ETPF) in patients with resectable nonmetastatic stage III or IV squamous cell carcinoma of the oropharynx. A GERCOR phase II ECHO-07 study. Cancer Med 2015; 4(5): 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesia R, Vazquez S, Grau JJ. et al. A phase 2 open label, single-arm trial to evaluate the combination of cetuximab plus taxotere, cisplatin, and 5-flurouracil as an induction regimen in patients with unresectable squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2016; 94(2): 289–296. [DOI] [PubMed] [Google Scholar]
- 24. Domenge C, Hill C, Lefebvre JL. et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tete et du Cou (GETTEC). Br J Cancer 2000; 83(12): 1594–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paccagnella A, Orlando A, Marchiori C. et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst 1994; 86(4): 265–272. [DOI] [PubMed] [Google Scholar]
- 26. Hitt R, Mesia R, Grau JJ. et al. Randomized phase III trial of induction chemotherapy (ICT) with docetaxel-cisplatin-5fluorouracil (DCF) followed by cisplatin-radiotherapy (CRT) or cetuximab-radiotherapy (CetRT) in patients (pts) with locally advanced unresectable head and neck cancer (LAUHNC). ASCO 2016, abstr 6001. [DOI] [PubMed]
- 27. Ghi MG, Paccagnella A, Ferrari D. et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced Head and Neck Cancer. A phase II-III trial. Ann Oncol 2017; 28(9): 2206–2212. [DOI] [PubMed] [Google Scholar]
- 28. Cohen EE, Karrison TG, Kocherginsky M. et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 2014; 32(25): 2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haddad R, O'Neill A, Rabinowits G. et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 2013; 14(3): 257–264. [DOI] [PubMed] [Google Scholar]
- 30. Chapman CH, Parvathaneni U, Yom SS.. Revisiting induction chemotherapy before radiotherapy for head and neck cancer, part I: carcinoma of non-nasopharyngeal sites. Future Oncol 2017; 13(6): 469–475. [DOI] [PubMed] [Google Scholar]
- 31. Geoffrois L, Martin L, Garaud P. et al. Induction docetaxel platinum 5-FU (TPF) followed by cetuximab-radiotherapy (cetux-RT) versus concurrent chemo-radiotherapy (CT/RT) in patients with N2b/c-N3 non operated stage III-IV squamous cell cancer of the head and neck (SCCHN): results of the GORTEC 2007-02 phase III randomized trial. ASCO 2016, abstr #6000.
- 32. Zhu G, Lin JC, Kim SB. et al. Asian expert recommendation on management of skin and mucosal effects of radiation, with or without the addition of cetuximab or chemotherapy, in treatment of head and neck squamous cell carcinoma. BMC Cancer 2016; 16(1): 42-016-2073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Budach V. TPF sequential therapy: when and for whom? Oncologist 2010; 15(Suppl 3): 13–18. [DOI] [PubMed] [Google Scholar]
- 34. Paccagnella A, Mastromauro C, D'Amanzo P, Ghi MG.. Induction chemotherapy before chemoradiotherapy in locally advanced head and neck cancer: the future? Oncologist 2010; 15 (Suppl 3): 8–12. [DOI] [PubMed] [Google Scholar]
- 35. Janoray G, Pointreau Y, Sire C. et al. Long-term results of a multicenter randomized phase II trial of induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil followed by either chemoradiotherapy or bioradiotherapy for larynx preservation. J Natl Cancer Inst 2016; 108(4): djv368. [DOI] [PubMed] [Google Scholar]
- 36. Linot B, Augereau P, Breheret R. et al. Efficacy and safety of early G-CSF administration in patients with head and neck cancer treated by docetaxel-cisplatin and 5-fluorouracil (DCF protocol): a retrospective study. Support Care Cancer 2014; 22(10): 2831–2837. [DOI] [PubMed] [Google Scholar]
- 37. Schrijvers D, Van Herpen C, Kerger J. et al. Docetaxel, cisplatin and 5-fluorouracil in patients with locally advanced unresectable head and neck cancer: a phase I-II feasibility study. Ann Oncol 2004; 15(4): 638–645. [DOI] [PubMed] [Google Scholar]
- 38. NICE. Improving outcomes in head and neck cancers. 2015; 2017 Published on 2004.
- 39. Wuthrick EJ, Zhang Q, Machtay M. et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol 2015; 33(2): 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim DH, Kim WT, Lee JH. et al. Analysis of the prognostic factors for distant metastasis after induction chemotherapy followed by concurrent chemoradiotherapy for head and neck cancer. Cancer Res Treat 1970; 47(1): 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L, Jiang N, Shi Y. et al. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Sci Rep 2015; 5(1): 10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Izawa N, Onozawa Y, Hikosaka T. et al. Efficacy and feasibility of docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy for locally advanced head and neck squamous cell carcinoma classified as clinical nodal stage N2c, N3, or N2b with supraclavicular lymph node metastases. Int J Clin Oncol 2015; 20(3): 455–462. [DOI] [PubMed] [Google Scholar]
- 43. Kreeft A, Tan IB, van den Brekel MW. et al. The surgical dilemma of ‘functional inoperability’ in oral and oropharyngeal cancer: current consensus on operability with regard to functional results. Clin Otolaryngol 2009; 34(2): 140–146. [DOI] [PubMed] [Google Scholar]
- 44. Amin MB, Edge S, Greene F. et al. The American Joint Committee on Cancer: The 8th Edition of the AJCC Cancer Staging Manual and the Future of TNM, Springer, 2017. [Google Scholar]
- 45. Misiukiewicz K, Gupta V, Posner M.. The Italian Collaborative Group sets a standard for the treatment of locally advanced head and neck cancer. Ann Oncol 2017; 28(9): 2051–2054. [DOI] [PubMed] [Google Scholar]
- 46. Spaulding MB, Fischer SG, Wolf GT.. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. The Department of Veterans Affairs Cooperative Laryngeal Cancer Study Group. J Clin Oncol 1994; 12(8): 1592–1599. [DOI] [PubMed] [Google Scholar]
- 47. Merlano M, Rosso R, Sertoli MR. et al. Randomized comparison of two chemotherapy, radiotherapy schemes for stage III and IV unresectable squamous cell carcinoma of the head and neck. Laryngoscope 1990; 100(5): 531–535. [DOI] [PubMed] [Google Scholar]
- 48. Lefebvre JL, Rolland F, Tesselaar M. et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst 2009; 101(3): 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Forastiere AA, Zhang Q, Weber RS. et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013; 31(7): 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Henriques De Figueiredo B, Fortpied C, Menis J. et al. Long-term update of the 24954 EORTC phase III trial on larynx preservation. Eur J Cancer 2016; 65: 109–112. [DOI] [PubMed] [Google Scholar]
- 51. Schuller DE, Metch B, Stem DW. et al. Preoperative chemotherapy in advanced resectable head and neck cancer: final report of the Southwest Oncology Group. Laryngoscope 1988; 98(11): 1205–1211. [DOI] [PubMed] [Google Scholar]
- 52. Villar A, Pera J, Arellano A. et al. Induction chemotherapy with cisplatin, bleomycin and methotrexate in advanced head and neck cancer – lack of therapeutic gain. Radiother Oncol 1987; 10(3): 175–181. [DOI] [PubMed] [Google Scholar]
- 53. Price KA, Cohen EE.. Current treatment options for metastatic head and neck cancer. Curr Treat Option Oncol 2012; 13(1): 35–46. [DOI] [PubMed] [Google Scholar]
- 54. Zhu DW, Liu Y, Yang X. et al. Low Annexin A1 expression predicts benefit from induction chemotherapy in oral cancer patients with moderate or poor pathologic differentiation grade. BMC Cancer 2013; 13: 301-2407-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saba NF, Magliocca KR, Kim S. et al. Acetylated tubulin (AT) as a prognostic marker in squamous cell carcinoma of the head and neck. Head Neck Pathol 2014; 8(1): 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang CZ, Ma J, Zhu DW. et al. GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma. Ann Oncol 2014; 25(6): 1215–1222. [DOI] [PubMed] [Google Scholar]
- 57. Yanamoto S, Yamada S, Takahashi H. et al. Expression of the cancer stem cell markers CD44v6 and ABCG2 in tongue cancer: effect of neoadjuvant chemotherapy on local recurrence. Int J Oncol 2014; 44(4): 1153–1162. [DOI] [PubMed] [Google Scholar]
- 58. Bossi P, Perrone F, Miceli R. et al. Tp53 status as guide for the management of ethmoid sinus intestinal-type adenocarcinoma. Oral Oncol 2013; 49(5): 413–419. [DOI] [PubMed] [Google Scholar]
- 59. Perrone F, Bossi P, Cortelazzi B. et al. TP53 mutations and pathologic complete response to neoadjuvant cisplatin and fluorouracil chemotherapy in resected oral cavity squamous cell carcinoma. J Clin Oncol 2010; 28(5): 761–766. [DOI] [PubMed] [Google Scholar]
- 60. Marta GN, Riera R, Bossi P. et al. Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: systematic review and meta-analysis. Eur J Cancer 2015; 51(17): 2596–2603. [DOI] [PubMed] [Google Scholar]
- 61. Bossi P, Lo Vullo S, Guzzo M. et al. Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Ann Oncol 2014; 25(2): 462–466. [DOI] [PubMed] [Google Scholar]
- 62. Ang KK, Harris J, Wheeler R. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363(1): 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Granata R, Miceli R, Orlandi E. et al. Tumor stage, human papillomavirus and smoking status affect the survival of patients with oropharyngeal cancer: an Italian validation study. Ann Oncol 2012; 23(7): 1832–1837. [DOI] [PubMed] [Google Scholar]
- 64. Bhatia A, Burtness B.. Human papillomavirus-associated oropharyngeal cancer: defining risk groups and clinical trials. J Clin Oncol 2015; 33(29): 3243–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rosenthal DI, Harari PM, Giralt J. et al. Impact of p16 status on the results of the phase III cetuximab (cet)/radiotherapy (RT). J Clin Oncol 2014; 32(15 suppl): 6001. [Google Scholar]
- 66. Fakhry C, Westra WH, Li S. et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008; 100(4): 261–269. [DOI] [PubMed] [Google Scholar]
- 67. Posner MR, Lorch JH, Goloubeva O. et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol 2011; 22(5): 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rainey H, Roy E, Selkridge I. et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients. ASCO 2017, abstr 6069. [DOI] [PubMed]
- 69. Marur S, Li S, Cmelak AJ. et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN cancer research group. J Clin Oncol 2017; 35(5): 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Driessen CM, de Boer JP, Gelderblom H. et al. Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil followed by randomization to two cisplatin-based concomitant chemoradiotherapy schedules in patients with locally advanced head and neck cancer (CONDOR study) (Dutch Head and Neck Society 08-01): a randomized phase II study. Eur J Cancer 2016; 52: 77–84. [DOI] [PubMed] [Google Scholar]
- 71. Ang KK, Zhang Q, Rosenthal DI. et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32(27): 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weidhaas JB, Harris J, Schaue D. et al. The KRAS-variant and cetuximab response in head and neck squamous cell cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2017; 3(4): 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Petrelli F, Coinu A, Riboldi V. et al. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: a systematic review and meta-analysis of published studies. Oral Oncol 2014; 50(11): 1041–1048. [DOI] [PubMed] [Google Scholar]
- 74. Guan J, Li Q, Zhang Y. et al. A meta-analysis comparing cisplatin-based to carboplatin-based chemotherapy in moderate to advanced squamous cell carcinoma of head and neck (SCCHN). Oncotarget 2016; 7(6): 7110–7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang J, Zhang J, Shi C. et al. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC Cancer 2016; 16(1): 689-016-2706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weber RS, Berkey BA, Forastiere A. et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg 2003; 129(1): 44–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.