Abstract
Intradural spinal tumors are rare tumors of the central nervous system. Due to the eloquence of the spinal cord and its tracts, the compact architecture of the cord and nerves, and the infiltrative nature of some of these tumors, surgical resection is difficult to achieve without causing neurological deficits. Likewise, chemotherapy and radiotherapy are utilized more cautiously in the treatment of intradural spinal tumors than their cranial counterparts. Targeted therapies aimed at the genetic alterations and molecular biology tailored to these tumors would be helpful but are lacking.
Here, we review the major types of intradural spinal tumors, with an emphasis on genetic alterations, molecular biology, and experimental therapies for these difficult to treat neoplasms.
Keywords: astrocytoma, ependymoma, meningioma, nerve sheath tumors, spinal cord tumors
Introduction
Primary spinal cord tumors are rare tumors of the central nervous system (CNS), comprising 2% to 4% of CNS tumors.1,2 Though many intracranial and spinal tumors share similar names, there is increasing evidence that the genetics and histopathological characteristics of intradural spinal tumors are different compared with their cranial counterparts.
In this review, we will discuss recent advances in our understanding of the most common intradural spinal tumors, namely astrocytomas, ependymomas, hemangioblastomas, meningiomas, and nerve sheath tumors, with an emphasis on molecular biology and experimental therapies for these neoplasms.
Intramedullary Spinal Cord Tumors
Intramedullary spinal cord tumors (IMSCTs) comprise approximately 5%–10% of tumors within the spinal canal.1,2 Gliomas, which include both ependymomas and astrocytomas, constitute 80%–90% of IMSCTs, with ependymomas constituting roughly two thirds of these and astrocytomas constituting one third.3 Hemangioblastomas are the other main type of IMSCTs, making up 8%–15% of IMSCTs, while approximately 2%–5% of IMSCTs are rare mass lesions such as neurenteric cysts and dermoid/epidermoids.
Astrocytomas
Epidemiology
Primary spinal cord astrocytomas are extremely rare, with an incidence of less than 0.1 per 100000 person-years.3,4 The average age of presentation is 35 years, with 60% of patients being male.3,5
Genetics
There have been tremendous advancements in our understanding of the underlying genetics and molecular biology of intracranial astrocytomas. This is reflected in the addition of some of these molecular parameters to the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System.6 Our understanding of spinal cord astrocytomas has lagged compared with their intracranial counterparts for several reasons. First, the rarity of this clinical entity makes it more difficult to obtain samples to run adequate analyses. Secondly, the location of these tumors in the parenchyma and their infiltrative nature render them extremely “eloquent,” making the task of obtaining enough tissue to do exhaustive analyses more difficult.
A comparison of several common molecular and genetic aberrations in intracranial astrocytomas with spinal cord astrocytomas reveals some commonalities as well as divergences (Table 1). For example, though isocitrate dehydrogenase 1 (IDH1) and IDH2 mutations have been found in intracranial astrocytomas,7,8 the incidence of IDH1 mutations in spinal cord astrocytomas is not clear. In a series of WHO grade II or III spinal cord astrocytomas, the R132H mutation in IDH1, which is the most common mutation found in intracranial astrocytoma, was not seen in any spinal cord astrocytomas (n = 9).9 Similarly, in another large multicenter discovery cohort of spinal cord astrocytomas (n = 17), IDH mutations were not observed.10 These findings would suggest that there are potential differences in the genetic makeup of intracranial and spinal cord tumors with similar histopathological grades. Although there is a paucity of data describing the genetic differences among the different grades of spinal cord astrocytomas, we review the relevant literature below.
Table 1.
Genetics of astrocytoma, with an emphasis on spinal cord astrocytomas*
| Gene | Locus | Location | Tumor Type | Comments |
|---|---|---|---|---|
| BRAF | 7q34 | Spinal cord > Brain | I, II | BRAF-KIAA1549 translocation, BRAF copy number gain, but not BRAF V600E mutation21,22 |
| H3F3A | 17q25 | Spinal cord > Brain | III, IV | H3F3A K27M mutation found in mainly midline gliomas, including spinal cord21,23–26 |
| CDKN2A | 9q21 | Spinal cord > Brain | All grades | More common in brainstem/spinal cord compared with other locations and associated with higher grades27,28 |
| IDH1 | 2q33.3 | Brain > Spinal cord | Grade III, IV | Mutations are thought to suggest secondary glioblastoma, with better prognosis.24,29 Much more prevalent in intracranial gliomas. |
| TP53 | 17p13 | Brain > Spinal Cord | All grades | P53 mutations thought to be secondary and not driver mutations21 |
*Modified and adapted from Tendulkar et al33 with permission.
Grade I Astrocytoma (Pilocytic)
BRAF is a member of the mitogen-activated protein kinase (MAPK) pathway which is important for cellular division, cell cycle progression, and malignant transformation and has proven important in our understanding of the molecular underpinnings of intracranial and spinal cord astrocytomas.11,12 Two major mutations have been noted in BRAF: a fusion oncogene consisting of BRAF and a previously uncharacterized gene, KIAA1549, and a valine to glutamate substitution at position 600 (BRAF V600E), causing constitutive activation of the MAPK pathway.13,14 Interestingly, numerous studies have shown that supratentorial pilocytic astrocytomas are more likely to harbor the BRAF V600E mutation, while posterior fossa and spinal cord pilocytic astrocytomas are more likely to harbor fusion oncogenes.15 A recent multi-institutional study of 17 spinal cord astrocytomas revealed that 80% of grade I astrocytomas harbored mutations in the BRAF genes, with 40% harboring the BRAF-KIAA1549 translocation and the other 60% having a BRAF copy number gain10; none of their specimens harbored the BRAF V600E mutation. These findings of different genetic underpinnings between intracranial and spinal cord astrocytomas will have important implications as targeted therapies for these tumors are investigated, as described below.
Another gene that has been found to be important in the tumorigenesis of astrocytomas is cyclin-dependent kinase inhibitor 2A (CDKN2A), which is located on 9q21 and encodes the p16 tumor suppressor protein.15,16 In an institutional cohort of over 140 pilocytic astrocytomas, Horbinski et al found that specific homozygous deletions of p16 were significantly more common in pilocytic astrocytomas of the brainstem and spinal cord compared with the cerebrum or cerebellum.17
Grade II Astrocytoma
Grade II spinal astrocytomas appear to harbor BRAF-KIAA1549 translocations and BRAF amplifications.10 There is limited information regarding spinal grade II astrocytomas, given the rarity with which they are resected or biopsied prior to transforming to higher-grade lesions.
Grade III/IV Astrocytoma
Another important gene in spinal cord astrocytomas is histone 3 variant H3.3 (H3F3A), which has been implicated in the tumorigenesis of both intracranial and spinal astrocytomas.18,19 The mutation of H3F3A K27M has predominantly been detected in malignant astrocytomas arising in structures of the midline, including the thalamus, brainstem, and spinal cord, and as such was listed as a separate entity in the 2016 WHO classification.6,20 Comparing low-grade (grades I and II) and high-grade (grades III and IV) spinal cord astrocytomas, a preponderance of the K27M mutation was found in grades III and IV spinal cord astrocytomas but without mutations in the BRAF gene, suggesting that BRAF and H3F3A may segregate based on the grading of the spinal cord astrocytoma and may have prognostic utility.10
Tumor suppressor protein 53 (TP53) has also been shown to be expressed in spinal cord glioblastomas, at a rate of 80%–90%.21,22 Interestingly, it appears that many patients may harbor mutations in TP53 but not IDH1, in contradistinction to patients with intracranial glioblastomas.22
Treatments
Surgery
Surgical resection remains the mainstay of treatment of patients with symptomatic spinal cord astrocytomas. Similar to surgery for intracranial astrocytomas in eloquent areas, the major goals of surgery are maximum resection while avoiding long-term neurological dysfunction.
There is increasing evidence that the grade of these tumors has a great influence on their infiltrative nature and, hence, the ability to find a good resection plane and the ability to provide gross total resection (GTR). Low-grade lesions, such as pilocytic astrocytomas (grade I), have a distinct surgical plane that separates them from surrounding eloquent spinal cord parenchyma, making GTR an accomplishable goal. Intraoperative monitoring in the form of motor evoked potentials, somatosensory evoked potentials, and electromyography is an important adjunct that helps the surgeon make important decisions about when to stop resection, particularly in cases where dissection planes are not clear.23,24
It is important to note that in patients with mild symptoms, if serial imaging shows a probable low-grade astrocytoma and no increase in size, surgical resection may be deferred (Fig. 1). In this case, serial imaging is important in the care and surveillance of these patients. The National Comprehensive Cancer Network (NCCN) encourages spine MRIs for patients who have imaging and clinical evidence of low-grade tumors every 3–6 months for 5 years and then at least annually.25
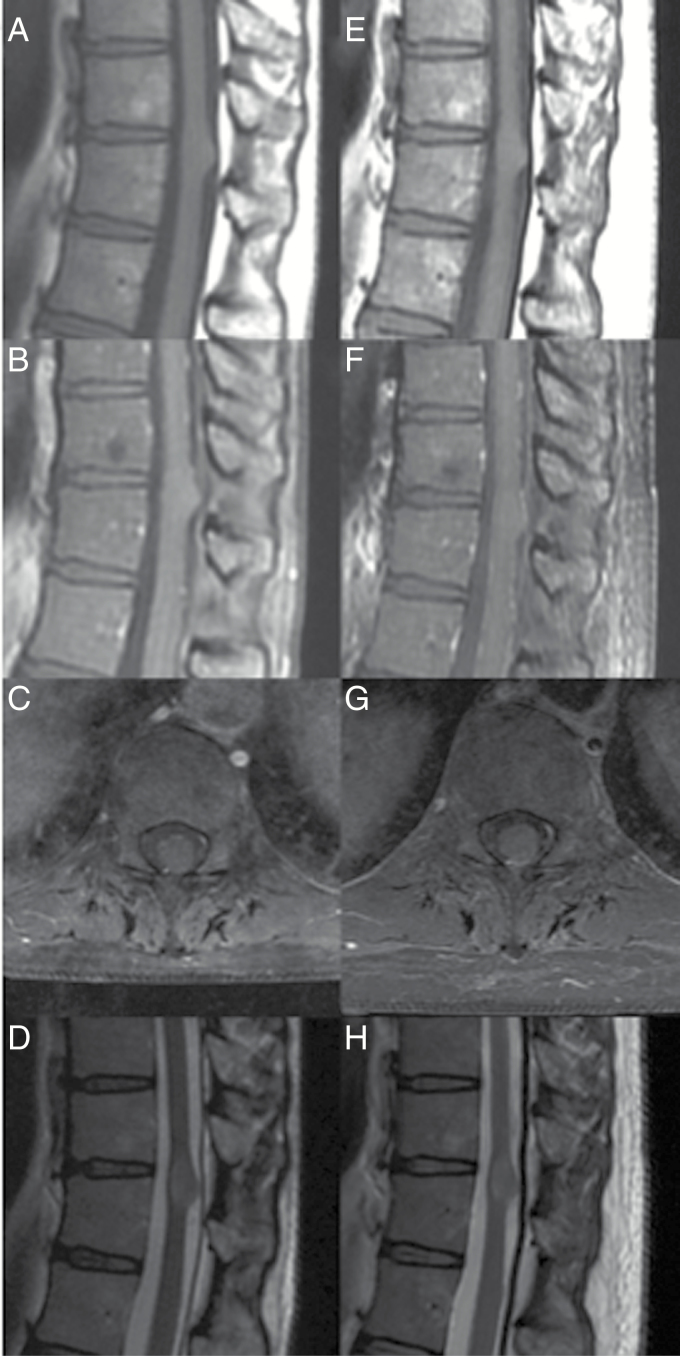
Fig. 1.
Low-grade astrocytoma. A 44-year-old woman underwent MRI of the lower back for low back pain. (A) Sagittal T1-weighted MRI revealed an exophytic/expansile lesion at T8–T9. (B) T1-weighted MRI with contrast revealed that the lesion did not enhance with contrast administration. (C) T1-weighted axial images with contrast through the lesion, showing the infiltrative nature of lesion. (D) T2- weighted sagittal images. (E) Two years later, T1-weighted sagittal images showed the lesion remained the same size. (F) There continued to be no enhancement on T1-weighted imaging with contrast administration. (G) Axial T1-weighted MRI with contrast. (H) T2-weighted sagittal images. Due to the patient’s continued asymptomatic presentation, no biopsy was performed. From Abd-El-Barr et al26 with permission.
Grade II lesions can be more infiltrative, but with a slower growth pattern.7 In many cases these are poorly enhancing but diffuse in nature and with indistinct borders. Biopsy can be performed, though it may leave patients with neurological deficits due to the invasive nature of these lesions.
For high-grade (WHO grade III or IV) spinal cord astrocytomas, it is our practice to attempt resection if a dissection or transition plane can be found, but often a biopsy and duraplasty (to allow for inevitable tumor growth) is performed due to the lack of distinct borders and high likelihood of rapid recurrence.26–28 In these cases, the NCCN recommends postoperative MRI 2–6 weeks after surgery, then every 2–4 months for 2–3 years and then less frequently.25 Similar to intracranial astrocytomas, lower-grade lesions may transform to higher-grade lesions, but the molecular mechanisms behind this are unclear (Fig. 2).
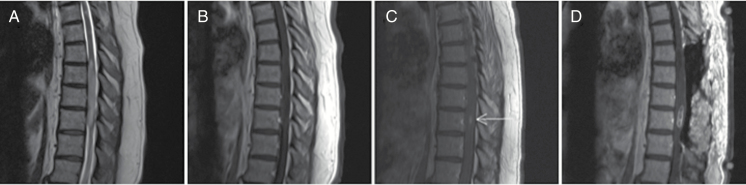
Fig. 2.
High-grade astrocytoma. A 59-year-old female who originally presented with gait instability and balance issues. (A) Initial MRI of her thoracic spine demonstrated an expansile intramedullary mass extending from T7 to T10 on T2 sagittal imaging. (B) Sagittal imaging demonstrating no contrast enhancement at original presentation. The patient was clinically observed for over a year until follow-up imaging demonstrated (C) new areas of contrast enhancement (arrow). The patient underwent multilevel laminectomies, duraplasty, and selective biopsy of the contrast enhancing lesion. (D) Postoperative T1-weighted contrast-enhanced imaging demonstrating postoperative changes and biopsy of the lesion. Final pathology demonstrated astrocytoma with high-grade features, negative for IDH1 mutation.
Radiation Therapy
The role of radiotherapy appears to be reserved for cases where GTR is not achievable, although one study showed that patients receiving radiotherapy had worse outcomes.3,29–31 As noted by the authors of that paper, this may signify a selection bias, as many of the patients who received radiotherapy were high risk and not candidates for surgery.3 Traditionally, conventional radiation was what was available for patients, and this required high doses (40–60 Gy) to show efficacy with a high rate of complications.32,33 However, the development of intensity-modulated radiation therapy and stereotactic radiosurgery has been exciting in that these techniques will allow for high doses to be delivered to the spine with a steep stepoff of radiation fields—limiting the complications associated with traditional radiation therapies.34,35
Systemic Therapy
Chemotherapy, in the form of temozolomide, an oral alkylating agent, appears to have a limited role in the treatment of spinal cord astrocytomas, in contradistinction to its important role in the treatment of intracranial astrocytomas.36,37 Studies have demonstrated only partial response to temozolomide in both low-grade and high-grade spinal cord astrocytomas, though some hematological side effects were noted.38–40 A systematic review of temozolomide in primary spinal cord glioblastoma did appear to show that patients treated with temozolomide had a slightly longer length of survival (16 mo vs 10 mo), but this did not reach statistical significance.41 Similarly, for pediatric patients with low-grade astrocytomas, patients who received adjuvant radiation therapy and chemotherapy after subtotal resection had longer survival times than those patients who only had subtotal resection, but not as long as those patients who underwent GTR.42
Anti-angiogenic agents are another important class of drugs against astrocytomas. Bevacizumab targets vascular endothelial growth factor (VEGF) and has been shown to have palliative effects in spinal cord astrocytomas that have failed surgical therapy, radiation therapy, and temozolomide treatment, but studies are limited.39,43
Experimental Therapies
Given our new understanding of the genetic underpinnings of astrocytomas, many clinical trials targeting these genetic mutations are under way related to intracranial astrocytomas. Experimental therapies for the treatment of spinal cord astrocytomas lag their intracranial counterpart due to the rarity of this disease.
Among the most exciting avenues are the use of BRAF inhibitors. The presence of BRAF mutations in intracranial gliomas has spurred clinical trials investigating the safety and efficacy of these inhibitors. Newer drugs that target the fusion protein BRAF: KIAA1549 are also being developed.44
As for high-grade astrocytomas, the finding of the preponderance of the H3F3A K27M mutation in spinal cord astrocytomas may make the use of a demethylation inhibitor a viable option—an option that has been shown to have good effect in a xenograft model of brainstem glioma.45
Another important experimental avenue that has been explored recently in the treatment of intracranial astrocytomas has been the use of neural stem cells (NSCs). Neural stem cells are pluripotent cells capable of generating gliogenic or neurogenic progeny.46 An important discovery is the tropism these cells have for tumors in vivo,47,48 which makes them great candidates to deliver toxic therapies targeted toward tumor cells. Thus, the concept of engineered NSCs which express an enzyme that activates a nontoxic prodrug and then the administration of the prodrug causing significant concentrations of the toxic drug at the tumor site has been a promising one.49 A phase I trial of patients with recurrent intracranial gliomas who were treated with a one-time intracranial administration of an immortalized NSC line that expressed cytosine deaminase (CD) (HB1.F3.CD.C21), which converts the prodrug 5-fluorocytosine (5-FC) to the cytotoxic 5-fluorouracil (5-FU) showed a good safety profile and, importantly, showed that these NSCs were nontumorigenic and were able to hone in on the intracranial tumor.50
We have shown the efficacy of using NSCs in a rat model of spinal cord glioma with one of our collaborators.51 Rats were injected with NSCs engineered to express either CD gene only (ie, F3.CD) or dual genes of CD and thymidine kinase (ie, F3.CD-TK) in the tumor epicenter 7 days after tumor seeding. We have shown that rats injected with dual-expressed NSCs survived longer than those that had the single engineered CD or debris and had improved autonomic function and decreased tumor growth.51
Many of these experimental therapies for intracranial glioblastoma require direct injection at the time of surgery for primary or recurrent tumors. Direct injection into spinal cord astrocytomas may not be practical, and as such, the use of other methods such as biodegradable polymers, convection enhanced delivery, and intrathecal delivery may prove to be more practical.
Ependymomas
Epidemiology
Ependymomas are the most common IMSCT in adults3,7 and have an incidence of 0.17 per 100000 person-years.4 They occur evenly between females and males.52 In adults, they have a propensity to be located in the cervical or cervicothoracic region, but in children, spinal ependymomas are mostly of the myxopapillary type found in the filum terminale or conus.53
Genetics
Recent discoveries in the genetics and tumorigenesis of ependymomas have distinguished spinal ependymomas from their intracranial counterpart (Table 2). Using advanced DNA methylation techniques on a large cohort of 500 tumors, Pajtler et al were able to identify 9 major molecular subgroups of ependymomas, with 3 in each compartment of the CNS, namely spine, posterior fossa, and supratentorial.54 The spinal cord ependymomas were divided into subependymomas (WHO grade I), myxopapillary ependymomas (WHO grade I), and (anaplastic) ependymomas (WHO grade II/III).
Table 2.
Genetics of ependymomas, with an emphasis on spinal cord ependymomas
For subependymomas, the most common mutation found was partial or complete loss of chromosome 6, which has been corroborated in other studies.55 Candidate genes for the pathogenesis of this tumor include T-complex protein 1 (TCP1) (involved in tubulin function) and adrenomedullin 1 (ADM1) and cyclin-dependent kinase 11 (CDK11) (involved in cell proliferation).55 For myxopapillary ependymomas, there is evidence of chromosomal instability, with some groups reporting an overexpression of the NEFL gene, which encodes the neurofilament light polypeptide more likely to be found in myxopapillary ependymomas compared with intracranial ependymomas.56
The most common genetic mutation in spinal cord ependymoma appears to be mutations in the neurofibromin (NF2) gene.57,58 In studies comparing both intracranial ependymomas and spinal cord ependymomas, mutation in the NF2 gene, which encodes the scaffolding protein merlin, also known as schwannomin, was found to be preferentially expressed in spinal cord ependymomas compared with intracranial ependymomas.57,59 It is thought that these mutations make cells less able to respond to contact inhibition, leading to uncontrolled cellular growth and proliferation.7
Treatments
Surgery
Surgery for symptomatic ependymomas appears to allow for longer survival, especially if GTR is possible.3,60–62 Subependymomas are usually intramedullary and, although low-grade, can be quite large; and although a surgical plane can often be found, their large size may not allow for GTR.63 Ependymomas are also intramedullary, but even if tumors are extensive, a surgical plane can often be found between the tumor and the spinal cord parenchyma, allowing for GTR63 (Fig. 3). The presence of a syrinx aids in identifying the surgical plane and should be exploited early in the surgery. There can be a vascular nidus along the tumor margin as well, which may share blood supply with normal spinal cord, and special care should be taken to preserve blood vessels not involved with the tumor.
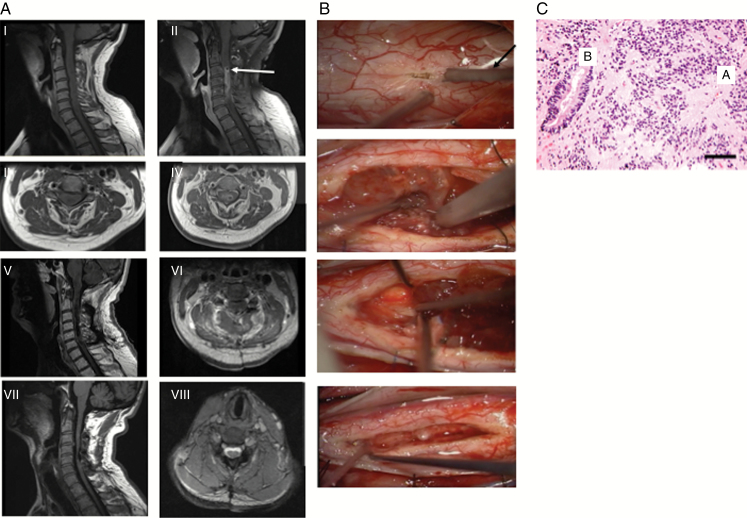
Fig. 3.
Cervical ependymoma. A 53-year-old male who presented with several months of bilateral hand numbness and problems with gait. (A) Radiographic findings. (I) MRI revealed cervical intradural intramedullary lesion. Sagittal T1-weighted MRI revealed some enlargement of cervical spinal cord. (II) Sagittal T1-weighted MRI with contrast revealed diffuse contrast enhancement from C3 to C6 with evidence of cystic changes at rostral portion of lesion (arrow). (III) Axial T1-weighted MRI at level of cyst. (IV) Axial T1-weighted MRI with contrast at level of cyst. Patient underwent C2 to C6 laminoplasty and resection of tumor. (V) Postoperative sagittal T1-weighted MRI with contrast revealed good resection. (VI) Axial T1-weighted MRI with contrast revealed removal of cyst. (VII) Two years later, patient’s symptoms have improved and sagittal T1-weighted MRI reveals continued absence of tumor. (VIII) Axial T2-weighted MRI reveals absence of tumor and resolution of cystic changes. (B) Intraoperative photographs. (I) Midline myelotomy was done with CO2 laser (arrow). (II) Tumor is debulked using ultrasonic aspirator (arrow). (III) A good surgical plane at rostral end of tumor was found, allowing for removal of tumor without invasion into spinal cord parenchyma. (IV) Inspection of resection cavity revealed GTR of tumor. (C) Classically demonstrate perivascular pseudorosettes (ie, tumor cells radially arranged around a central vessel, with their pink fibrillary processes extending like spokes inward to the vessel, A) and true ependymal rosettes (ie, tumor cells radially arranged a nonvascular lumen, B). Scale bar = 50 μm. Magnification = 200×.
Myxopapillary ependymomas are often located in the thoraco-lumbar region, as they originate from the filum terminale or conus medullaris.7 As such, they can be considered intradural extramedullary lesions, making their complete removal easier than intramedullary ependymomas (Fig. 4), but they do carry a higher potential for drop metastasis and CSF spread. Care should be taken in surgery to prevent morselization of the tumor.
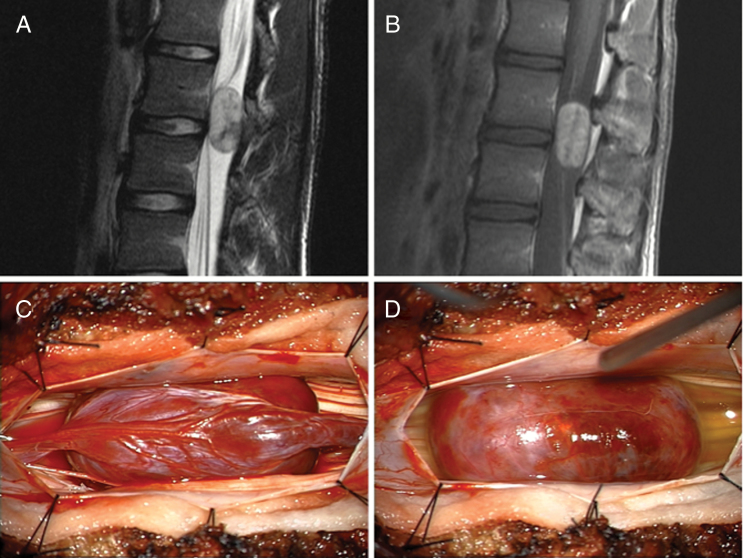
Fig. 4.
Myxopapillary ependymoma. A 17-year-old male who presented with two months of progressive back pain. (A) Initial MRI demonstrated an intradural, extramedullary mass at the L2–L3 level that was well circumscribed but slightly heterogeneous on T2-weighted imaging. (B) On contrast administration the lesion enhanced prominently. (C) Intraoperatively, the lesion was found to be intimately associated with the filum terminale. (D) Sectioning of the filum allowed for easy dissection of the tumor away from the surrounding nerve roots.
Although neurofibromatosis type 2 (NF2) is the most common mutation seen in spinal cord ependymomas, only around 20% of patients with the clinical diagnosis of NF2 will present with symptomatic ependymomas.64 However, 30%–50% of NF2 patients will have imaging evidence of spinal cord ependymomas.65 This difference is thought to be because of the indolent nature of the ependymomas in NF2 patients.64 As such, the treatment paradigms of patients with sporadic ependymomas and those with NF2 may vary, as symptom progression may be slower in NF2 patients.64
Radiation Therapy
The use of adjuvant radiotherapy is controversial, as some studies have shown worse overall survival, while others have shown beneficial responses.3,66 Radiotherapy appears to be limited to cases in which GTR is not possible or for asymptomatic lesions that show slow interval growth. For low-grade and anaplastic ependymomas, there is limited evidence to suggest the efficacy of fractionated radiotherapy if only subtotal resection is accomplished and there is no evidence of brain lesions. Craniospinal radiation is reserved for cases of leptomeningeal metastases.25
Systemic Therapy
Adjuvant chemotherapy, similar to treatment of cranial ependymoma, may be an option in cases where GTR is not possible. A single study reported that etoposide may have positive effects in patients with recurrent spinal cord ependymomas, but supporting evidence is lacking.67
Targeted therapies for ependymomas are currently being studied. Imatinib in a recurrent spinal cord ependymoma that overexpressed platelet derived growth factor (PDGF) appeared promising, but this overexpression in all spinal cord ependymomas has not been shown.68 Due to merlin’s interaction with rapamycin, it has been hypothesized that mammalian target of rapamycin (mTOR) inhibitors, such as everolimus, may play a role in treatment of NF2 mutated ependymomas, and a phase II trial is currently under way looking at the efficacy of everolimus in children with recurrent ependymomas (NCT02155920).
Interestingly, it has been noted that in patients with NF2, receiving bevacizumab stabilizes their known intracranial schwannomas. Bevacizumab has also been shown to decrease the size of cystic spinal cord ependymomas in NF2 patients, leading to clinical improvement in 7 out of 12 (58%) patients.69 It is unclear whether bevacizumab will have similar efficacy in sporadic spinal cord ependymomas.
Hemangioblastomas
Epidemiology
Spinal cord hemangioblastomas are benign vascular lesions that account for less than 10% of IMSCTs.70,71 They occur equally in males and females and the average age of presentation is 48 years.71 Approximately 20%–40% of patients who present with hemangioblastomas have evidence of von Hippel–Lindau (VHL) disease, which is characterized by the VHL mutation.70 The majority of patients present with neurological deficits thought to be due to tumor growth and spinal cord edema, while others may be found on workup of systemic VHL.
Of the VHL disease diagnosed in patients who have hemangioblastomas, 20% will be located in the spinal cord, while 80% are in the posterior fossa.70,72 VHL disease has an autosomal dominant inheritance pattern with 90% penetrance and is caused by a mutation or loss of the tumor suppressor gene at 3p25–26.73
Genetics
The VHL gene encodes an E3 ubiquitin ligase that targets hypoxia-inducible factor 1a (HIF-α), a known modulator of vascular proliferation. As a result of mutations or loss of the gene, cells cannot adequately degrade HIF-α, causing a proliferation of blood vessels.7,70 As has been the case with intracranial astrocytomas, there has been a correlation between activated HIF-α and VEGF, and thus both have been found to be increased in VHL mutated cells.74,75
Treatments
Surgery
As with other intramedullary tumors, surgical resection is recommended in cases of symptomatic lesions or in lesions that are seen to grow on serial imaging. Because spinal cord hemangioblastomas are common in patients with VHL, early MRI screening is recommended in young patients known to harbor the disease.76 Lesions that are found in asymptomatic patients should be followed with serial MRIs and clinical examinations. Importantly, because 10%–20% of patients with VHL will also have a pheochromocytoma, patients with VHL should be screened for elevated catecholamines prior to any surgery, as endocrine active tumors may require surgical excision or blockade before surgery.77 VHL patients are also prone to developing renal cell carcinoma, retinal hemangioblastomas, and pancreatic neuroendocrine tumors as well.
Hemangioblastomas are thought to arise from the pial layer and are classified as juxtamedullary, though they may have an encapsulated intramedullary component as well.7,78 Using precise microsurgical techniques, it is usually possible to achieve GTR of the mass and mural nodule.79
Radiation Therapy
Based on experience with posterior fossa hemangioblastomas, radiosurgery has been proposed as a method to treat spinal cord hemangioblastomas.80 Such a treatment is usually reserved for cases in which a diagnosis of VHL has already been made and a tissue diagnosis is not required.81,82 A recent meta-analysis was unable to compare surgery versus radiosurgery, due to the rarity of these lesions.83 Precise targeting is difficult for intramedullary lesions, as the area is typically compact and cord tolerance is limited with such close margins.
Systemic Therapy
The increased VEGF and HIF-α noted in hemangioblastomas are obvious targets for treatment of vascular lesions. The VEGF inhibitor bevacizumab has shown some promise for the treatment of spinal cord hemangioblastomas,84 but the experience has been limited to a few case reports and appears to be a possibility for salvage treatment. Pozapanib, which is a multiple tyrosine kinase inhibitor (TKI), blocking both the VEGF and PDGF receptor pathways, has shown some promise for VHL-related hemangioblastomas and is currently undergoing a phase II trial (NCT01436227).85 VEGF inhibitors are also associated with side effects, including poor wound healing and increased bleeding, as well as rebound malignant edema after discontinuing the agent.86 The use of anti-angiogenic drugs such as thalidomide and SU5416 or sunitinib, which are inhibitors of VEGF, has also been shown to be helpful in limited studies.7,87 Vorinostat, a histone deacetylase inhibitor, is being investigated as a potential therapy for VHL-related hemangioblastomas (NCT02108002).
Intradural Extramedullary Spinal Tumors – Meningiomas
Epidemiology
Spinal meningiomas are the most common primary tumors of the adult spine.4,88 Meningiomas arise from the arachnoidal cap cells of the meninges and occur less frequently in the spine than their intracranial counterpart, with at least one study citing a 1:8 ratio of spinal:intracranial meningiomas.89 Females are more often affected than males, and these tumors tend to arise between the fourth and fifth decades of life; this sex predilection is particularly more pronounced when comparing spinal with intracranial meningiomas.89,90
Genetics
The most consistent genetic abnormality found among spinal meningiomas is complete or partial loss of chromosome 22, followed by loss of 1p, 9p, and 10q and gains in 5p and 17q.91,92 A single institutional study looking at 14 spinal and 141 intracranial meningiomas found that the genetic mutations in spinal meningiomas tended to arise from a single tumor cell clone and were not as complex or heterogeneous as the intracranial variety.91 Using RNA microarray techniques, 1555 genes (out of 2 × 10^4 genes analyzed) were identified as displaying a differential expression pattern and 35 of these genes showed a significantly different expression in cranial meningiomas when matched for histologic subtype against spinal meningiomas. Some of these included genes expressing transcription factors or proteins involved in cell proliferation and differentiation, such as the HOX genes, as well as TCF8, CYR61, FHL2, KFL4, JUNB, and FOSL2 genes.91 Similarly, study of 58 spinal meningiomas that focused on matrix metalloproteinase 9 and hormone status discovered that unlike their intracranial counterparts, the progesterone expression of spinal meningiomas did not correlate with proliferative index as assessed by Ki-67 staining.93,94
Another important mutation that has been identified in cases of familial spinal meningiomas without NF2 mutations is SMARCE1 (switch/sucrose nonfermentable related, matrix associated, actin dependent regulator of chromatin, subfamily E, member 1), a chromatin-remodeling complex gene, also known to be part of a family of genes whose mutation has been implicated in schwannomatosis.95 Thus far this mutation has only been associated with clear cell histology.95 It is important to note that recent high-impact work has been conducted in intracranial meningioma and identified mutations in tumor necrosis factor receptor–associated factor 1, Krüppel-like factor 4, Akt1, and Smoothened, identifying new potential therapeutic targets.96,97 These findings have yet to be identified in spinal meningiomas.
Treatments
Surgery
Surgical resection is the primary treatment of spinal meningioma given the low rate of operative morbidity and the low recurrence rates (~1.3%–4%) for these often benign tumors (Fig. 5).89,90,98 As with its intracranial counterpart, surgeons should strive for a Simpson grade I resection (ie, complete removal of the tumor and involved dura) in spinal meningioma if feasible and safe. In one study of surgical resection for spinal meningioma based on Simpson grade, it was found that while recurrences were lower with Simpson grade I removals, complications were higher, whereas the opposite held with Simpson grade II removal.99 Removal of dura is more difficult to reconstruct with duraplasty given the constraints of spinal canal anatomy, especially in the ventral and lateral portions of the canal, and can lead to higher CSF leak/fistula rates.
Fig. 5.
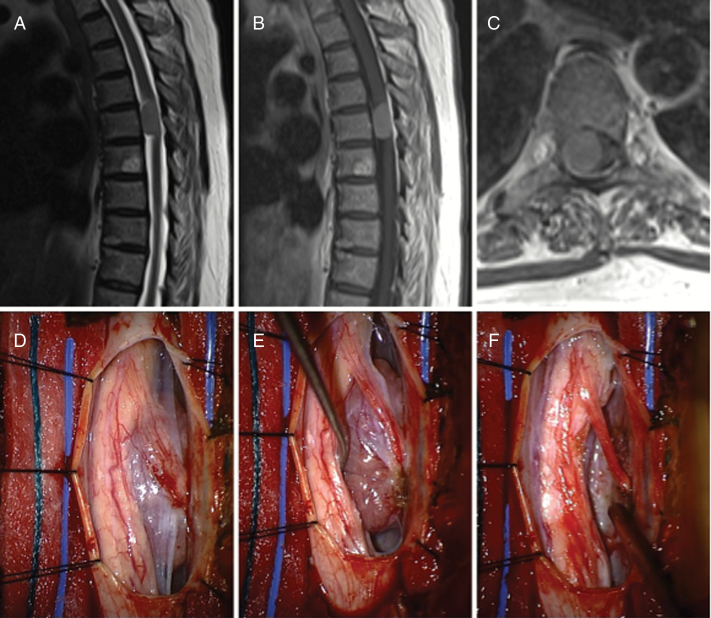
Meningioma. A 64-year-old female who presented with increasing gait instability as well as right-sided radiating rib pain. (A) Initial MRI demonstrated an intradural, extramedullary mass at the T5–T6 level on T2-weighted sequences. (B) T1-weight contrast-enhanced imaging showed homogeneous enhancement. (C) Axial imaging demonstrated significant mass effect on the associated cord. (D) Intraoperatively, a distinct mass was encountered ventral to the exiting nerve root on the right. (E) Opening of the dentate ligament allowed for direct access to the tumor, (F) which was able to be resected while leaving the overlying nerve root intact.
Radiation Therapy
The use of conventional radiation therapy is limited and controversial given the often indolent nature of the tumor and risk of radiation. Some authors reserve its use for malignant meningiomas, whereas others have advocated its use in early recurrence after subtotal or total resection and in patients where surgery is not possible (eg, medical comorbidities).98,100–102 Previously, radiosurgery was confined to use in the intracranial space given the need for frame-based targeting, which is untenable for the spine; however, more recently with frameless technology, its use has been reported in extracranial locations, including spinal meningiomas.103
Systemic Therapy
The genetic alterations found in intracranial meningioma have spurred a great deal of interest in formulating targeted medical therapies for this disease. While genetic differences as discussed above have been highlighted, the adoption of newer treatments for spinal meningiomas is likely to be slow. Currently, chemotherapy has a more extensive experimental history in the use of adjuvant treatment for recurrent or malignant intracranial meningioma, with a range of drugs including hydroxyurea, temozolomide, hormone modulators (eg, mifepristone, tamoxifen, octreotide, sandostatin), and targeted therapy (eg, the TKIs imatinib and erlotinib, and the anti-VEGF bevacizumab).104
The use of experimental chemotherapeutic agents in the treatment of spinal meningioma, however, has been even more limited owing to the predominance of good outcomes with surgery alone and low mitotic activity of tumor cells. There are several scattered reports of its use in spinal meningioma. Some advocate using adjuvant therapy in spinal meningioma, as is done for intracranial meningioma by WHO grade and resection status.105
Nerve Sheath Tumors
Epidemiology
Nerve sheath tumors, consisting predominantly of schwannomas and neurofibromas, are the second most common primary spinal cord tumor in adults.2,106 These tumors have an estimated incidence of 0.26 cases per 100000 in the United States, with a slightly higher rate found in males (odds ratio, 1.11). Greater than 98% of these tumors are benign, but malignant nerve sheath tumors, though rare, are a distinct and notoriously difficult-to-treat subset associated with a high burden of morbidity and mortality.2,107
Genetics
Though most of these tumors occur sporadically, there are known associations of both neurofibromas and schwannomas with both neurofibromatosis type 1 (NF1) and dysfunction of the NF1 tumor suppressor gene, as well as with NF2 and loss of NF2 gene function, which in turn produces the scaffolding protein merlin. Spinal tumors are common in NF2, with greater than 67% of patients estimated to have a spinal tumor detectable by MRI.65,108,109 Schwannomas can be encountered in NF1 patients, and likewise neurofibromas can be seen in the setting of NF2. Moreover, tumors exist of hybrid or mixed pathology that are difficult to pathologically classify clearly into schwannomas or neurofibromas. Though uncommon, when encountered, these tumors are highly suggestive of an underlying genetic disorder.110
A third major neurofibromatosis syndrome, schwannomatosis, has recently been recognized. Though the exact definitions and diagnostic criteria for this syndrome are still evolving, the recognition of this syndrome acknowledges the observation that many patients present with either multiple schwannomas or familial schwannomas who do not fulfill the diagnostic criteria of NF2.111,112 The SMARCB1 tumor suppressor gene, which is found on chromosome 22 and is involved in chromatin remodeling, has been implicated in many of these cases, and up to 45% of the cases of familial schwannomas have been noted to have SMARCB1 mutations.113
Treatments
Surgery
Treatment of nerve sheath tumors not associated with neurofibromatosis has traditionally consisted of maximal safe surgical resection, though certain patients with asymptomatic lesions may be observed for growth or symptom/sign development (Fig. 6). Complete resection is often feasible if the proximal and distal ends of the tumor are identified and surrounding nerve rootlets dissected free from the tumor wall. Sacrifice of 1–2 nerve rootlets enveloped by the tumor is tolerable with minimal neurologic deficit. Complete resections are typical for schwannomas, but neurofibromas are more challenging to remove due to involved nerve rootlets and fibers.
Fig. 6.
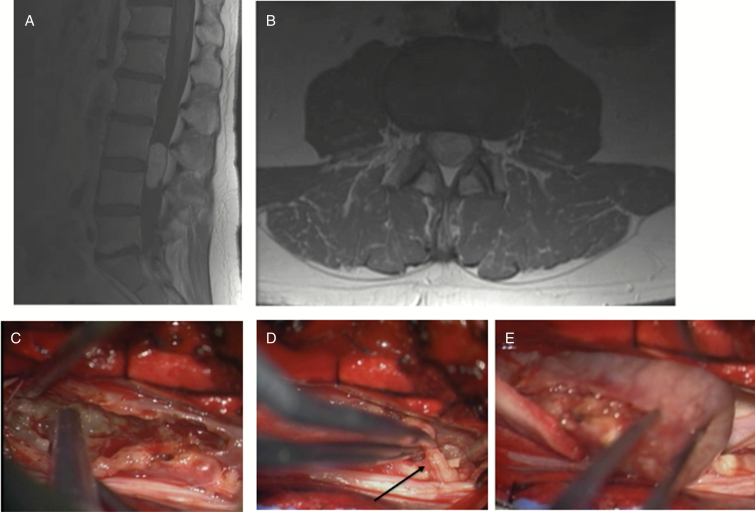
Schwannoma. A 44-year-old male with several months of right-sided lower extremity pain and numbness. (A, B) MRI revealed large intradural tumor. (C) Tumor was debulked using ultrasonic aspirator. (D) A nerve root was found to be involved with tumor, and on stimulation, no motor responses were elicited, allowing for sacrifice of root (arrow). (E) Tumor was able to be totally resected.
Radiation Therapy
Recent improvements in frameless stereotaxic accuracy have resulted in increased interest in the use of radiosurgery and hypofractionated radiation in the treatment of both spinal tumors in general and specifically nerve sheath tumors.114 Shin et al investigated the use of stereotactic radiosurgery in 66 spinal nerve sheath tumor patients.115 With an average follow-up of 44 months, they reported local control rates of 95.4%, with significant improvements in visual analog scale pain scores and no reported complications or radiation-induced neurotoxicity. Similar results have been reported by several other case series, all featuring very high rates of local control with little to no reported neurotoxicity.116,117 It should be noted, however, that the promising results achieved by these investigators nevertheless still have limitations, most prominently in the duration of follow-up. As nerve sheath tumors are generally slow growing, shorter follow-up times may miss many cases of treatment failure or delayed malignant transformation.
Systemic Therapies
As slow-growing tumors, spinal nerve sheath tumors are generally not thought to be responsive to standard chemotherapy regimens. However, investigators have continued to explore possible medical therapies in patients with neurofibromatosis, particularly in preventing the progression of plexiform neurofibromas and in cases of unresectable tumors. The TKI imatinib has been tested in a phase II clinical trial with some demonstrated benefit. Twenty-six percent of patients receiving the study drug experienced a ≥20% reduction in plexiform neurofibroma volume.118 The mTOR inhibitor sirolimus (rapamycin), in one phase II clinical trial of 46 patients, has also demonstrated some modest benefit.119 Patients on sirolimus had a slower time to progression of their neurofibromas of on average 4 months. Recently, an inhibitor of mitogen-activated protein kinase kinase, selumetinib, has shown promise as well.120 Twenty-four children recently underwent treatment in a phase I trial, with 71% achieving partial response (>20% reduction in tumor volume) and 0% reporting disease progression, with most children able to tolerate prolonged treatment regimens. It may be that some of these treatments will be effective against intraspinal neurofibromas as well.
Other chemotherapeutic options and molecular targets have also been tested, but with limited demonstrated benefits. Tipifarnib, a farnesyltransferase inhibitor, has been used to inhibit the dysregulated Ras signaling pathway that occurs in plexiform neurofibromas in NF1 patients.121 Though initial phase I trial data demonstrated that the drug was well tolerated in the pediatric population, subsequent phase II trials failed to demonstrate improvements in time to progression in plexiform neurofibromas.122
Few trials have investigated medical therapy for tumors in NF2-related schwannomas, but so far success has been limited when evaluating agents targeting epidermal growth factor receptor (EGFR) activity.123 Lapatinib, a selective EGFR inhibitor, has been shown to have some activity in ependymomas,124 but follow-up results have been mixed.125
Conclusions
Intradural spinal tumors include a wide range of histopathological subtypes arising from the spinal cord, spinal nerves, and meninges. They can be daunting entities to treat due to the eloquence of the spinal cord and its compact tracts and the infiltrative properties of some of these tumors. Currently, the mainstay of treatment involves surgical resection for maximal removal when possible, and chemotherapy and radiation therapy are reserved for residual or recurrent disease.
Discoveries in the genetic and molecular mechanisms behind spinal tumors, and their cranial counterparts, have opened a path for experimenting with more targeted treatments, which can help with residual or recurrent disease and may even one day supplant surgical resection. Advances in radiotherapy targeting and dose delivery are also evolving rapidly and may have a combinatorial effect with surgery to increase safety and minimize complications.
As a rare subset of tumors, intradural spinal tumors are difficult to study and investigate. Discovering similarities among cranial and spine tumors can help accelerate research, but distinguishing differences between them are equally important and critical. Thus, sharing of resources and pooling of data are essential for the discovery of safer and more effective treatments for these rare and challenging spine tumors.
Funding
Portions of this work were supported by the Daniel J. Sandman, PhD Memorial Fund.
Conflict of interest statement. Dr Chi serves as a consultant to K2M.
References
- 1. Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 2011;11(3):320–328. [DOI] [PubMed] [Google Scholar]
- 2. Duong LM, McCarthy BJ, McLendon RE, et al. . Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer. 2012;118(17):4220–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Milano MT, Johnson MD, Sul J, et al. . Primary spinal cord glioma: a surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98(1):83–92. [DOI] [PubMed] [Google Scholar]
- 4. Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87(2):173–179. [DOI] [PubMed] [Google Scholar]
- 5. Minehan KJ, Brown PD, Scheithauer BW, Krauss WE, Wright MP. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2009;73(3):727–733. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 7. Zadnik PL, Gokaslan ZL, Burger PC, Bettegowda C. Spinal cord tumours: advances in genetics and their implications for treatment. Nat Rev Neurol. 2013;9(5):257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sturm D, Witt H, Hovestadt V, et al. . Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 9. Ellezam B, Theeler BJ, Walbert T, et al. . Low rate of R132H IDH1 mutation in infratentorial and spinal cord grade II and III diffuse gliomas. Acta Neuropathol. 2012;124(3):449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shankar GM, Lelic N, Gill CM, et al. . BRAF alteration status and the histone H3F3A gene K27M mutation segregate spinal cord astrocytoma histology. Acta Neuropathol. 2016;131(1):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penman CL, Faulkner C, Lowis SP, Kurian KM. Current understanding of BRAF alterations in diagnosis, prognosis, and therapeutic targeting in pediatric low-grade gliomas. Front Oncol. 2015;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Deimling A, Korshunov A, Hartmann C. The next generation of glioma biomarkers: MGMT methylation, BRAF fusions and IDH1 mutations. Brain Pathol. 2011;21(1):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones DT, Kocialkowski S, Liu L, et al. . Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies H, Bignell GR, Cox C, et al. . Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 15. Horbinski C, Nikiforova MN, Hagenkord JM, Hamilton RL, Pollack IF. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol. 2012;14(6):777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horbinski C, Hamilton RL, Nikiforov Y, Pollack IF. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119(5):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartzentruber J, Korshunov A, Liu XY, et al. . Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 19. Wu G, Broniscer A, McEachron TA, et al. ; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solomon DA, Wood MD, Tihan T, et al. . Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;26(5):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yanamadala V, Koffie RM, Shankar GM, et al. . Spinal cord glioblastoma: 25years of experience from a single institution. J Clin Neurosci. 2016;27:138–141. [DOI] [PubMed] [Google Scholar]
- 22. Nagaishi M, Nobusawa S, Yokoo H, et al. . Genetic mutations in high grade gliomas of the adult spinal cord. Brain Tumor Pathol. 2016;33(4):267–269. [DOI] [PubMed] [Google Scholar]
- 23. Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16(Suppl 2):S130–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng JS, Ivan ME, Stapleton CJ, Quinones-Hinojosa A, Gupta N, Auguste KI. Intraoperative changes in transcranial motor evoked potentials and somatosensory evoked potentials predicting outcome in children with intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014;13(6):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nabors LB, Portnow J, Ammirati M, et al. . Central nervous system cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(10):1191–1202. [DOI] [PubMed] [Google Scholar]
- 26. Abd-El-Barr MM, Huang KT, Chi JH. Infiltrating spinal cord astrocytomas: epidemiology, diagnosis, treatments and future directions. J Clin Neurosci. 2016;29:15–20. [DOI] [PubMed] [Google Scholar]
- 27. Garcés-Ambrossi GL, McGirt MJ, Mehta VA, et al. . Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11(5):591–599. [DOI] [PubMed] [Google Scholar]
- 28. Karikari IO, Nimjee SM, Hodges TR, et al. . Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2015;76(Suppl 1):S4–S13; discussion S13. [DOI] [PubMed] [Google Scholar]
- 29. Isaacson SR. Radiation therapy and the management of intramedullary spinal cord tumors. J Neurooncol. 2000;47(3):231–238. [DOI] [PubMed] [Google Scholar]
- 30. Guss ZD, Moningi S, Jallo GI, Cohen KJ, Wharam MD, Terezakis SA. Management of pediatric spinal cord astrocytomas: outcomes with adjuvant radiation. Int J Radiat Oncol Biol Phys. 2013;85(5):1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abdel-Wahab M, Etuk B, Palermo J, et al. . Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys. 2006;64(4):1060–1071. [DOI] [PubMed] [Google Scholar]
- 32. Merchant TE, Kiehna EN, Miles MA, Zhu J, Xiong X, Mulhern RK. Acute effects of irradiation on cognition: changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. Int J Radiat Oncol Biol Phys. 2002;53(5):1271–1278. [DOI] [PubMed] [Google Scholar]
- 33. Tendulkar RD, Pai Panandiker AS, Wu S, et al. . Irradiation of pediatric high-grade spinal cord tumors. Int J Radiat Oncol Biol Phys. 2010;78(5):1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dea N, Gokaslan Z, Choi D, Fisher C. Spine oncology‒primary spine tumors. Neurosurgery. 2017;80(3S):S124–S130. [DOI] [PubMed] [Google Scholar]
- 35. Purvis TE, Goodwin CR, Lubelski D, Laufer I, Sciubba DM. Review of stereotactic radiosurgery for intradural spine tumors. CNS Oncol. 2017;6(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 37. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 38. Chamberlain MC. Temozolomide for recurrent low-grade spinal cord gliomas in adults. Cancer. 2008;113(5):1019–1024. [DOI] [PubMed] [Google Scholar]
- 39. Kaley TJ, Mondesire-Crump I, Gavrilovic IT. Temozolomide or bevacizumab for spinal cord high-grade gliomas. J Neurooncol. 2012;109(2):385–389. [DOI] [PubMed] [Google Scholar]
- 40. Kim WH, Kim WH, Yoon SH, et al. . Temozolomide for malignant primary spinal cord glioma: an experience of six cases and a literature review. J Neurooncol. 2011;101(2):247–254. [DOI] [PubMed] [Google Scholar]
- 41. Hernández-Durán S, Bregy A, Shah AH, Hanft S, Komotar RJ, Manzano GR. Primary spinal cord glioblastoma multiforme treated with temozolomide. J Clin Neurosci. 2015;22(12):1877–1882. [DOI] [PubMed] [Google Scholar]
- 42. Ahmed R, Menezes AH, Torner JC. Role of resection and adjuvant therapy in long-term disease outcomes for low-grade pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr. 2016;18(5):594–601. [DOI] [PubMed] [Google Scholar]
- 43. Chamberlain MC, Johnston SK. Recurrent spinal cord glioblastoma: salvage therapy with bevacizumab. J Neurooncol. 2011;102(3):427–432. [DOI] [PubMed] [Google Scholar]
- 44. Sun Y, Alberta JA, Pilarz C, et al. . A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol. 2017;19(6):774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hashizume R, Andor N, Ihara Y, et al. . Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20(12):1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Llorens-Bobadilla E, Martin-Villalba A. Adult NSC diversity and plasticity: the role of the niche. Curr Opin Neurobiol. 2017;42:68–74. [DOI] [PubMed] [Google Scholar]
- 47. Aboody KS, Brown A, Rainov NG, et al. . Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97(23):12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim SK, Cargioli TG, Machluf M, et al. . PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11(16):5965–5970. [DOI] [PubMed] [Google Scholar]
- 49. Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron. 2011;70(4):597–613. [DOI] [PubMed] [Google Scholar]
- 50. Portnow J, Synold TW, Badie B, et al. . Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res. 2017;23(12):2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ropper AE, Zeng X, Haragopal H, et al. . Targeted treatment of experimental spinal cord glioma with dual gene-engineered human neural stem cells. Neurosurgery. 2016;79(3):481–491. [DOI] [PubMed] [Google Scholar]
- 52. Garrido E, Stein BM. Microsurgical removal of intramedullary spinal cord tumors. Surg Neurol. 1977;7(4):215–219. [PubMed] [Google Scholar]
- 53. Vera-Bolanos E, Aldape K, Yuan Y, et al. ; CERN Foundation Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015;17(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pajtler KW, Witt H, Sill M, et al. . Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olsen TK, Gorunova L, Meling TR, et al. . Genomic characterization of ependymomas reveals 6q loss as the most common aberration. Oncol Rep. 2014;32(2):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee CH, Chung CK, Ohn JH, Kim CH. The similarities and differences between intracranial and spinal ependymomas: a review from a genetic research perspective. J Korean Neurosurg Soc. 2016;59(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singh PK, Gutmann DH, Fuller CE, Newsham IF, Perry A. Differential involvement of protein 4.1 family members DAL-1 and NF2 in intracranial and intraspinal ependymomas. Mod Pathol. 2002;15(5):526–531. [DOI] [PubMed] [Google Scholar]
- 58. Lamszus K, Lachenmayer L, Heinemann U, et al. . Molecular genetic alterations on chromosomes 11 and 22 in ependymomas. Int J Cancer. 2001;91(6):803–808. [DOI] [PubMed] [Google Scholar]
- 59. Palm T, Figarella-Branger D, Chapon F, et al. . Expression profiling of ependymomas unravels localization and tumor grade-specific tumorigenesis. Cancer. 2009;115(17):3955–3968. [DOI] [PubMed] [Google Scholar]
- 60. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993;79(2):204–209. [DOI] [PubMed] [Google Scholar]
- 61. Aghayev K, Vrionis F, Chamberlain MC. Adult intradural primary spinal cord tumors. J Natl Compr Canc Netw. 2011;9(4):434–447. [DOI] [PubMed] [Google Scholar]
- 62. Lin YH, Huang CI, Wong TT, et al. . Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71(2):205–210. [DOI] [PubMed] [Google Scholar]
- 63. Karikari IO, Nimjee SM, Hodges TR, et al. . Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2011;68(1):188–197; discussion 197. [DOI] [PubMed] [Google Scholar]
- 64. Plotkin SR, O’Donnell CC, Curry WT, Bove CM, MacCollin M, Nunes FP. Spinal ependymomas in neurofibromatosis type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011;14(4):543–547. [DOI] [PubMed] [Google Scholar]
- 65. Patronas NJ, Courcoutsakis N, Bromley CM, Katzman GL, MacCollin M, Parry DM. Intramedullary and spinal canal tumors in patients with neurofibromatosis 2: MR imaging findings and correlation with genotype. Radiology. 2001;218(2):434–442. [DOI] [PubMed] [Google Scholar]
- 66. Harrop JS, Ganju A, Groff M, Bilsky M. Primary intramedullary tumors of the spinal cord. Spine (Phila Pa 1976). 2009;34(22 Suppl):S69–S77. [DOI] [PubMed] [Google Scholar]
- 67. Chamberlain MC. Etoposide for recurrent spinal cord ependymoma. Neurology. 2002;58(8):1310–1311. [DOI] [PubMed] [Google Scholar]
- 68. Fakhrai N, Neophytou P, Dieckmann K, et al. . Recurrent spinal ependymoma showing partial remission under Imatimib. Acta Neurochir (Wien). 2004;146(11):1255–1258. [DOI] [PubMed] [Google Scholar]
- 69. Morris KA, Afridi SK, Evans DG, et al. . The response of spinal cord ependymomas to bevacizumab in patients with neurofibromatosis Type 2. J Neurosurg Spine. 2017;26(4):474–482. [DOI] [PubMed] [Google Scholar]
- 70. Gläsker S. Central nervous system manifestations in VHL: genetics, pathology and clinical phenotypic features. Fam Cancer. 2005;4(1):37–42. [DOI] [PubMed] [Google Scholar]
- 71. Westwick HJ, Giguère JF, Shamji MF. Incidence and prognosis of spinal hemangioblastoma: a surveillance epidemiology and end results study. Neuroepidemiology. 2016;46(1):14–23. [DOI] [PubMed] [Google Scholar]
- 72. Ostrom QT, Gittleman H, Farah P, et al. . CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Latif F, Tory K, Gnarra J, et al. . Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. [DOI] [PubMed] [Google Scholar]
- 74. Bader HL, Hsu T. Systemic VHL gene functions and the VHL disease. FEBS Lett. 2012;586(11):1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34(17):2239–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shuin T, Yamasaki I, Tamura K, Okuda H, Furihata M, Ashida S. Von Hippel-Lindau disease: molecular pathological basis, clinical criteria, genetic testing, clinical features of tumors and treatment. Jpn J Clin Oncol. 2006;36(6):337–343. [DOI] [PubMed] [Google Scholar]
- 77. Lonser RR, Glenn GM, Walther M, et al. . von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. [DOI] [PubMed] [Google Scholar]
- 78. Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98(1):106–116. [DOI] [PubMed] [Google Scholar]
- 79. Weil RJ, Lonser RR, DeVroom HL, Wanebo JE, Oldfield EH. Surgical management of brainstem hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98(1):95–105. [DOI] [PubMed] [Google Scholar]
- 80. Moss JM, Choi CY, Adler JR Jr, Soltys SG, Gibbs IC, Chang SD. Stereotactic radiosurgical treatment of cranial and spinal hemangioblastomas. Neurosurgery. 2009;65(1):79–85; discussion 85. [DOI] [PubMed] [Google Scholar]
- 81. Ryu SI, Kim DH, Chang SD. Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg Focus. 2003;15(5):E10. [DOI] [PubMed] [Google Scholar]
- 82. Selch MT, Tenn S, Agazaryan N, Lee SP, Gorgulho A, De Salles AA. Image-guided linear accelerator-based spinal radiosurgery for hemangioblastoma. Surg Neurol Int. 2012;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bridges KJ, Jaboin JJ, Kubicky CD, Than KD. Stereotactic radiosurgery versus surgical resection for spinal hemangioblastoma: a systematic review. Clin Neurol Neurosurg. 2017;154:59–66. [DOI] [PubMed] [Google Scholar]
- 84. Omar AI. Bevacizumab for the treatment of surgically unresectable cervical cord hemangioblastoma: a case report. J Med Case Rep. 2012;6:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Migliorini D, Haller S, Merkler D, et al. . Recurrent multiple CNS hemangioblastomas with VHL disease treated with pazopanib: a case report and literature review. CNS Oncol. 2015;4(6):387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sardi I, Sanzo M, Giordano F, et al. . Monotherapy with thalidomide for treatment of spinal cord hemangioblastomas in a patient with von Hippel-Lindau disease. Pediatr Blood Cancer. 2009;53(3):464–467. [DOI] [PubMed] [Google Scholar]
- 88. Helseth A, Mork SJ. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71(6):842–845. [DOI] [PubMed] [Google Scholar]
- 89. Solero CL, Fornari M, Giombini S, et al. . Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25(2):153–160. [PubMed] [Google Scholar]
- 90. Levy WJ Jr, Bay J, Dohn D. Spinal cord meningioma. J Neurosurg. 1982;57(6):804–812. [DOI] [PubMed] [Google Scholar]
- 91. Sayagués JM, Tabernero MD, Maíllo A, et al. . Microarray-based analysis of spinal versus intracranial meningiomas: different clinical, biological, and genetic characteristics associated with distinct patterns of gene expression. J Neuropathol Exp Neurol. 2006;65(5):445–454. [DOI] [PubMed] [Google Scholar]
- 92. Arslantas A, Artan S, Oner U, et al. . Detection of chromosomal imbalances in spinal meningiomas by comparative genomic hybridization. Neurol Med Chir (Tokyo). 2003;43(1):12–18; discussion 19. [DOI] [PubMed] [Google Scholar]
- 93. Barresi V, Alafaci C, Caffo M, Barresi G, Tuccari G. Clinicopathological characteristics, hormone receptor status and matrix metallo-proteinase-9 (MMP-9) immunohistochemical expression in spinal meningiomas. Pathol Res Pract. 2012;208(6):350–355. [DOI] [PubMed] [Google Scholar]
- 94. Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86(1):113–120. [DOI] [PubMed] [Google Scholar]
- 95. Smith MJ, O’Sullivan J, Bhaskar SS, et al. . Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45(3):295–298. [DOI] [PubMed] [Google Scholar]
- 96. Brastianos PK, Horowitz PM, Santagata S, et al. . Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Clark VE, Erson-Omay EZ, Serin A, et al. . Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Setzer M, Vatter H, Marquardt G, Seifert V, Vrionis FD. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus. 2007;23(4):E14. [DOI] [PubMed] [Google Scholar]
- 99. Kim CH, Chung CK, Lee SH, et al. . Long-term recurrence rates after the removal of spinal meningiomas in relation to Simpson grades. Eur Spine J. 2016;25(12):4025–4032. [DOI] [PubMed] [Google Scholar]
- 100. Gezen F, Kahraman S, Canakci Z, Bedük A. Review of 36 cases of spinal cord meningioma. Spine (Phila Pa 1976). 2000;25(6):727–731. [DOI] [PubMed] [Google Scholar]
- 101. Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17(8):1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roux FX, Nataf F, Pinaudeau M, Borne G, Devaux B, Meder JF. Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46(5):458–463; discussion 463–464. [DOI] [PubMed] [Google Scholar]
- 103. Gerszten PC, Ozhasoglu C, Burton SA, et al. . CyberKnife frameless stereotactic radiosurgery for spinal lesions: clinical experience in 125 cases. Neurosurgery. 2004;55(1):89–98; discussion 98–99. [PubMed] [Google Scholar]
- 104. Kaley T, Barani I, Chamberlain M, et al. . Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goldbrunner R, Minniti G, Preusser M, et al. . EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 106. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Albayrak BS, Gorgulu A, Kose T. A case of intra-dural malignant peripheral nerve sheath tumor in thoracic spine associated with neurofibromatosis type 1. J Neurooncol. 2006;78(2):187–190. [DOI] [PubMed] [Google Scholar]
- 108. Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mautner VF, Tatagiba M, Lindenau M, et al. . Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol. 1995;165(4):951–955. [DOI] [PubMed] [Google Scholar]
- 110. Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123(3):295–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. MacCollin M, Chiocca EA, Evans DG, et al. . Diagnostic criteria for schwannomatosis. Neurology. 2005;64(11):1838–1845. [DOI] [PubMed] [Google Scholar]
- 112. Plotkin SR, Blakeley JO, Evans DG, et al. . Update from the 2011 International Schwannomatosis Workshop: from genetics to diagnostic criteria. Am J Med Genet A. 2013;161A(3):405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Smith MJ, Wallace AJ, Bowers NL, et al. . Frequency of SMARCB1 mutations in familial and sporadic schwannomatosis. Neurogenetics. 2012;13(2):141–145. [DOI] [PubMed] [Google Scholar]
- 114. Hsu W, Nguyen T, Kleinberg L, et al. . Stereotactic radiosurgery for spine tumors: review of current literature. Stereotact Funct Neurosurg. 2010;88(5):315–321. [DOI] [PubMed] [Google Scholar]
- 115. Shin DW, Sohn MJ, Kim HS, et al. . Clinical analysis of spinal stereotactic radiosurgery in the treatment of neurogenic tumors. J Neurosurg Spine. 2015;23(4):429–437. [DOI] [PubMed] [Google Scholar]
- 116. Gerszten PC, Quader M, Novotny J Jr, Flickinger JC. Radiosurgery for benign tumors of the spine: clinical experience and current trends. Technol Cancer Res Treat. 2012;11(2):133–139. [DOI] [PubMed] [Google Scholar]
- 117. Marchetti M, De Martin E, Milanesi I, Fariselli L. Intradural extramedullary benign spinal lesions radiosurgery. Medium- to long-term results from a single institution experience. Acta Neurochir (Wien). 2013;155(7):1215–1222. [DOI] [PubMed] [Google Scholar]
- 118. Robertson KA, Nalepa G, Yang FC, et al. . Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13(12):1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Weiss B, Widemann BC, Wolters P, et al. . Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015;17(4):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dombi E, Baldwin A, Marcus LJ, et al. . Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Khalaf WF, Yang FC, Chen S, et al. . K-ras is critical for modulating multiple c-kit-mediated cellular functions in wild-type and Nf1+/- mast cells. J Immunol. 2007;178(4):2527–2534. [DOI] [PubMed] [Google Scholar]
- 122. Widemann BC, Dombi E, Gillespie A, et al. . Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16(5):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Karajannis MA, Legault G, Hagiwara M, et al. . Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012;14(9):1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fouladi M, Stewart CF, Blaney SM, et al. . Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(27):4221–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. DeWire M, Fouladi M, Turner DC, et al. . An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: a Collaborative Ependymoma Research Network study (CERN). J Neurooncol. 2015;123(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]