Abstract
Background
Driven by reduced nutritional intakes and metabolic alterations, malnutrition in cancer patients adversely affects quality of life, treatment tolerance and survival. We examined evidence for oral nutritional interventions during chemo(radio)therapy.
Design
We carried out a systematic review of randomized controlled trials (RCT) with either dietary counseling (DC), high-energy oral nutritional supplements (ONS) aiming at improving intakes or ONS enriched with protein and n-3 polyunsaturated fatty acids (PUFA) additionally aiming for modulation of cancer-related metabolic alterations. Meta-analyses were carried out on body weight (BW) response to nutritional interventions, with subgroup analyses for DC and/or high-energy ONS or high-protein n-3 PUFA-enriched ONS.
Results
Eleven studies were identified. Meta-analysis showed overall benefit of interventions on BW during chemo(radio)therapy (+1.31 kg, 95% CI 0.24–2.38, P = 0.02, heterogeneity Q = 21.1, P = 0.007). Subgroup analysis showed no effect of DC and/or high-energy ONS (+0.80 kg, 95% CI −1.14 to 2.74, P = 0.32; Q = 10.5, P = 0.03), possibly due to limited compliance and intakes falling short of intake goals. A significant effect was observed for high-protein n-3 PUFA-enriched intervention compared with isocaloric controls (+1.89 kg, 95% CI 0.51–3.27, P = 0.02; Q = 3.1 P = 0.37). High-protein, n-3 PUFA-enriched ONS studies showed attenuation of lean body mass loss (N = 2 studies) and improvement of some quality of life domains (N = 3 studies). Overall, studies were limited in number, heterogeneous, and inadequately powered to show effects on treatment toxicity or survival.
Conclusion
This systematic review suggests an overall positive effect of nutritional interventions during chemo(radio)therapy on BW. Subgroup analyses showed effects were driven by high-protein n-3 PUFA-enriched ONS, suggesting the benefit of targeting metabolic alterations. DC and/or high-energy ONS were less effective, likely due to cumulative caloric deficits despite interventions. We highlight the need and provide recommendations for well-designed RCT to determine the effect of nutritional interventions on clinical outcomes, with specific focus on reaching nutritional goals and providing the right nutrients, as part of an integral supportive care approach.
Keywords: chemo(radio)therapy, oral nutrition intervention, systematic review, meta-analysis, clinical outcomes, nutritional outcomes
Key Message
Driven by reduced intake and metabolic alterations, cancer-related malnutrition negatively impacts clinical outcomes. This systematic review shows overall benefit of nutritional support during chemo(radio)therapy on body weight, mostly driven by high-protein n-3 PUFA-enriched ONS. Well-designed trials are required to study the effects of nutritional support on treatment toxicities or survival.
Introduction
Cancer-related malnutrition frequently develops, with prevalence ranging from 30% to 90% depending on tumor site, stage of disease and treatment [1–4]. Major causes are cancer-induced metabolic alterations and/or cancer-induced symptoms (e.g. anorexia, nausea, pain) resulting in decreased food intake. Malnutrition can be exacerbated by the side-effects of anticancer drugs such as fatigue, anorexia, altered hedonic input and a wide range of GI symptoms [5], and/or by physical inactivity resulting from physical and psychosocial distress, which may lead to further loss of muscle mass. Malnutrition impairs tolerance to anticancer treatments including chemotherapy and is associated with decreased response to treatment [6–9], decreased quality of life (QoL) [6, 8, 10] and shorter survival [6, 8–15].
Due to the scale and importance of cancer-associated malnutrition, evidence-based position papers and clinical guidelines have been published to raise awareness and provide practical guidance to ensure that nutritional screening, nutritional intervention and ongoing monitoring are incorporated into treatment programs early and throughout the patient journey [16–20].
To inform clinical decisions about the impact of nutritional intervention during chemotherapy, it is of key importance to review the evidence base for nutritional support in this context. Trials have been undertaken in selected patient groups with or at-risk of cancer-associated malnutrition to investigate the effectiveness of dietary counseling (DC) and/or high energy oral nutritional supplements (ONS). ONS may be fortified with protein or anti-inflammatory n-3 long-chain polyunsaturated fatty acids (n-3 PUFAs). Although some of these trials have been included in previous reviews [21–24], none of the prior reviews focused specifically on patients undergoing chemo(radio)therapy, none included all first line volitional nutritional interventions, i.e. DC and/or ONS and none undertook meta-analyses using these specific criteria.
We aimed to examine the evidence from randomized controlled trials (RCT), in patients undergoing chemo(radio)therapy, investigating the effect of oral nutritional interventions on a range of nutritional and clinical outcomes. A meta-analysis was carried out to determine the overall effect of oral nutritional interventions. In addition, subgroup meta-analyses were undertaken to investigate the impact of (i) DC and/or high-energy ONS, or (ii) high-protein, n-3 PUFA-enriched ONS, which also aim to modulate cancer-related metabolic alterations. Finally, based on the analysis of available evidence, we aimed to outline guidance for future studies. For the purpose of this review, the terms ‘cancer-associated malnutrition’ and ‘cachexia’ are not differentiated and are taken to designate the same pathophysiological condition.
Methods
Literature search and study selection
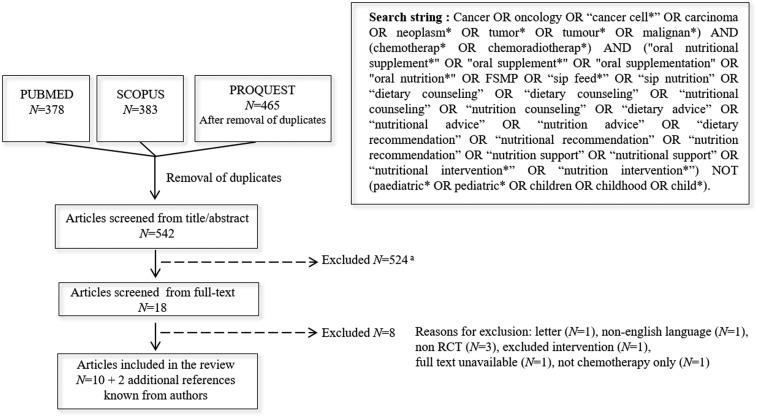
The search was carried out on 22 July 2016 using PubMed, Scopus and Proquest, which contains 96 databases including Medline and Embase. Databases were screened for search terms in titles and abstracts, referring to cancer, chemo(radio)therapy and different types of oral nutritional intervention. The search string is described in Figure 1. The pre-specified inclusion and exclusion criteria are outlined in Table 1.
Figure 1.
Search string and flow chart of screening and study selection areasons for exclusion from title/abstract included review articles, study not in cancer patients, not intervention study, not adult patients, inadequate intervention, not chemo(radio)therapy only and non RCT.
Table 1.
Summary of inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Publications |
|
|
Two reviewers (HB and MdvdS) independently carried out the selection of articles for inclusion, as described (Figure 1). Titles and abstracts of all records were screened and eligible publications were retrieved in full. Hand searching of reference lists of relevant studies and reviews was used to identify additional articles. Differences in judgment during the selection process were settled by discussion and consensus.
The methodological quality of the studies was assessed independently by two authors (AL and JA) using the Jadad scoring system [25]. Differences in judgment during the evaluation of study quality were settled by consensus.
Data extraction
Data were extracted for the following outcomes: nutritional intake, compliance, body weight (BW) response, lean body mass (LBM), QoL, circulating C-reactive protein (CRP) levels, chemotherapy toxicities, treatment delays or survival. Some raw data for BW were retrieved directly from investigators [26–28].
Meta-analysis
Meta-analysis of BW response to nutritional intervention was carried out on data from nine studies. Data were not available to perform meta-analysis on other outcomes. Data from Evans et al. [29] could not be included in the meta-analysis due to missing baseline BW and standard deviation (SD) for BW response. To avoid overlap between groups due to methodological design in Baldwin et al. [30], we included only the comparison between DC and no DC. Mean and SD values for BW response in Guarcello et al. [31] were calculated from published data on the median (range) according to the method described by Hozo et al. for small sample sizes [32]. Subsequently, results for BW response from baseline were calculated using a correlation coefficient of 0.96 based on other studies [26–28, 33, 34], as recommended in the Cochrane Handbook (Section 16.1.3.2) [35]. Sensitivity analyses were carried out before including these data in the meta-analysis.
Due to heterogeneity among studies, a random-effects model was fitted with mean difference (MD) as outcome using restricted maximum likelihood estimation (REML) with the SAS® software to acquire an overall estimate for the intervention effect. Due to the small number of studies involved, t-distribution was used to obtain a 95% confidence interval (CI) for the overall effect. A meta-regression was conducted to assess the effect of mean BW difference between the groups at baseline. Additionally, subgroup analyses were carried out; however, meta-regression was not possible due to the limited number of studies in the subgroups.
Results
Characteristics of included studies
Figure 1 outlines the results of the literature search and study selection. A total of 12 articles reporting the results of 11 RCT (1350 patients) were included. Included cancer types were diverse, with most studies conducted in lung (N = 3) or gastrointestinal (GI) (N = 2) cancers, or both tumor sites (N = 3). Enrolment ranged from 13 subjects (two-arm study) [28] to 358 subjects (four-arm study) [30]. Mean or median age ranged from 57 to 69 years, with the exception of Bourdel-Marchasson et al. [26] which included patients above 70 years old (mean age 78 years). Studies included both well-nourished and malnourished patients. Chemo(radio)therapy regimens varied according to cancer type, treatment intention and year of publication (Table 2). Duration of intervention ranged from 4 weeks to 6 months, with one study conducted over 12 months.
Table 2.
Summary of included studies
| Reference | Jadad score | N | Age (median range) or (mean±SD) | Tumor type | Chemotherapy treatment | Nutritional status at inclusion | % patients (at risk of) malnutrition | Interventiona | Control | Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Elkort [36] | 1 | 47 | NR | Breast | 5-FU+Cytoxan+ methotrexate or l-PAM+5-FU | Well-nourished N=24 and moderately malnourished N=2, severely malnourished excluded (reported for N=26 completers) | 8 | High-energy ONS | Routine care | 12 months |
| Evans [29] | 1 | 180 | 59 (33–78) (CRC) and 61 (37–78) (NSCLC) | CRC- NSCLC | 5-FU+ methotrexate for CRC; vindesine+ cisplatin for NSCLC | Malnourished based on >5% BW loss: 39% (CRC) and 43% (NSCLC) | 39 (CRC) – 43 (NSCLC) | DC+high- energy ONS | Routine care | 12 weeks |
| Ovesen [39] | 3 | 137 | 58 (22–80) Ctr and 59 (29–80) Int | Lung ovary breast | Diverse | Half of patients had >5% BW loss in previous 3 mo | 50 | DC+ high-energy ONS if required | Routine care | 5 months |
| Breitkreutz [37] | 1 | 23 | 60.6±3.1 Ctr and 57.8±1.3 Int | GI | 5-FU and leucovorin | Inclusion criteria: moderate malnutrition, based on 3 out of 5 criteria (BW <90% of IBW or WL ≥10%/6 mo; triceps skinfold <90% of ideal; arm muscle circumference <90% of ideal; creatinine index <80% of ideal; serum albumin <35 g/l) | 100 | DC+ high-energy ONS | DC | 8 weeks |
| Baldwin [30] | 3 | 358 | 66.8 (24–88) | Mostly GI some NSCLC | Diverse single agents and combination- Palliative intent | Inclusion criterion: any weight loss in previous 3 mo. Mean % BW loss was 11.2±6.4 (GI) and 9.8±6 (lung) | 100 | DC and/or high- energy ONS | Routine care | 6 weeks |
| Bourdel- Marchasson [26] | 3 | 343 | 78±4.9 | Any | Diverse single agents and combination | Inclusion criterion: at risk of malnutrition according to MNA. MNA 20.4±2.1 (Ctr) and 20.1±2 (Int), Malnourished patients (MNA<17) were excluded. % BW loss was 8.6±7.9 (Ctr) and 8.9±6.6 (Int) | 100 | DC+high-energy ONS if required | Routine care | 3–6 months |
| Reference | Jadad score | N | Age (median range) or (mean± SD) | Tumor type | Chemo(radio)therapy treatment | Nutritional status at inclusion | % patients (at risk of) malnutrition | Interventionb | Control | Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Guarcello [31] | 2 | 46 | 68.7 (51–79) Ctr and 65.6 (43–79) Int | Lung stage IIIb–IV | Not reported | Inclusion criterion: malnutrition, BW loss >10% in previous 6 mo. % BW loss was 11.8 (4.3-18) (Ctr) and 12.9 (3.8-32.8) (Int) | 100 | High-protein, n-3 PUFA- enriched ONS | Isocaloric isonitrogeneous ONS | 8.5 weeks |
| Trabal [28] | 1 | 13 | 68.2±15.6 Ctr and 61.5±15.8 Int | CRC stage IV | FOLFOX or capecitabin | Two patients at risk of malnutrition, exclusion of severely malnourished patients (according to SGA scores). % BW loss was 3.1±3.3 (Ctr) and 8.1±6.9 (Int) | 15 | High-protein, n-3 PUFA- enriched ONS+DC | DC | 12 weeks |
| Van der Meij [27, 33] | 5 | 42 | 57.2±8.1 Ctr and 58.4±12 Int | NSCLC stage III | Cisplatin+ docetaxel or cisplatin +bevacizumab with concurrent thoracic radiotherapy | % BW loss in past mo: 1.5±4.7 (Ctr) and 0.3±2.4 (Int) 20% patients were considered malnourished based on BMI <18.5 and/or BW loss >5% in previous mo and/or BW loss >10% in previous 6 mo | 20 | High-protein, n-3 PUFA- enriched ONS | Isocaloric ONS | 5 weeks |
| Sanchez-Lara [34] | 3 | 92 | 61±12.4 Ctr and 58.8±14 Int | NSCLC stage III–IV | Paclitaxel+cisplatin/ carboplatin | % BW loss was 7.1±9 (Ctr) and 8.8±8 (Int) Malnutrition based on SGA: none (N=48), moderate (N=21), severe (N=23) | 48 | High-protein, n-3 PUFA- enriched ONS | Isocaloric diets | 6–8 weeks |
| Pastore [38] | 3 | 69 | 63.5±11.8 | GI–lung | Not reported | Malnutrition based on SGA: none (N=9), moderate (N=48), severe (N=12) | 87 | High-protein, n-3 PUFA- enriched ONS | Isocaloric ONS | 4 weeks |
Intervention nutritional goals are detailed in Table 3.
Intervention aims to provide about 590–600 kcal, 32–33 g protein and 2–2.2 g EPA per day.
CRC, colorectal cancer; Ctr, control group; DC, dietary counseling; 5-FU, 5-fluorouracil; FOLFOX, folinic acid, 5-fluorouracil and oxaliplatin; GI, gastrointestinal; (I)BW, (initial) body weight; Int, intervention group; l-PAM, Melphalan; MNA, mini nutritional assessment; mo, month; NSCLC, non-small-cell lung carcinoma; ONS, oral nutritional supplement; SGA, subjective global assessment.
Assessment of study methodological quality with the Jadad scale showed that quality was overall low, with only one study scoring the maximum five points [27]. The main limitation was the absence of blinding in most studies. The method of randomization was also inadequate or insufficiently described in five studies [28, 29, 31, 36, 37].
In six studies, the objective of the intervention was to increase energy (and protein) with DC and/or high-energy ONS, most often compared with routine care [26, 29, 30, 36, 37, 39]. Nutritional goals were heterogeneous among studies, as reported in Table 3. In two studies, some patients received ONS in addition to DC, based on study staff assessment of patient needs [26, 39]. Routine care was not always clearly defined, and was most probably heterogeneous among centers. In one trial, both groups had similar target goals and received nutritional counseling, however only the intervention group received ONS [37]. Five other studies investigated the effect of ONS enriched with protein and n-3 PUFA, providing 590–600 kcal, 32–33 g protein and 2–2.2 g of eicosapentaenoic acid (EPA) per day, usually compared with an isocaloric control [27, 28, 31, 33, 34, 38]. In these studies, there was some heterogeneity in control groups; in one RCT patients received DC only [28], and another RCT included an isocaloric, isonitrogeneous control [31].
Table 3.
Summary table of nutritional goals, achieved intakes and compliance data for studies conducted with DC and/or high-energy ONS
| Values in parentheses were calculated from published dataa |
Energy intake |
Protein intake |
Information on compliance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Summary intervention | Intake at baseline | Study goal | Achieved intake after intervention | Intake at baseline | Study goal | Achieved intake after intervention | Compliance | Consumption of ONS |
| Elkort [36] | high-energy ONS | NR | Meet requirements according to RDA 1974 | NR | NR | Meet/exceed requirements according to RDA 1974 | NR | Compliance records were monitored and home visits were planned as needed, but no record of actual compliance | NR |
| Evans [29] | DC+high- energy ONS | NR |
|
|
NR |
|
NR | Tube feeding was indicated for 68% of patients but provided to only 6% (high refusal rate) | NR |
| Ovesen [39] | DC+high- energy ONS if required |
|
1.5–1.7×BEE (2103–2383 kcal/day) |
|
|
|
|
Achieved intake was close to targets, but remained lower i.e. 1.5 × BEE and 0.9 g/kg protein | 21% of patients |
| Breitkreutz [37] | high-energy ONS | NR |
|
|
NR |
|
NR | NR | NR |
| Baldwin [30] | DC and/or high-energy ONS | NR |
|
NR | NR | – | NR | Not enough food diaries returned to be analyzed. Compliance to ONS fell after one week | At the end of intervention, 19% of patients reported to consume all prescribed ONS |
| Bourdel Marchasson [26] | DC+high- energy ONS if required |
|
|
|
|
|
|
At visit 2, energy goals were reached by 40% and protein goals by 47% of patients | Up to 25% of patients |
In order to provide a better visualization beyond the heterogeneity in the type of information available, values in parentheses were calculated from available published data. Calculations were made for a ‘standard person’ with a body weight of 70 kg and a height of 1.70 m.
ONS, oral nutritional supplement; NR, not reported; RDA, recommended daily amount; DC, dietary counseling; TCI, total caloric intake; BEE, basal energy expenditure.
Effect of nutritional intervention on study outcomes
Outcomes relating to nutritional status (e.g. dietary intakes, BW response, body composition) were examined in addition to clinical outcomes (QoL, treatment-related toxicity, treatment response and survival). Since compliance with the study protocol, and thus achievement of the study goals for nutritional intake, has a major impact on trial outcomes this factor is considered first below. It is important to note that clinical outcomes were most often secondary or exploratory end points.
Dietary intake and compliance
In RCT conducted with DC and/or high-energy ONS achieved energy intake was reported in four studies [26, 29, 37, 39] with a significant increase in energy intake compared with controls reported in three studies [26, 29, 39]. Target dietary intakes, and the methods used to determine these goals, were heterogeneous among studies which hampered efforts to establish if subjects had actually fully achieved study targets. To make comparisons clearer we calculated target and actual intakes based on the available published data and using an illustrative example of a ‘standard’ person (BW 70 kg, height 1.70 m) (Table 3). In studies by Ovesen et al. [39], Bourdel Marchasson et al. [26] and Breitkreutz et al. [37], achieved intakes (1700–2000 kcal) fell short of target intake goals (2100–2800 kcal), and therefore energy requirements were not met by the intervention. In Evans et al. [29], patients met 90% of the target intake. Data on protein intake were not generally reported but in two studies a significant increase in protein intake compared with controls was found, although not sufficient to reach protein intake targets [26, 39]. Compliance was described in five RCT conducted with DC and/or high-energy ONS and was generally poor (Table 3). For example, Baldwin et al. [30] reported that compliance fell after 1 week, and at the end of intervention only 19% of subjects reported consuming all of the prescribed ONS.
The five RCT conducted with high-protein, n-3 PUFA-enriched ONS aimed to provide two or three cans per day and reported compliance to the recommended ONS dose. In Guarcello et al. [31], non-compliant patients were excluded (20%) and therefore all remaining subjects (80%) consumed the prescribed dose. Sanchez Lara et al. [34] and Trabal et al. [28] reported good compliance of 70% and 80%, respectively. Compliance was suboptimal in the study by Van der Meij et al. [27], where patients in the intervention arm took only half of the prescribed dose. In Pastore et al. [38] compliance was poor, 36% of patients in the intervention group and 14% patients in the control group stopped taking the ONS. Three of these RCT reported achieved dietary intake [27, 31, 34]. In Sanchez Lara et al. [34], achieved intakes were 2195 kcal and 88 g protein/day after intervention, significantly higher compared with controls receiving isocaloric menus based on usual food [34]. Van der Meij et al. [27] reported significant increases in intakes compared with controls at some but not all timepoints, with achieved intakes of 1827 kcal and 77 g protein/day at the end of the intervention. Compliant subjects in the intervention group in Guarcello et al. [31] reached intakes of 2000 kcal and 60 g protein/day, significantly increased compared with baseline.
BW response
All six of the RCT conducted with DC and/or high-energy ONS reported data for BW response [26, 29, 30, 36, 37, 39]. Only one study reported significant improvement in BW [37]. Despite an increase in energy and/or protein intake, this did not translate into consistent improvements in BW, most likely due to poor compliance and failure to meet study goals for nutritional intake as described above. In four RCT the effect of high-protein, n-3 PUFA-enriched ONS on BW response was reported [27, 28, 31, 34]. In three studies significant improvements in BW were reported compared with an isocaloric control [27, 28, 34]. In the fourth study by Guarcello et al. [31] in malnourished patients, compliant subjects in the intervention group showed significant increases in BW compared with baseline.
A meta-analysis was carried out on BW response data from nine studies. Most included trials were based on modified intention to treat populations while one study was clearly labeled as a per protocol analysis [31]. Strict intention to treat analysis, with imputation for missing data, was carried out in only one study [30]. Due to the heterogeneity in approaches, and the limited number of studies, we chose to include all available data in our analyses, and to also report analyses after exclusion of the study reporting per-protocol data only.
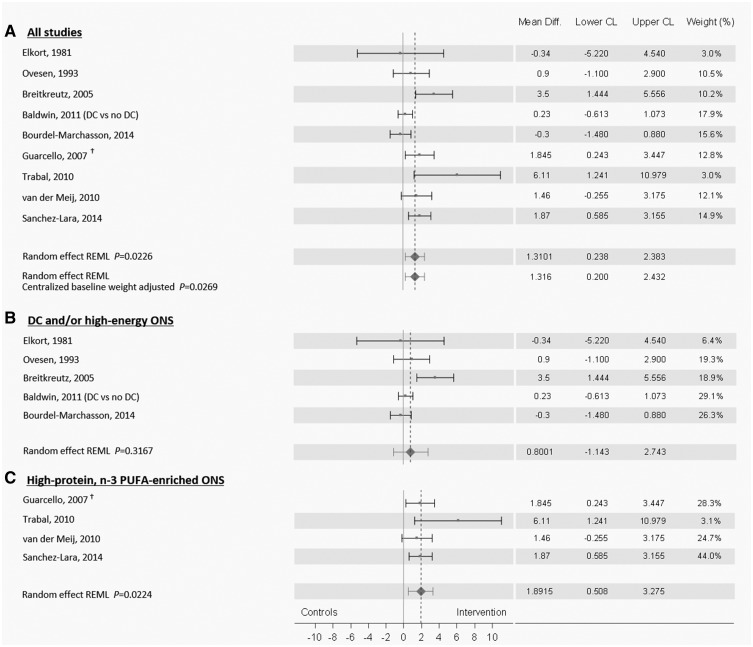
Results show that oral nutritional intervention led to significant improvement in BW compared with controls (MD 1.31 kg, 95% CI 0.24–2.38, P = 0.02) (Figure 2A). The meta-regression did not show an effect of baseline weight differences between groups. Heterogeneity was present in this analysis (Q = 21.1, P = 0.007), however, CI of most of the studies overlap to some degree. In addition, the weight contribution of studies suggests that the overall conclusion was not driven by only a few dominant studies. Exclusion of the study reporting only per-protocol data [31] from the meta-analysis led to an MD of 1.26 kg, 95% CI 0.01–2.52, P = 0.049 with heterogeneity Q = 19.7, P = 0.006. Subgroup meta-analysis of studies conducted with DC including high-energy ONS for some patients (N = 3) [26, 30, 39] or with high-energy ONS alone (N = 2) [36, 37] showed no significant impact of the intervention (MD 0.80 kg, 95% CI −1.14 to 2.74, P = 0.32) (Figure 2B). Heterogeneity within studies was confirmed by the analysis (heterogeneity Q = 10.5, P = 0.03). Second subgroup meta-analysis of data on BW response showed that intervention with high-protein, n-3 PUFA-enriched ONS led to significant improvement in BW compared with isocaloric controls (MD 1.89 kg, 95% CI 0.50–3.27, P = 0.022; heterogeneity Q = 3.1 P = 0.37) (Figure 2C). Exclusion of the study reporting only per-protocol data [31] from the subgroup meta-analysis led to a MD of 1.91 kg, 95% CI −0.30 to 4.12, P = 0.065, with heterogeneity Q = 3.1 P = 0.21).
Figure 2.
Meta-analysis of the effects of oral nutritional intervention on body weight (BW) response. A random effect model was run on mean difference of BW response data from studies investigating the effect of oral nutritional intervention (A). Subgroup analysis were subsequently carried out for studies conducted with DC and ONS when required [26, 41, 30] (N = 3) or with high-energy ONS (N = 2) [36, 37] (B) and for studies conducted with high-protein, n-3 PUFA-enriched ONS (N = 4) [27, 28, 31, 34] (C). †Guarcello et al. [31] reported only per-protocol data. When excluding this study from the meta-analysis, results were MD 1.26 kg, 95% CI 0.01–2.52, P = 0.049 (A) and MD 1.91 kg, 95% CI −0.30 to 4.11, P = 0.065 (C). Limitations in the meta-analysis pertain to the limited amount of data available. Publication bias could not be assessed as there were not enough studies available to perform a funnel plot. In addition, there is heterogeneity in analyses A and B, and we cannot rule out the impact of the variety of cancer types and stages, nutritional status, length of intervention and variations in the intervention per se.
Body composition
Two studies conducted with DC and/or high-energy ONS reported body composition parameters. There was no effect of the intervention on fat free mass (FFM) in Ovesen et al. [39], whereas Breitkreutz et al. [37] observed a significant benefit of the intervention on FFM compared with controls (P < 0.05) using bioelectrical impedance analysis (BIA).
Body composition was reported using BIA in two RCT with high-protein n-3 PUFA-enriched ONS. Van der Meij et al. [27] found that FFM fell less in the intervention group over the course of study than in the control group, with a difference between groups of 1.9 kg (P = 0.02). Sanchez-Lara et al. [34] showed a large difference in baseline LBM between groups, however there was a mean gain of 1.6 kg in the intervention group versus a mean loss of 2 kg in controls (P = 0.01).
Quality of life
QoL was measured using a variety of tools [e.g. European Organization for Research and Treatment of Cancer (EORTC) C-30, Functional Assessment of Cancer Therapy (FAACT) [30], the Linear Analog Self-Assessment (LASA) [37] and ‘QoL Index’ [39]].
In three RCT with DC and/or high-energy ONS reporting QoL, no significant effect of the intervention was observed on QoL scores compared with controls [30, 37, 39].
QoL was measured in four RCT conducted with high-protein, n-3 PUFA-enriched ONS using the EORTC C-30 questionnaire [28, 31, 33, 34]. Van der Meij et al. [33] reported significantly better Global Health Score (GHS) (P = 0.04) and pre-defined domains for cognitive (P < 0.01), social (P = 0.04) and physical (P < 0.01) functions in the intervention group compared with controls. Sanchez-Lara et al. [34] reported a significant improvement in GHS from baseline only in intervention group (P = 0.02), but difference from controls was not significant (P = 0.13). In two studies, intervention had no significant effect on GHS [28, 31]. Some significant improvements in pre-defined domains and individual symptoms were also reported but these should be interpreted with caution [28, 31, 34].
Inflammation markers
Effects of high-protein, n-3 PUFA-enriched ONS on circulating levels of CRP were reported in four RCT [27, 31, 34, 38]. Serum CRP levels significantly decreased compared with baseline in a study in lung cancer patients (P < 0.05) [31] and a further study in NSCLC patients (P = 0.02), while no change was observed in the control groups in either study [34]. Others trials did not report a significant impact of the intervention [27, 38]. In the study by Van der Meij et al. [27], CRP levels were negatively correlated with plasma phospholipid EPA levels in patients in whom the intervention had led to >1.5% increase in circulating EPA levels.
Tumor response
Tumor response was reported in five RCT with DC and/or high-energy ONS, none of which reported a statistically significant effect of intervention on rates of complete remission, partial remission, stabilization, recurrence or progression of disease compared with controls [26, 29, 36, 37, 39].
One RCT investigated the effect of high protein, n-3 PUFA-enriched ONS on tumor response and showed no significant difference in response rate compared with an isocaloric control [34].
Treatment-related toxicity
The effect of nutritional intervention on treatment-related toxicity was reported on various types of outcomes in studies conducted with DC and/or high-energy ONS (N = 3) or high-protein, n-3 PUFA-enriched ONS (N = 3).
Evans et al. [29] showed no effect of the intervention with DC and/or high-energy ONS on frequency and magnitude of treatment delays, or on the degree of toxicities, and Elkort et al. [36] reported no difference between groups in GI and hematological toxicities. Bourdel-Marchasson et al. [26] showed that patients receiving DC, including high-energy ONS when required, had less grade three to four infections compared with control patients receiving routine care (4% versus 10%, respectively; P = 0.03). Results indicated no differences in hospitalization rate and no change in chemotherapy protocol or rate of falls, pressure ulcers or fractures [26].
Studies with high-protein, n-3 PUFA-enriched ONS did not show a significant effect of intervention on chemotherapy delays [28, 33], dose reduction [33], hospital admissions [33] or biochemical and hematological toxicities [34].
Survival
Four RCT conducted with DC and/or high-energy ONS reported survival and did not observe a significant effect of nutritional intervention on overall survival [29, 39] and 1- and 2-year survival [26, 30].
Sanchez-Lara et al. [34] reported no impact of high-protein, n-3 PUFA-enriched ONS on overall survival. After adjustment for multiple baseline parameters such as stage or gender, high-protein, n-3 PUFA-enriched ONS was independently associated with better progression free survival (P = 0.05) [34].
Discussion
In this systematic review of RCT, we assessed the nutritional and clinical effectiveness of all first line, volitional oral nutritional interventions, including DC, high-energy ONS, or high-protein, n-3 PUFA-enriched ONS in cancer patients undergoing chemo(radio)therapy.
Effectiveness of nutritional intervention on nutritional outcomes
The pathogenesis of cancer cachexia is complex and multifactorial, but reduced food intake almost invariably contributes to progressive wasting. Filling the gap between recommended and actual food intake, which is the first aim of oral nutritional interventions, remains a key step in the prevention and treatment of cachexia. Our meta-analysis shows that oral nutritional intervention has an overall significant beneficial impact on BW response during chemo(radio)therapy, however, because heterogeneity was present in the analysis, subgroup analyses were subsequently carried out for this outcome. A subgroup meta-analysis with studies conducted with DC and/or high-energy ONS reported no significant positive effect on BW response. In this group of studies, the difficulty in reaching energy requirements may partially explain the absence of significant effect on BW, as reported achieved intakes (1700–2000 kcal) usually fell short of target intake goals (2100–2800 kcal) due to poor compliance. It may be assumed that similarly, protein goals were not reached in these studies, though specific data for this outcome were unavailable. Therefore, outcomes in these studies might have been impacted by low compliance to the nutritional intervention. In addition, the heterogeneity of nutritional goals may reflect the difficulty in setting adequate and realistic targets at various stages of the clinical journey. As a consequence, it seems likely that weight gain could only be achieved in studies with appropriate target intake and high compliance. In this regard, further research may investigate the effectiveness for cancer patients of tight caloric control, as piloted in the TiCaCo trial [40], a paradigm of intensive individualized dietary intervention which includes close monitoring of intake versus expenditure, with options for DC, ONS and escalation to artificial nutrition support.
Intervention with high-energy ONS containing high protein levels and n-3 PUFA led to improvements in BW response and muscle mass during chemotherapy compared with isocaloric controls (isocaloric ONS/diets). In addition to adversely impacting dietary intake, disease and treatment may also cause metabolic alterations, such as systemic inflammation that leads to increased protein turnover and catabolism, and will accelerate loss of muscle mass. Loss of muscle is a major component of weight loss in malnourished cancer patients and is known to be an independent prognostic factor for toxicities [41–45] or tumor progression [43] during chemotherapy. Cachexia is distinct from starvation, and thus adequate nutritional intake alone may not be sufficient to prevent the worsening of nutritional status in cancer patients [46, 47], due to tumor-induced metabolic perturbations. In light of this, our systematic review and meta-analysis shows that cancer-associated cachexia appears to be better prevented and treated when formulations minimize the caloric and protein gaps but also include increased amounts of high quality proteins and/or nutrients aimed at modulating the inflammatory response. These reported benefits of ONS enriched in protein and n-3 PUFA on BW response and muscle mass support the recommendations of recent ESPEN guidelines, which highlight the increased protein needs (1–1.5 g/kg/day) in cancer patients, and the benefit of n-3 PUFA during chemotherapy [19]. Supplying more protein is aimed at overcoming anabolic resistance; specific benefits of n-3 PUFA supplementation are likely to be mediated by modulation of systemic inflammation, as described in recent reviews [24, 48, 49].
Effectiveness of nutritional intervention on clinical outcomes
Improvements in clinically relevant outcomes such as QoL, treatment-related toxicity and survival are key targets for nutritional intervention. High-protein, n-3 PUFA-enriched ONS were reported to improve some aspects of QoL, but evidence remains limited. Studies did not show significant difference between groups in tumor response and overall survival, although Sanchez Lara et al. [34] reported benefit on disease free survival. Due to the paucity of the data and heterogeneity of the outcomes reported, it is not yet possible to reach conclusion about the effect on treatment-related toxicities. A more recent trial of n-3 PUFA-enriched ONS in patients with GI cancer receiving chemotherapy showed improved nutritional and clinical outcomes [50]. However, the specific roles of n-3 PUFA, proteins, and calories remain uncertain since the trial did not include an isocaloric, isonitrogeneous comparator.
Factors impacting outcomes of nutritional intervention trials
It is not intuitively obvious as to why nutritional intervention would lack other clinical benefits in populations of patients whose dietary intake is known to be severely compromised, and some key points need to be considered that can impact outcome of nutritional intervention studies.
The duration of nutritional intervention ranged from 4 to 12 weeks in most studies. A significant impact on clinical outcome may not be achievable in trials where the intervention is given for a short time or a much shorter duration than that of the anticancer treatment. For example, in a treatment plan with 4 monthly cycles of chemotherapy, an average daily deficit of 400 kcal adds up to a total cumulative deficit of 48 000 kcal which may result in at least 7 kg BW loss [51]. If, owing to low compliance and/or a short period of intervention, the majority of this deficit fails to be addressed, clinical benefits cannot be attained. In addition, a recent retrospective analysis of esophageal cancer patients receiving chemo(radio)therapy showed that nutritional intervention may improve survival when initiated before chemotherapy, suggesting the importance of early assessment and initiation of nutritional support [52].
The most widely accepted definition of cancer cachexia acknowledges the presence of different stages from pre-cachexia to refractory cachexia with severely malnourished cancer patients less likely to respond to nutritional intervention than those at nutrition risk [18]. When reported, severity of malnutrition was heterogenous within and across studies. Progression of cancer-related malnutrition over time also varies depending on the type and stage of cancer and tolerance to treatment. The impact of nutritional status at baseline on the efficacy of nutritional intervention during chemotherapy could not be assessed in isolation in this review and should be further examined in future trials.
Malnutrition in patients with advanced cancer is frequently, but not always, associated with chronic cancer-induced systemic inflammation resulting in anorexia, insulin resistance, anabolic resistance and muscle loss [20]. These metabolic derangements will interfere with nutritional interventions and may limit the effect of feeding in this subgroup of patients. None of the analyzed trials selected or stratified for inflammatory response syndrome, thus no data on differential treatment effects were available, neither for standard interventions nor for interventions including anti-inflammatory ingredients. These aspects should be included in the design of future trials.
Reaching caloric and protein targets is clearly difficult during chemotherapy, even in the protected arena of a clinical trial. Ways to improve compliance to the nutritional intervention might be explored in further studies. For example, flexible nutritional intervention goals during the course of chemotherapy could allow for lower intake during treatment days, and higher nutritional goals to catch up between cycles. In addition, compliance might be improved by focusing on patient education and awareness, or strategies to support patient convenience, such as more concentrated ONS or improved palatability of supplements, as sensory and taste challenges are often experienced during chemotherapy [53, 54]. Anabolic (e.g. drugs, exercise and specific nutrients) and anticatabolic (e.g. anti-inflammatory) therapies could also potentiate effects of nutritional intervention. Nutritional support as a stand-alone intervention is possibly not optimal and patients would benefit more from a multimodal approach also including management of symptoms such as pain, GI symptoms or psychological distress [55]. The ENABLE III study confirms that early integration of supportive and palliative care, which includes among other approaches psychological support and nutrition intervention, with anticancer treatments reduces morbidity and mortality [56].
Poor study design rather than no effect of the intervention per se may also contribute to the inability to evaluate the effect of nutrition intervention on treatment-related outcomes. In this review, only one trial was double-blinded; however, blinding of subjects and care-givers to different modes of nutrition intervention in many cases is very difficult or even impossible. Randomization was problematic in many of the studies and patients enrolled in the control arms of the trials of DC and/or high-energy ONS received ‘routine care’, the heterogeneity of which across cancer centers worldwide is well known. There was also heterogeneity in terms of study population (cancer type and baseline nutritional status), duration of intervention and intervention strategy. Finally, failure to consider clinical outcomes as primary study parameters led to inadequate powering and stratification to detect changes.
This review offers insights into the limitations of the RCT that have been conducted on this topic and highlights the urgent need for further well-designed trials. Many of these insights should be considered in future trial design and we outline recommendations accordingly.
Recommendations for study design
| 1. Study design |
|
| 2. Study population |
|
| 3. Duration of trial |
|
| 4. Interventions |
|
| 5. Compliance | Report compliance to nutritional intervention by:
|
| 6. Outcome | Consider the following outcome parameters and ensure that the trial is carefully powered for the chosen primary outcome:
|
| 7. Analysis | Use intention-to-treat analysis and then perform subgroup analyses based on pre-trial weight loss, inflammation status and compliance to nutritional intervention. |
Conclusion
Although solid data suggest that malnutrition is a prognostic factor for poor clinical outcome during chemo(radio)therapy, evidence supporting nutritional intervention during oncological treatment remains limited. The overall positive effect of nutritional interventions during chemo(radio)therapy on BW was mostly driven by high-protein n-3 PUFA-enriched ONS, suggesting the benefit of targeting metabolic alterations while supporting energy and protein intakes. Cumulative caloric deficits remained despite interventions and likely hampered the effect of DC and/or high-energy ONS on BW. Contributing causes to shortfalls of intake require study and these may be intrinsic (i.e. anorexia, altered hedonic inputs), chemotherapy-related or due to insufficient quality or quantity of nutritional support. There is a pressing need for well-designed RCT to investigate the impact of nutritional intervention on treatment toxicities or survival, and we provide recommendations for design of such trials. Optimal timing and duration of nutritional intervention, and strategies to improve compliance to nutritional support should be further explored. In addition, future research should investigate the effect of nutritional intervention as part of a multimodal care approach with special emphasis on combining interventions with anti-inflammatory and anabolic components.
Acknowledgements
The authors thank Fionna Page for editorial support and Dr Aysun Yavuz-Cetinyurek for performing the meta-analysis. The authors are also grateful to Drs van der Meij, Bourdel-Marchasson, Trabal and Pastore for sharing additional data.
Funding
This work was supported by Danone Nutricia Research who provided editorial support for this review (no grant number applies).
Disclosure
MdvdS received honoraria for independent lectures at educational and scientific events organized by Abbott, Fresenius Kabi, Nutricia. AL received honoraria for independent lectures at educational and scientific events organized by Abbott, Baxter, Fresenius Kabi, Nutricia, Smartfish. AL has received research funding from Nestlé Health Science and from Fresenius Kabi. HB and MJ are employees of Danone Nutricia Research. JA has received honoraria for independent lectures at educational and scientific events organized by Baxter, B. Braun, Falk, Fresenius Kabi and Nutricia. VEB has declared no conflict of interest.
References
- 1. Nitenberg G, Raynard B.. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol/Hematol 2000; 34(3): 137–168. [DOI] [PubMed] [Google Scholar]
- 2. Laviano A, Meguid MM.. Nutritional issues in cancer management. Nutrition 1996; 12(5): 358–371. [DOI] [PubMed] [Google Scholar]
- 3. Hébuterne X, Lemarié E, Michallet M. et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014; 38(2): 196–204. [DOI] [PubMed] [Google Scholar]
- 4. Gyan E, Raynard B, Durand JP. et al. Malnutrition in patients with cancer. JPEN J Parenter Enteral Nutr 2017; 148607116688881 [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Arends J.. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 2005; 9: S51–S63. [DOI] [PubMed] [Google Scholar]
- 6. Andreyev HJ, Norman AR, Oates J, Cunningham D.. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998; 34(4): 503–509. [DOI] [PubMed] [Google Scholar]
- 7. DeWys WD, Begg C, Lavin PT. et al. ; Eastern Cooperative Oncology Group Prognostic effect of weight loss prior to chemotherapy in cancer patients. AmJ Med 1980; 69(4): 491–497. [DOI] [PubMed] [Google Scholar]
- 8. Persson C, Glimelius B.. The relevance of weight loss for survival and quality of life in patients with advanced gastrointestinal cancer treated with palliative chemotherapy. Anticancer Res 2002; 22(6B): 3661–3668. [PubMed] [Google Scholar]
- 9. Di Fiore A, Lecleire S, Gangloff A. et al. Impact of nutritional parameter variations during definitive chemoradiotherapy in locally advanced oesophageal cancer. Dig Liver Dis 2014; 46(3): 270–275. [DOI] [PubMed] [Google Scholar]
- 10. Sánchez-Lara K, Turcott JG, Juárez E. et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer 2012; 64(4): 526–534. [DOI] [PubMed] [Google Scholar]
- 11. Clavier JB, Antoni D, Atlani D. et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 2014; 27(6): 560–567. [DOI] [PubMed] [Google Scholar]
- 12. Topkan E, Parlak C, Selek U.. Impact of weight change during the course of concurrent chemoradiation therapy on outcomes in stage IIIB non-small cell lung cancer patients: retrospective analysis of 425 patients. Int J Radiat Oncol Biol Phys 2013; 87(4): 697–704. [DOI] [PubMed] [Google Scholar]
- 13. Vashi PG, Gupta D, Lammersfeld CA. et al. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutr J 2013; 12(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buskermolen S, Langius JA, Kruizenga HM. et al. Weight loss of 5% or more predicts loss of fat-free mass during palliative chemotherapy in patients with advanced cancer: a pilot study. Nutr Cancer 2012; 64(6): 826–832. [DOI] [PubMed] [Google Scholar]
- 15. Caillet P, Liuu E, Raynaud Simon A. et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr 2017; 36(6): 1473–1482. [DOI] [PubMed] [Google Scholar]
- 16. Caccialanza R, Pedrazzoli P, Cereda E. et al. Nutritional support in cancer patients: a position paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J Cancer 2016; 7(2): 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aapro M, Arends J, Bozzetti F. et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 2014; 25(8): 1492–1499. [DOI] [PubMed] [Google Scholar]
- 18. Fearon K, Strasser F, Anker SD. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12(5): 489–495. [DOI] [PubMed] [Google Scholar]
- 19. Arends J, Bachmann P, Baracos V. et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017; 36(1): 11–48. [DOI] [PubMed] [Google Scholar]
- 20. Arends J, Baracos V, Bertz H. et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 2017; 36(5): 1187–1196. [DOI] [PubMed] [Google Scholar]
- 21. Baldwin C, Spiro A, Ahern R, Emery PW.. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012; 104(5): 371–385. [DOI] [PubMed] [Google Scholar]
- 22. van der Meij BS, van Bokhorst-de van der Schueren MA, Langius JA, Brouwer IA. et al. n-3 PUFAs in cancer, surgery, and critical care: a systematic review on clinical effects, incorporation, and washout of oral or enteral compared with parenteral supplementation. Am J Clin Nutr 2011; 94(5): 1248–1265. [DOI] [PubMed] [Google Scholar]
- 23. Ries A, Trottenberg P, Elsner F. et al. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: an EPCRC cachexia guidelines project. Palliat Med 2012; 26(4): 294–304. [DOI] [PubMed] [Google Scholar]
- 24. de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL.. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: a systematic review. Clin Nutr 2015; 34(3): 359–366. [DOI] [PubMed] [Google Scholar]
- 25. Jadad AR, Moore RA, Carroll D. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 26. Bourdel-Marchasson I, Blanc-Bisson C, Doussau A. et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One 2014; 9(9): e108687.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Meij BS, Langius JA, Smit EF. et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr 2010; 140(10): 1774–1780. [DOI] [PubMed] [Google Scholar]
- 28. Trabal J, Leyes P, Forga M, Maurel J.. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutr Hosp 2010; 25(5): 736–740. [PubMed] [Google Scholar]
- 29. Evans WK, Nixon DW, Daly JM. et al. A randomized study of oral nutritional support versus ad lib nutritional intake during chemotherapy for advanced colorectal and non-small-cell lung cancer. JCO 1987; 5(1): 113–124. [DOI] [PubMed] [Google Scholar]
- 30. Baldwin C, Spiro A, McGough C. et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 2011; 24(5): 431–440. [DOI] [PubMed] [Google Scholar]
- 31. Guarcello M, Riso S, Buosi R, D'Andrea F.. EPA-enriched oral nutritional support in patients with lung cancer: effects on nutritional status and quality of life. Nutr Ther Metabol 2007; 25: 25–30. [Google Scholar]
- 32. Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Meij BS, Langius JA, Spreeuwenberg MD. et al. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr 2012; 66(3): 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sánchez-Lara K, Turcott JG, Juárez-Hernández E. et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014; 33(6): 1017–1023. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JPT, Green S(editors). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration [on-line], 2011; www.handbook.cochrane.org. (11 April 2018, date last accessed). [Google Scholar]
- 36. Elkort RJ, Baker FL, Vitale JJ, Cordano A.. Long-term nutritional support as an adjunct to chemotherapy for breast cancer. JPEN J Parenter Enteral Nutr 1981; 5(5): 385–390. [DOI] [PubMed] [Google Scholar]
- 37. Breitkreutz R, Tesdal K, Jentschura D. et al. Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wien Klin Wochenschr 2005; 117(19-20): 685–692. [DOI] [PubMed] [Google Scholar]
- 38. Pastore CA, Orlandi SP, Gonzalez MC.. Introduction of an omega-3 enriched oral supplementation for cancer patients close to the first chemotherapy: may it be a factor for poor compliance? Nutr Cancer 2014; 66(8): 1285–1292. [DOI] [PubMed] [Google Scholar]
- 39. Ovesen L, Allingstrup L, Hannibal J. et al. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. JCO 1993; 11(10): 2043–2049. [DOI] [PubMed] [Google Scholar]
- 40. De Waele E, Mattens S, Honore PM. et al. Nutrition therapy in cachectic cancer patients. The Tight Caloric Control (TiCaCo) pilot trial. Appetite 2015; 91: 298–301. [DOI] [PubMed] [Google Scholar]
- 41. Antoun S, Baracos VE, Birdsell L. et al. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010; 21(8): 1594–1598. [DOI] [PubMed] [Google Scholar]
- 42. Prado CM, Baracos VE, McCargar LJ. et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 2007; 13(11): 3264–3268. [DOI] [PubMed] [Google Scholar]
- 43. Prado CM, Baracos VE, McCargar LJ. et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009; 15(8): 2920–2926. [DOI] [PubMed] [Google Scholar]
- 44. Prado CM, Lieffers JR, McCargar LJ. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9(7): 629–635. [DOI] [PubMed] [Google Scholar]
- 45. Barret M, Antoun S, Dalban C. et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014; 66(4): 583–589. [DOI] [PubMed] [Google Scholar]
- 46. Cohn SH, Vartsky D, Vaswani AN. et al. Changes in body composition of cancer patients following combined nutritional support. Nutr Cancer 1982; 4(2): 107–119. [DOI] [PubMed] [Google Scholar]
- 47. Giles KH, Kubrak C, Baracos VE. et al. Recommended European Society of Parenteral and Enteral Nutrition protein and energy intakes and weight loss in patients with head and neck cancer. Head Neck 2016; 38(8): 1248–1257. [DOI] [PubMed] [Google Scholar]
- 48. Laviano A, Rianda S, Molfino A, Rossi Fanelli F.. Omega-3 fatty acids in cancer. Curr Opin Clin Nutr Metab Care 2013; 16(2): 156–161. [DOI] [PubMed] [Google Scholar]
- 49. Morland S, Martins J, Mazurak V.. n-3 polyunsaturated fatty acid supplementation during cancer chemotherapy. J Nutr Intermediary Metabolism 2016; 5: 107–116. [Google Scholar]
- 50. Shirai Y, Okugawa Y, Hishida A. et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci Rep 2017; 7: 4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wishnofsky M. Caloric equivalents of gained or lost weight. Am J Clin Nutr 1958; 6(5): 542–546. [DOI] [PubMed] [Google Scholar]
- 52. Cox S, Powell C, Carter B. et al. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: outcomes from SCOPE1. Br J Cancer 2016; 115(2): 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. IJpma I, Renken RJ, Ter Horst GJ, Reyners AK.. The palatability of oral nutritional supplements: before, during, and after chemotherapy. Support Care Cancer 2016; 24(10): 4301–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen J, Wakefield CE, Laing DG.. Smell and taste disorders resulting from cancer and chemotherapy. CPD 2016; 22(15): 2253–2263. [DOI] [PubMed] [Google Scholar]
- 55. MacDonald N, Easson AM, Mazurak VC. et al. Understanding and managing cancer cachexia. J Am Coll Surg 2003; 197(1): 143–161. [DOI] [PubMed] [Google Scholar]
- 56. Bakitas MA, Tosteson TD, Li Z. et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III Randomized Controlled Trial. JCO 2015; 33(13): 1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]