Abstract
Background
Human immunodeficiency virus (HIV)–infected pregnant women increasingly receive antiretroviral therapy (ART) to prevent mother-to-child transmission (PMTCT). Studies suggest HIV-exposed uninfected (HEU) children face higher mortality than HIV-unexposed children, but most evidence relates to the pre-ART era, breastfeeding of limited duration, and considerable maternal mortality. Maternal ART and prolonged breastfeeding while on ART may improve survival, although this has not been reliably quantified.
Methods
Individual data on 19 219 HEU children from 21 PMTCT trials/cohorts undertaken from 1995 to 2015 in Africa and Asia were pooled to estimate the association between 24-month mortality and maternal/infant factors, using random-effects Cox proportional hazards models. Adjusted attributable fractions of risks computed using the predict function in the R package “frailtypack” were used to estimate the relative contribution of risk factors to overall mortality.
Results
Cumulative incidence of death was 5.5% (95% confidence interval, 5.1–5.9) by age 24 months. Low birth weight (LBW <2500 g, adjusted hazard ratio (aHR, 2.9), no breastfeeding (aHR, 2.5), and maternal death (aHR, 11.1) were significantly associated with increased mortality. Maternal ART (aHR, 0.5) was significantly associated with lower mortality. At the population level, LBW accounted for 16.2% of 24-month mortality, never breastfeeding for 10.8%, mother not receiving ART for 45.6%, and maternal death for 4.3%; combined, these factors explained 63.6% of deaths by age 24 months.
Conclusions
Survival of HEU children could be substantially improved if public health practices provided all HIV-infected mothers with ART and supported optimal infant feeding and care for LBW neonates.
Keywords: HIV-exposed uninfected, children, infants, mortality, Asia, Africa
Pooled results from 21 studies involving more than 19 000 human immunodeficiency virus–exposed but uninfected children show that an estimated two-thirds of infant deaths are attributable to lack of antiretroviral treatment for mothers, low birth weight, never being breastfed, and mother’s death.
Antiretroviral therapy (ART) to prevent mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV) has dramatically reduced the number of HIV-infected infants [1, 2]. As more women living with HIV survive and become pregnant, the number of HIV-exposed uninfected (HEU) children continues to increase [3]. Several studies have shown that HEU children may experience worse health outcomes than HIV-unexposed uninfected (HUU) children in the same setting [4–10], although this has not been confirmed elsewhere [11–14]. Most evidence relates to the era before widespread use of ART for PMTCT and for treatment, when increased mortality in HEU children was associated with poor maternal health and lack of prolonged breastfeeding. Maternal ART for life and prolonged breastfeeding with the protection of ART could ameliorate such negative associations [15, 16], but this has not yet been reliably quantified.
By pooling available individual data on HEU children from clinical trials and observational studies, from both the pre- and post-ART era, we assessed mortality risk in HEU children in Africa and Asia and associated factors. We also estimated the relative importance of identified risk factors in mediating poor outcomes among HEU children.
METHODS
In a recent systematic review [17], we electronically searched 2 bibliographic databases, PubMed and Scopus, for articles published from 2004 to 2015 using the following keywords: HIV, Mortality, and Child or Infant, without restrictions on type or region of study, limited to English and French. Titles and abstracts were assessed; retained articles were subject to full-text reviews with identification of additional references. Additionally, we identified PMTCT trials with potential data on mortality in HEU children. A total of 29 studies were identified, and their principal investigators were contacted. One declined participation [18], 5 were unable to share data [5, 19–22] and 2 did not meet the inclusion criteria [23, 24], leaving 21 studies for the pooled 24-month mortality analysis: 16 from sub-Saharan Africa [4, 8, 25–38] and 5 from Asia [39–42]. Of these, 17 were randomized trials and 4 were observational studies conducted at different times (Supplementary Table S1), with varying sample sizes and follow-up durations (Table 1).
Table 1.
Characteristics of the Mothers and Children in Included in the Studies/Trials (N = 19219)
| Trial/Study | No. of Children | Follow-up Duration (days) | Birth Weight (<2500 g) | Ever Breastfed | Breastfeeding Duration (days) | Antenatal Maternal CD4 (cells/mm3) | Maternal Death | Child Death | Child Age at Death (days) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Q1 | Q3 | n | % | n | % | Median | Q1 | Q3 | Median | Q1 | Q3 | n | % | n | % | Median | Q1 | Q3 | |
| BAN | 2250 | 336 | 254 | 338 | 174 | 7.7 | 2250 | 100 | 169 | 167 | 196 | 439 | 330 | 582 | 9 | 0.4 | 56 | 2.5 | 190 | 61 | 283 |
| Ditrame(ANRSa) | 312 | 551 | 385 | 641 | 43 | 13.8 | 306 | 98.1 | 283 | 188 | 505 | 596 | 403 | 781 | 18 | 4.5 | 32 | 10.3 | 77 | 4 | 130 |
| Ditrame(ANRSb) | 90 | 546 | 284 | 604 | 15 | 16.7 | 89 | 98.9 | 342 | 237 | 484 | 576 | 420 | 809 | 9 | 8.7 | 16 | 17.8 | 71 | 36 | 176 |
| Ditrame Plus | 688 | 730 | 538 | 734 | 83 | 12.1 | 395 | 57.4 | 124 | 96 | 200 | 411 | 258 | 569 | 13 | 1.7 | 53 | 7.7 | 2 | 1 | 79 |
| HIVIGLOB/SWEN Uganda | 575 | 546 | 541 | 549 | 62 | 10.8 | 574 | 99.9 | 105 | 77 | 150 | 436 | 292 | 596 | 2 | 0.3 | 21 | 3.7 | 70 | 15 | 207 |
| HIVNET024 | 1457 | 377 | 340 | 401 | 139 | 9.5 | 1457 | 100 | 284 | 108 | 366 | 366 | 240 | 523 | 40 | 2.4 | 67 | 4.6 | 173 | 116 | 273 |
| Good Start | 259 | 252 | 252 | 252 | 31 | 12.0 | 259 | 52.8 | 252 | 252 | 252 | . | . | . | 13 | 2.7 | 8 | 3.1 | 74 | 56 | 123 |
| Kesho Bora | 965 | 561 | 543 | 730 | 90 | 9.3 | 723 | 74.9 | 156 | 80 | 192 | 341 | 258 | 435 | 10 | 1.0 | 66 | 6.8 | 126 | 29 | 213 |
| Mashi | 1102 | 1096 | 730 | 1461 | 80 | 7.3 | 533 | 48.3 | 176 | 119 | 182 | 370 | 244 | 517 | 31 | 2.8 | 83 | 7.5 | 75 | 12 | 224 |
| Mma Bana | 702 | 731 | 730 | 733 | 102 | 14.5 | 680 | 96.9 | 178 | 141 | 181 | 347 | 231 | 484 | 11 | 1.6 | 35 | 5.0 | 151 | 6 | 243 |
| PEP | 779 | 210 | 153 | 246 | 128 | 16.4 | 405 | 52.0 | 81 | 38 | 123 | 477 | 323 | 664 | 11 | 1.4 | 18 | 2.3 | 109 | 71 | 138 |
| PHPT-1 | 1283 | 551 | 546 | 558 | 126 | 9.8 | 0 | 0 | . | . | . | 362 | 240 | 510 | 49 | 3.8 | 3 | 0.2 | 185 | 182 | 548 |
| PHPT-2 | 1805 | 368 | 365 | 373 | 172 | 9.5 | 0 | 0 | . | . | . | 376 | 247 | 531 | 6 | 0.3 | 4 | 0.2 | 283 | 180 | 289 |
| PHPT-5 1st | 407 | 731 | 553 | 737 | 51 | 12.5 | 0 | 0 | . | . | . | 454 | 366 | 569 | 0 | 0 | 0 | 0 | . | . | . |
| PHPT-5 2nd | 310 | 189 | 184 | 207 | 62 | 20.0 | 0 | 0 | . | . | . | 361 | 250 | 489 | 0 | 0 | 1 | 0.3 | 156 | 156 | 156 |
| PROMOTE2 | 361 | 404 | 310 | 408 | 69 | 19.1 | 357 | 98.9 | 365 | 280 | 392 | 377 | 280 | 506 | 9 | 2.5 | 9 | 2.5 | 138 | 22 | 238 |
| SWEN | 628 | 366 | 364 | 368 | 177 | 28.2 | 627 | 99.8 | 101 | 98 | 182 | 472 | 324 | 667 | 8 | 1.3 | 14 | 2.2 | 46 | 27 | 188 |
| Tshipidi | 429 | 735 | 731 | 774 | 60 | 14.0 | 34 | 7.93 | 181 | 44 | 184 | 426 | 320 | 577 | 7 | 1.6 | 22 | 5.1 | 23 | 2 | 166 |
| VTS | 936 | 703 | 485 | 779 | 101 | 10.8 | 854 | 91.2 | 224 | 177 | 281 | 479 | 342 | 642 | 44 | 4.7 | 39 | 4.2 | 125 | 54 | 261 |
| ZEBS | 763 | 729 | 368 | 730 | 82 | 10.8 | 763 | 100 | 182 | 126 | 487 | 361 | 237 | 498 | 47 | 6.2 | 93 | 12.2 | 245 | 129 | 394 |
| Zvitambo | 3118 | 464 | 365 | 729 | 474 | 15.2 | 3113 | 99.8 | 456 | 365 | 553 | 423 | 279 | 593 | 134 | 4.3 | 245 | 7.9 | 82 | 38 | 201 |
Maternal antiretroviral exposure was categorized as none; single/double peripartum antiretrovirals for PMTCT; 3-drug ART for PMTCT given antenatally and postnatally until cessation of breastfeeding when breastfeeding or until delivery when exclusively formula-fed; or 3-drug ART for life, prescribed beyond breastfeeding cessation per World Health Organization (WHO) HIV treatment and prevention recommendations [43, 44]. Mothers with missing information on antiretroviral use (n = 44) were assumed to have followed the relevant study protocol [28, 35] and thus categorized into the single/double antiretroviral PMTCT. The final HIV status of each child was defined by study-specific criteria. In our analyses, each child contributed from birth to 24 months of age, with right-censoring in case of death, end of study follow-up, and loss to follow-up. We restricted analyses to HEU children with information on breastfeeding and excluded 457 children with unknown infant feeding status. Mortality rates per 100 child-years of follow-up were estimated by maternal and child characteristics. We used the Kaplan-Meier method to estimate survival curves and the log-rank test to test for differences between groups.
Associations between 24-month mortality and the following factors were assessed: residence (rural vs urban/peri-urban), sex, low birth weight (LBW; <2500 g), breastfeeding (ever/never), maternal education (none/primary vs above), maternal age at delivery (5-year categories), maternal antiretroviral exposure (fixed), and maternal vital status (time-dependent). Children known to have initiated breastfeeding but with unknown weaning date (n = 1032) were considered to have been breastfed from birth to either age 6 months per WHO feeding guidance at the time [45], study exit date, or date of mother’s death, whichever occurred first. We used random-effects Cox proportional hazards models to estimate the association between 24-month mortality and potential risk factors, accounting for heterogeneity between studies. The final multivariable model included region (Africa vs Asia) as a fixed effect and adjusted for maternal antenatal CD4 cell count (categorical) because CD4 counts and ART eligibility varied widely between studies. Data from different sites in Kesho Bora [31] and HIVNET024 [30] were treated separately. Missing data were included as a separate category to maintain sample size. We used a stepwise-descending approach for selection of variables in multivariable models, which included variables that were statistically significant in univariate analyses (at a P value < .1, except for maternal antiretroviral exposure, which was maintained in the model independent of statistical significance). In the final model, statistical significance was reached when the P value was < .05. We also analyzed the association between weaning and survival among breastfed children only (n = 13418), with breastfeeding cessation defined in a time-varying manner.
We assessed the combined effects of breastfeeding and maternal 3-drug ART (for PMTCT or for life) on mortality, classifying observation time for each HEU child into 4 categories defined by child being breastfed (yes/no) and mother being on 3-drug ART (yes/no), with breastfeeding and ART variables being time dependent. When the date of ART end was unknown, ART was assumed to have continued until the weaning date or 6 months post-partum [45], whichever came first. The association between 24-month mortality and breastfeeding/maternal 3-drug ART was assessed in multivariable analyses using Cox proportional hazards models and allowing for heterogeneity between studies/trials and adjusting for region as fixed effect and maternal antenatal CD4 cell count and birth weight (<2500 g) as categorical variables.
Finally, to investigate the relative contribution of risk factors to overall 24-month mortality in HEU children, we estimated the adjusted attributable fractions (aAFs) of risks based on our final multivariable model [46, 47]. The AF for a given factor was the number of deaths attributable to the factor divided by the total number of deaths in our population if the prevalence of other factors remained at the same level. To do this, we first obtained the total number of deaths at a given time by summing the individual predicted probabilities of survival for each child based on the predict function in the R package “frailtypack” [48], then we subtracted this number from the total population to derive the number of deaths. To estimate the number of deaths attributable to the exposure of interest, we computed the number of deaths in the population as if it was not exposed to the factor while exposures to other risk factors were unchanged. Nonexposure was simulated by setting all children to the reference category. For example, for deaths associated with LBW, all children were classified into the category of having birth weight great than 2500 g. The number of deaths attributable to a specific factor was the difference between the total number of deaths calculated previously and the number of deaths in the unexposed population. We estimated the aAFs of the identified risk factors at 6, 12, and 24 months of age and computed 95% confidence intervals (CIs) using bootstrapping [49]. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina). For the estimates of AF, we used the R package’s “frailtypack” [48] and “boot” [49] using R version 3.3.2 (R Development Core Team, 2004).
RESULTS
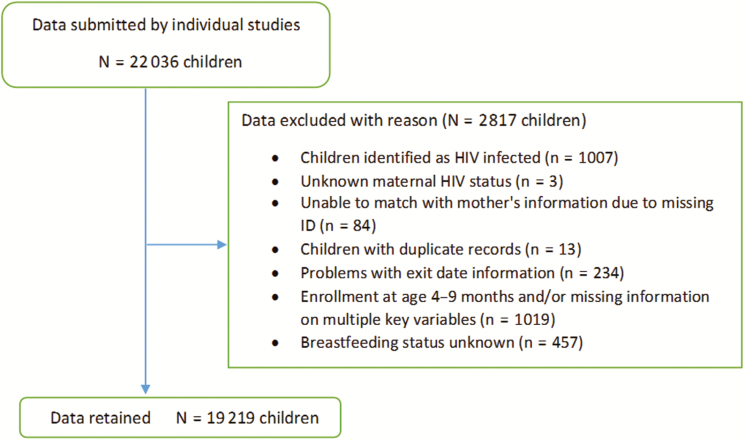
A total of 19219 HEU children were part of the analyses (Figure 1). Maternal/child baseline characteristics are shown in Table 2. Median child follow-up was 404 days (interquartile range [IQR], 336–712). More than 75% of children were born in sub-Saharan Africa, mostly Southern Africa; nearly 70% were born prior to 2005. Most children were ever breastfed (69.8%) for a median 181 days (IQR, 126–365). Maternal antiretroviral exposure varied across studies (Supplementary Table S2), reflecting the timing of the study and prevailing ART and PMTCT recommendations [44]. Overall, 23% of mothers received no antiretrovirals, 61% received mono/dual peripartum antiretrovirals for PMTCT, 12% received 3-drug ART for PMTCT, and only 4% were on ART for life. Median antenatal CD4 count was 405 cells/mm3 (IQR, 280–563); 58% of mothers had a CD4 count >350 cells/mm3 at the first antenatal visit. Median antenatal CD4 count in women who received ART for life was low at 214 cells/mm3 (IQR, 147–361). Median duration of ART was 178 (IQR, 152–196) and 443 (IQR, 371–730) days for 3-drug ART for PMTCT and ART for life, respectively. Information on maternal viral load was missing for 16%; among those with available information, median antenatal viral load was 4.0 log10 copies/mL (IQR, 3.3–4.6).
Figure 1.
Flow chart of the children included in the pooled analyses. Abbreviations: HIV, human immunodeficiency virus; ID, identification.
Table 2.
Baseline Characteristics of the Study Population (N = 19219)
| Child Characteristics | ||
|---|---|---|
| Follow-up duration (days) (median, IQR) | ||
| 404 | 336–712 | |
| Region (N, %) | ||
| East Africa | 1620 | 8.4 |
| Southern Africa | 11769 | 61.2 |
| West Africa | 1397 | 7.3 |
| South Asia | 628 | 3.3 |
| Southeast Asia | 3805 | 19.8 |
| Residence (N, %) | ||
| Rural | 1868 | 9.8 |
| Urban/Peri-urban | 17351 | 90.2 |
| Access to piped water at home (N, %) | ||
| Yes | 6182 | 32.2 |
| No | 4268 | 22.2 |
| Unknown | 8769 | 45.6 |
| Years of birth (N, %)a | ||
| 1995–1999 | 4659 | 24.2 |
| 2000–2004 | 8419 | 43.8 |
| 2005–2009 | 4852 | 25.3 |
| 2010–2014 | 1289 | 6.7 |
| Sex (N, %) | ||
| Male | 9839 | 51.2 |
| Female | 9376 | 48.8 |
| Unknown | 4 | 0.0 |
| Birth weight (N, %) | ||
| ≥2500 g | 16653 | 86.6 |
| <2500 g | 2321 | 12.1 |
| Unknown | 245 | 1.3 |
| Child ever breastfed (N, %) | ||
| Yes | 13418 | 69.8 |
| No | 5801 | 30.2 |
| Breastfeeding duration (days) (median, IQR) (N = 13418) | ||
| 181 | 126–365 | |
| Maternal characteristics | ||
| Education (N, %) | ||
| None/Primary | 8676 | 45.1 |
| Secondary or above | 9688 | 50.4 |
| Unknown | 855 | 4.5 |
| Maternal age at delivery (years) (N, %) | ||
| 15–20 | 2074 | 10.8 |
| 21–25 | 6159 | 32.1 |
| 26–30 | 5175 | 26.9 |
| 31–35 | 2545 | 13.2 |
| >35 | 1011 | 5.3 |
| Unknown | 2255 | 11.7 |
| Maternal ARVs/ART (N, %) | ||
| No ARVs | 4392 | 22.8 |
| Single or double ARVs for PMTCT | 11805 | 61.4 |
| 3-drug ART for PMTCT | 2248 | 11.7 |
| ART for life | 774 | 4.0 |
| Antenatal CD4 count above 350 cells/mm 3 (N, %) | ||
| Yes | 11129 | 57.9 |
| No | 7010 | 36.5 |
| Unknown | 1080 | 5.6 |
| Antenatal CD4 count (median, IQR) (N = 18139) | ||
| 405 | 280–563 | |
| Antenatal viral load above 5 log 10 copies/mL (N, %) | ||
| Yes | 2097 | 10.9 |
| No | 14037 | 73.0 |
| Unknown | 3085 | 16.1 |
| Antenatal viral load (log 10 copies/mL) (median, IQR) (N = 16134) | ||
| 4.0 | 3.3–4.6 | |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; IQR, interquartile range; PMTCT, prevent mother-to-child transmission.
aInformation on the date of birth was not available in HIVNET024 and ZEBS. As the enrollment into these studies took place in 2001–2003 and 2001–2004, respectively, these children were classified into the 2000–2004 category.
HEU Child Mortality
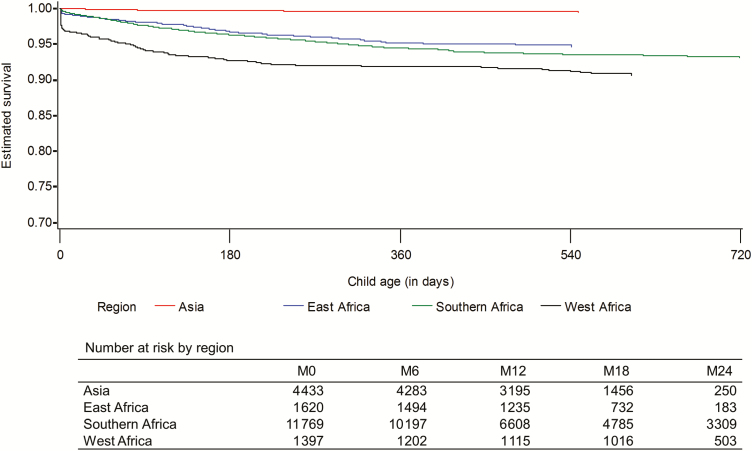
Cumulative incidence of death was 2.1% (394/18012; 95% CI, 1.9–2.3), 3.1% (575/17176; 95% CI, 2.9–3.4), 4.5% (797/12153; 95% CI, 4.2–4.8), and 5.5% (884/4245; 95% CI, 5.1–5.9) by age 3, 6, 12, and 24 months, respectively. Median age at death was 111 days (IQR, 37–244). Mortality varied from 0% in PHPT-5 1st [42] to 17.8% in Ditrame-ANRSb [26] (Table 1). Stratified by geographical region (Figure 2), 24-month survival probability was significantly higher in Asia than in Africa (P < .0001). Of the 300 children whose mothers died, 17% (n = 51) did not survive after mother’s death. Child mortality declined with increasing age at the time of mother’s death: 52% if mother died within 1 month of delivery, 36% if she died between 1 and 3 months, 20% between 3 and 6 months, 6% between 6 and 12 months, and 4% between 12 and 24 months. Mortality was highest among children with mothers not being on any antiretrovirals (6.1/100 child-years), with mortality in single/dual antiretrovirals for PMTCT, 3.1/100; 3-drug ART for PMTCT, 2.7/100; and ART for life, 3.4/100 child-years. Of note, one- third of the single/dual antiretrovirals for PMTCT and the 3-drug ART for PMTCT groups, respectively, were comprised of mothers of children in PHPT trials where child deaths were rarely observed, which might explain lower mortality rates in these 2 groups.
Figure 2.
Kaplan-Meier estimates of 24-month survival from birth by geographical region.
Association With Maternal/Child Characteristics
Univariably, LBW children were at 3-fold risk of dying as were never-breastfed children (Table 3). Children whose mother had died were 16 times as likely to die compared to children whose mothers survived; maternal antiretroviral exposure was associated with reduced child mortality, but this did not reach statistical significance. Adjusting for region, maternal antenatal CD4 count, maternal antiretroviral exposure, and maternal vital status, LBW and never breastfeeding remained significantly associated with increased mortality (Table 3). The association between maternal ART for life and reduced child mortality became statistically significant (adjusted hazard ratio [aHR], 0.5; 95% CI, 0.3–0.9) after adjusting for maternal CD4 count (HR, 0.72 in univariate analysis, declining to 0.54 after adjusting for maternal CD4 count only). Associations between mortality and the other antiretroviral categories did not reach statistical significance (single/dual antiretrovirals: aHR, 0.78; 95% CI, 0.50–1.22 and 3-drug ART for PMTCT: aHR, 0.66; 95% CI, 0.39–1.13). Children whose mother had died remained at a substantially increased risk of death (aHR, 11.1).
Table 3.
Factors Associated With Mortality Through 24 Months of Age in Human Immunodeficiency Virus–Exposed Uninfected Children (n = 19219)
| Factor | Total Number of Children (n = 19219) | Total Number of Deaths (n = 884) | Mortality Rate per 100 Child-Years of Follow-up | Univariate Model | Multivariable Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Adjusted Hazard Ratio | 95% CI | P Value | ||||||
| Region | <.0001 | <.0001 | |||||||||
| Africa | 14786 | 862 | 4.6 | Ref. | Ref. | ||||||
| Asia | 4433 | 22 | 0.4 | 0.08 | 0.04 | 0.17 | 0.05 | 0.02 | 0.11 | ||
| Residence | 0.22 | ||||||||||
| Rural | 1868 | 101 | 3.5 | Ref. | |||||||
| Urban/Peri-urban | 17351 | 783 | 3.7 | 0.84 | 0.64 | 1.11 | |||||
| Sex a | 0.28 | ||||||||||
| Male | 9839 | 467 | 3.8 | Ref. | |||||||
| Female | 9376 | 417 | 3.6 | 0.93 | 0.82 | 1.06 | |||||
| Birth weight | <.0001 | <.0001 | |||||||||
| ≥2500 g | 16553 | 613 | 2.9 | Ref. | Ref. | ||||||
| <2500 g | 2321 | 246 | 9.1 | 3.09 | 2.66 | 3.58 | 2.92 | 2.51 | 3.39 | ||
| Unknown | 245 | 25 | 8.1 | 2.21 | 1.47 | 3.33 | 2.40 | 1.60 | 3.61 | ||
| Breastfeeding | <.0001 | <.0001 | |||||||||
| Ever breastfed | 13418 | 700 | 4.2 | Ref. | Ref. | ||||||
| Never breastfed | 5801 | 184 | 2.5 | 2.76 | 2.14 | 3.57 | 2.48 | 1.95 | 3.16 | ||
| Maternal age at delivery (years) | 0.42 | ||||||||||
| 15–20 | 2074 | 103 | 4.4 | Ref. | |||||||
| 21–25 | 6159 | 276 | 3.9 | 0.91 | 0.73 | 1.15 | |||||
| 26–30 | 5175 | 231 | 3.8 | 0.91 | 0.72 | 1.16 | |||||
| 31–35 | 2545 | 99 | 3.2 | 0.80 | 0.61 | 1.06 | |||||
| >35 | 1011 | 35 | 2.8 | 0.73 | 0.50 | 1.08 | |||||
| Unknown | 2255 | 140 | 3.5 | 1.25 | 0.43 | 3.68 | |||||
| Maternal education | 0.03 | ||||||||||
| None/Primary | 8676 | 389 | 3.7 | Ref. | |||||||
| Secondary or above | 9688 | 477 | 3.7 | 0.88 | 0.76 | 1.03 | |||||
| Unknown | 855 | 18 | 3.3 | 0.23 | 0.05 | 1.05 | |||||
| Maternal CD4 count (cells/ mm 3) | <.0001 | <.0001 | |||||||||
| ≥350 | 11129 | 417 | 3.0 | Ref. | Ref. | ||||||
| <350 | 7010 | 403 | 4.6 | 1.58 | 1.38 | 1.82 | 1.43 | 1.24 | 1.66 | ||
| Unknown | 1080 | 64 | 5.6 | 1.55 | 1.16 | 2.07 | 1.49 | 1.12 | 1,99 | ||
| Maternal antiretroviral category | 0.28 | 0.13 | |||||||||
| No ARVs | 4392 | 305 | 6.0 | Ref. | Ref. | ||||||
| Single or double ARV for PMTCT | 11805 | 469 | 3.1 | 0.67 | 0.42 | 1.07 | 0.78 | 0.50 | 1.22 | ||
| 3-drug ART for PMTCT | 2248 | 73 | 2.7 | 0.61 | 0.34 | 1.07 | 0.66 | 0.39 | 1.13 | ||
| ART for life | 774 | 37 | 3.4 | 0.72 | 0.38 | 1.37 | 0.51 | 0.28 | 0.94 | ||
| Maternal vital status (time-dependent) | <.0001 | <.0001 | |||||||||
| Alive | b | 833 | 3.5 | Ref. | Ref. | ||||||
| Dead | 51 | 35.7 | 15.9 | 11.9 | 21.2 | 11.08 | 8.25 | 14.89 | |||
Variable trial/study was included in all models as random effects.
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; CI, confidence interval; HR, hazard ratio; PMTCT, prevent mother-to-child transmission.
aExcluding n = 4 with no information on sex.
bNot applicable for time-dependent variables.
Additional analyses, including ever-breastfed children only (n = 13418) and treating breastfeeding cessation as a time-dependent variable, showed mortality risk to be significantly increased after breastfeeding cessation. Adjusting for region, birth weight, maternal CD4 count, and maternal antiretroviral exposure, breastfeeding cessation was associated with a 12.5-fold (95% CI, 10.3–15.3) risk of death. In this model, children whose mothers received 3-drug ART (both PMTCT and for life) were at significantly lower risk of death (aHR, 0.51; 95% CI, 0.30–0.85 for 3-drug ART for PMTCT and aHR, 0.45; 95% CI, 0.22–0.92 for ART for life) than children whose mothers did not receive antiretrovirals (ARVs).
Sensitivity Analyses
To investigate the sensitivity of our assumption on 44 women with no information on ARV exposure, we ran the analyses and excluded these women; the aHRs were virtually unchanged. Further, 2 additional analyses were carried out to verify the effects of the inclusion of 1032 children with no information on weaning date and our assumption on their breastfeeding cessation at 6 months. After excluding these children, in the model with breastfeeding treated as a fixed effect, all aHRs were comparable to results shown in Table 3 (aHR, 3.0; 95% CI, 2.3–3.9 vs aHR, 2.5; 95% CI, 2.0–3.2). When breastfeeding was treated as a time-dependent variable, the risk related to breastfeeding cessation increased slightly but remained comparable to results presented in Table 3 (aHR, 16.9; 95% CI, 13.5–21.1 vs aHR, 13.1; 95% CI, 10.7–16.0).
Combined Effects of Maternal ART and Breastfeeding
Mortality by age 24 months differed significantly by breastfeeding and maternal 3-drug ART status at a given time (P < .0001; Table 4). Compared to not currently breastfed children with mothers not receiving 3-drug ART (category A in Table 4), mortality risk in not currently breastfed children with mothers receiving 3-drug ART (category B) was significantly reduced (HR, 0.6). In the absence of maternal 3-drug ART, currently breastfed children (category C) were significantly less likely to die (HR, 0.07). Currently breastfed children whose mothers were receiving 3-drug ART (category D) had the lowest mortality risk (HR, 0.04).
Table 4.
Multivariate Analysis on the Effects of Breastfeeding and Maternal 3-Drug Antiretroviral Therapy on Child Mortality (n = 24186 child-years)
| Category | Child Currently Breastfeda | Mother Being on 3-Drug Antiretroviral Therapya | Number of Child-Years | Number of Deaths | Adjusted Hazard Ratiob | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|---|---|---|
| <.0001 | ||||||||
| A | No | No | 14119 | 552 | Ref. | |||
| B | No | Yes | 1027 | 44 | 0.63 | 0.45 | 0.87 | |
| C | Yes | No | 7874 | 269 | 0.08 | 0.06 | 0.09 | |
| D | Yes | Yes | 1166 | 19 | 0.04 | 0.03 | 0.07 | |
aTime-dependent variables.
bVariable trial/study was included as random effects. Also adjusted for region, maternal antenatal CD4 count, and birth weight.
Adjusted Attributable Fractions of Risks
To investigate the impact of LBW, never breastfeeding, mother not on 3-drug ART for life, and maternal death, we estimated the aAFs of risks based on the parameter estimates obtained from our final model (Table 3). Mother not receiving 3-drug ART for life accounted for 45.6% (95% CI, 19.1–63.9) of child deaths by age 24 months. LBW accounted for an estimated 16.2% of child deaths by age 24 months, never breastfeeding for 10.8%, and maternal death for 4.3%. Combined, these 4 factors explained 63.6% (95% CI, 45.7–76.6) of deaths by age 24 months. The aAFs of risks at 6 and 12 months related to these 4 factors did not significantly differ from those at 24 months (Table 5).
Table 5.
Estimated Adjusted Attributable Fractions and 95% Confidence Intervals of Risk Factors for Mortality in Human Immunodeficiency Virus–Exposed Uninfected Children at Different Time Points
| Expected Number of Deaths Given the Distribution at 6, 12, and 24 Months | 0–6 Months | 0–12 Months | 0–24 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 755 | 1054 | 1444 | |||||||
| Number of Deaths Attributable to the Risk Factor(s) | aAF (%) | 95% CI | Number of Deaths Attributable to the Risk Factor(s) | aAF (%) | 95% CI | Number of Deaths Attributable to the Risk Factor(s) | aAF (%) | 95% CI | |
| Mother not receiving 3-drug antiretroviral therapy for life | 342 | 47.9 | 20.0–65.8 | 466 | 46.9 | 15.6–64.9 | 620 | 45.6 | 19.1–63.9 |
| Low birth weight | 140 | 18.5 | 15.1–21.9 | 184 | 17.4 | 14.2–20.6 | 234 | 16.2 | 13.1–19.2 |
| Breastfeeding never initiated | 88 | 11.7 | 5.2–19.6 | 119 | 11.3 | 5.0–18.9 | 157 | 10.8 | 4.9–17.9 |
| Mother not being alive | 44 | 5.9 | 3.1–13.5 | 53 | 5.1 | 2.7–11.5 | 62 | 4.3 | 2.3–9.3 |
| All 4 factors above | 486 | 66.2 | 48.6–78.4 | 666 | 65.0 | 47.2–77.7 | 891 | 63.6 | 45.7–76.6 |
Abbreviations: aAF; adjusted attributable fraction; CI, confidence interval.
DISCUSSION
Using data from 21 studies/trials undertaken between 1995 and 2015 in Africa and Asia, our findings suggest that where mothers are alive, on ART for life, and breastfeed their infants, 24-month mortality in HEU children is substantially reduced.
As reported previously [50–52], LBW, prevalent in 12% of these HEU children, was a major risk factor for mortality. However, the negative consequences of LBW and non-breastfeeding may be even greater in settings outside the context of well-resourced research studies. Almost half of HEU deaths occurred in the first 3 months of life and two-thirds before age 6 months, highlighting the importance of intervening programmatically in this early period.
The survival of mothers living with HIV had a major effect on the survival of HEU children; this association has also been reported among HUU children [53]. In our analyses, the death of a mother shortly after delivery was most hazardous for the survival of her HEU child.
Our results suggest that the risk of mortality in HEU children is reduced when mothers are on ART either until breastfeeding cessation or for life. Mother’s initiation and continuation of ART likely improves her own health, which in turn increases the chances of child survival through better breastfeeding practices, reduced exposure to comorbidities, improved mother’s care capacity, and other unmeasured benefits at the household level.
The estimated AFs differed from the aHRs. This indicates that the impact at the population level, which reflects prevalence of risk factors, differs from that at the individual level. The CI around the AF estimate of mother not receiving 3-drug ART for life was particularly wide, and caution is required in interpreting this result. Our estimated AFs show that 36% of HEU child mortality at age 24 months could not be accounted for by the 4 risk factors identified and highlight that HEU children are also at risk of death from other common causes of child mortality. The lack of contemporaneous mortality data from HUU children meant that we were unable to categorically comment whether HEU children are at any greater mortality risk if these 4 risk factors are fully addressed.
Although the analyses include a large number of HEU children from diverse settings, interpretation of findings is hindered by lack of detail on potentially important variables including gestational age, neonatal care practices (early initiation and type of breastfeeding), and household exposure to opportunistic infections (such as tuberculosis). These factors may account for much of the remaining 36% mortality.
Relatively few women were on ART for life, and studies generally followed earlier WHO guidelines on HIV and infant feeding, recommending breastfeeding for about 6 months only. Although we confirm the associations between reduced mortality risk and maternal ART for life and breastfeeding, our data does not allow us to fully capture the complex associations between different factors that influence a child’s survival outcome. The women on ART in our study are a highly select population, and we cannot comment on the extent to which such reductions are facilitated by study-specific factors and whether such associations would be equally observed in women on ART in standard-of-care settings. Further, due to lack of data, we were unable to allow for cotrimoxazole prophylaxis and childhood immunization, which are used to prevent infectious morbidity in young children.
We were also unable to differentiate small-for-gestational age from premature infants in those with LBW. About 11% of all infants in Eastern and Southern Africa are born with LBW; in Southeast Asia it is around 28% [54]. LBW has been associated with HIV infection in pregnant women in sub-Saharan Africa and with the protease inhibitor class of ART during pregnancy [55–57]. While the primary drivers for LBW may vary by region and HIV exposure, the relationship between LBW and mortality in both HEU and HUU children is clear and strong and has immediate programmatic implications.
The combination of 3-drug ART was introduced in about the middle of the timespan covered by the studies included; ART eligibility criteria varied over time as did inclusion for trials. Although we could not allow for these trends, these factors could have introduced selection bias. Each study had its own criteria for eligibility, including CD4 counts. Data included in our analyses were heterogeneous in terms of feeding and duration of follow-up, which limits our ability to generalize the results beyond these studies. Finally, exclusion of 457 children whose breastfeeding status was unknown might have led to underestimation of mortality rates as almost 29% (132/457) of these children died before age 12 months; 105 died before age 1 month, 17 between age 1 and 3 months, and 10 died thereafter.
We show substantial regional differences in 24-month mortality, which deserves further investigation, as do the issues surrounding breastfeeding. Explaining the missing fraction of HIV-related and other external mortality risk factors requires prospectively collected data from both HEU and HUU populations. It remains unclear whether HEU infants and children
are immunologically impaired at a clinically significant level or whether increased exposure to opportunistic infections because of living in HIV-affected households would explain the missing fraction. Perhaps the most germane question is whether increasing rollout of lifelong ART among women living with HIV and fully supporting optimal infant feeding practices will mitigate the patterns of risks identified in these historical cohorts. With more and more women living with HIV being initiated on ART, understanding the interactions between fetal HIV and ART exposure, the effects of prematurity or small-for-gestational age on mortality, and the effects of other long-term outcomes including early child development, infectious morbidity, and the risk of noncommunicable diseases will be increasingly important.
CONCLUSIONS
Our findings show that not-breastfeeding and LBW were associated with considerable mortality risk and suggest that maternal ART, initiated before or during pregnancy, may substantially reduce child mortality in the first 2 years of life. With increasing numbers of HIV-infected pregnant women being initiated on ART, this would provide hope for reducing overall child mortality in settings of high HIV prevalence. The importance of delivering effective integrated care so that women living with HIV are not only initiated on ART but are also linked with other essential elements of maternal and child healthcare is clear. Eliminating pediatric HIV and improving the survival, health, and development of HEU children should not be separate from improving the well-being of mothers and children not affected by HIV, and our metric of success needs to evolve to “HIV-free survival and development.” While integrated programs and coordinated research and monitoring are unquestionably possible, continued global investment in these responses is perhaps the greatest challenge.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. R. B., M. L. N., and N. R. initiated and set the objectives of the collaboration, defined the statistical analysis plans, and substantially contributed to the writing of the manuscript. S. A. undertook the literature review, managed the data pooling, participated in the definition of the statistical analysis plan, performed statistical analysis, and wrote the first draft of the paper. M. R. and P. J. substantially contributed to the statistical analysis. G. J., J. H., T. F., G. G., L. K., R. S., V. L., S. L., R. C. B., T. D., and S. L. C. critically reviewed the manuscript and substantially contributed to the interpretation of the results. All other coauthors reviewed the manuscript.
Acknowledgments. BAN: Charles van der Horst, Denise Jamieson; Ditrame-ANRSa: Christiane Welffens-Ekra, Philippe Msellati; Ditrame-ANRSb: Nicolas Meda, Philippe Van de Perre; Ditrame Plus: Marguerite Timité-Konan, Clarisse Bosse; Good Start: Mickey Chopra, Debra Jackson, Vundli Ramokolo, Ameena Goga; HIVIGLOB: J. Brooks Jackson, Laura A. Guay, Philippa Musoke, Mary Glenn Fowler, Michael C. Mubiru; PEP: Mike Urban (University of Stellenbosch); PHPT: Marc Lallemant, principal investigator of the PHPT clinical trials, all PHPT coinvestigators, the PHPT staff who helped collect and manage the data, and Nicolas Salvadori for the preparation of the data-set of the PHPT studies. PROMOTE2: Diane Havlir, Theodore Ruel. SWEN: Amita Gupta, Jayagowri Sasty, Harjot K. Singh; ZEBS: The late Moses Sinkala (Lusaka District Health Management Team), Chipepo Kankasa (University Teaching Hospital), Donald M. Thea (Boston University), Grace M. Aldrovandi (University of California–Los Angeles).
Disclaimer. The World Health Organization (WHO) had no role in the study design, data collection, data analysis, or interpretation of data. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of WHO or the Centers for Disease Control and Prevention.
Funding. This work was funded by the WHO.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. Prevention Gap Report. Geneva: UNAIDS, 2016. [Google Scholar]
- 2. UNAIDS. On the Fast-track to an AIDS-Free Generation. Geneva: UNAIDS, 2016. [Google Scholar]
- 3. Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis 2016; 16:e92–e107. [DOI] [PubMed] [Google Scholar]
- 4. Marinda E, Humphrey JH, Iliff PJ, et al. . Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007; 26:519–26. [DOI] [PubMed] [Google Scholar]
- 5. Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. . Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr 2006; 41:504–8. [DOI] [PubMed] [Google Scholar]
- 6. Powis KM, Kitch D, Ogwu A, et al. . Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis 2011; 204:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koyanagi A, Humphrey JH, Moulton LH, et al. . Predictive value of weight loss on mortality of HIV-positive mothers in a prolonged breastfeeding setting. AIDS Res Hum Retroviruses 2011; 27:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shapiro RL, Lockman S, Kim S, et al. . Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis 2007; 196:562–9. [DOI] [PubMed] [Google Scholar]
- 9. von Mollendorf C, von Gottberg A, Tempia S, et al. . Increased risk for and mortality from invasive pneumococcal disease in HIV-exposed but uninfected infants aged <1 year in South Africa, 2009–2013. Clin Infect Dis 2015;60:1346–56. [DOI] [PubMed] [Google Scholar]
- 10. Henderson RA, Miotti PG, Saavedra JM, et al. . Longitudinal growth during the first 2 years of life in children born to HIV-infected mothers in Malawi, Africa. Pediatr AIDS HIV Infect 1996; 7:91–7. [PubMed] [Google Scholar]
- 11. Moraleda C, de Deus N, Serna-Bolea C, et al. . Impact of HIV exposure on health outcomes in HIV-negative infants born to HIV-positive mothers in sub-Saharan Africa. J Acquir Immune Defic Syndr 2014; 65:182–9. [DOI] [PubMed] [Google Scholar]
- 12. Landes M, van Lettow M, Chan AK, et al. . Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One 2012; 7:e47337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel D, Bland R, Coovadia H, et al. . Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS 2010; 24:437–45. [DOI] [PubMed] [Google Scholar]
- 14. Bailey RC, Kamenga MC, Nsuami MJ, et al. . Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol 1999; 28:532–40. [DOI] [PubMed] [Google Scholar]
- 15. Ndirangu J, Newell ML, Thorne C, Bland R. Treating HIV-infected mothers reduces under 5 years of age mortality rates to levels seen in children of HIV-uninfected mothers in rural South Africa. Antivir Ther 2012; 17:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thorne C IP, Chamla D, Romano S, Luo C, Newell ML. Morbidity and mortality in HIV-exposed uninfected children. Future Virol 2015;10:1077–100. [Google Scholar]
- 17. Arikawa S, Rollins N, Newell ML, Becquet R. Mortality risk and associated factors in HIV-exposed, uninfected children. Trop Med Int Health 2016; 21:720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taha TE, Hoover DR, Chen S, et al. . Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis 2011; 53:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owor M, Mwatha A, Donnell D, et al. . Long-term follow-up of children in the HIVNET 012 perinatal HIV prevention trial: five-year growth and survival. J Acquir Immune Defic Syndr 2013; 64:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kafulafula G, Hoover DR, Taha TE, et al. . Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr 2010; 53:6–13. [DOI] [PubMed] [Google Scholar]
- 21. Chatterjee A, Bosch RJ, Hunter DJ, Fataki MR, Msamanga GI, Fawzi WW. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-infected women in Tanzania. J Acquir Immune Defic Syndr 2007; 46:599–606. [DOI] [PubMed] [Google Scholar]
- 22. Heidkamp RA, Stoltzfus RJ, Fitzgerald DW, Pape JW. Growth in late infancy among HIV-exposed children in urban Haiti is associated with participation in a clinic-based infant feeding support intervention. J Nutr 2012; 142:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutcliffe CG, Scott S, Mugala N, et al. . Survival from 9 months of age among HIV-infected and uninfected Zambian children prior to the availability of antiretroviral therapy. Clin Infect Dis 2008; 47:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marquez C, Okiring J, Chamie G, et al. . Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. J Trop Pediatr 2014; 60:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dabis F, Msellati P, Meda N, et al. . 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d’Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mère-Enfant. Lancet 1999; 353:786–92. [DOI] [PubMed] [Google Scholar]
- 26. Msellati P, Meda N, Leroy V, et al. . Safety and acceptability of vaginal disinfection with benzalkonium chloride in HIV infected pregnant women in West Africa: ANRS 049b phase II randomized, double blinded placebo controlled trial. DITRAME Study Group. Sex Transm Infect 1999; 75:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kourtis AP, Wiener J, Kayira D, et al. . Health outcomes of HIV-exposed uninfected African infants. AIDS 2013; 27:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Becquet R, Bequet L, Ekouevi DK, et al. . Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d’Ivoire. PLoS Med 2007; 4:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onyango-Makumbi C, Bagenda D, Mwatha A, et al. . Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J Acquir Immune Defic Syndr 2010; 53:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chilongozi D, Wang L, Brown L, et al. . Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. Pediatr Infect Dis J 2008; 27:808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bork KA, Cournil A, Read JS, et al. . Morbidity in relation to feeding mode in African HIV-exposed, uninfected infants during the first 6 mo of life: the Kesho Bora study. Am J Clin Nutr 2014; 100:1559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shapiro RL, Kitch D, Ogwu A, et al. . HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana. AIDS 2013; 27:1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Venkatesh KK, de Bruyn G, Marinda E, et al. . Morbidity and mortality among infants born to HIV-infected women in South Africa: implications for child health in resource-limited settings. J Trop Pediatr 2011; 57:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Natureeba P, Ades V, Luwedde F, et al. . Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 2014; 210:1938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chopra M, Doherty T, Goga A, Jackson D, Persson LA. Survival of infants in the context of prevention of mother to child HIV transmission in South Africa. Acta Paediatr 2010; 99:694–8. [DOI] [PubMed] [Google Scholar]
- 36. Kammerer B, Kacanek D, Mayondi GK, Lockman S.. Pediatric neurodevelopmental functioning following in utero exposure to triple-NRTI-vs. PI-based ART in a randomized trial, Botswana. Washington, D.C: AIDS, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rollins NC, Ndirangu J, Bland RM, Coutsoudis A, Coovadia HM, Newell ML. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS One 2013; 8:e81307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuhn L, Sinkala M, Semrau K, et al. . Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis 2010; 50:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cressey TR, Punyawudho B, Urien S, et al. . Pharmacokinetics of daily nevirapine in neonates at high risk of HIV acquisition. CROI; 22–25 Feb 2016;Boston, Massachusetts. [Google Scholar]
- 40. Lallemant M, Jourdain G, Le Coeur S, et al. . Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med 2004; 351:217–28. [DOI] [PubMed] [Google Scholar]
- 41. Singh HK, Gupte N, Kinikar A, et al. . High rates of all-cause and gastroenteritis-related hospitalization morbidity and mortality among HIV-exposed Indian infants. BMC Infect Dis 2011; 11:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lallemant M, Le Coeur S, Sirirungsi W, et al. . Randomized noninferiority trial of two maternal single-dose nevirapine-sparing regimens to prevent perinatal HIV in Thailand. AIDS 2015; 29:2497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach (2006 revision). Geneva, Switzerland:World Health Organization; 2006. [PubMed] [Google Scholar]
- 44. World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach (2010 version). Geneva, Switzerland:World Health Organization; 2010. [PubMed] [Google Scholar]
- 45. Inter-agency Task Team on Prevention of HIV Infections in Pregnant Women MatI. HIV and infant feeding new evidence and programmatic experience: report of a technical consultation. Geneva: WHO, UNICEF, UNAIDS, UNFPA, 2006. [Google Scholar]
- 46. Rückinger S, von Kries R, Toschke AM. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol 2009; 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res 2001;10:195–216. [DOI] [PubMed] [Google Scholar]
- 48. Rondeau V, Marzroui Y, Gonzalez JR frailtypack: An R Package for the Analysis of Correlated Survival Data with Frailty Models Using Penalized Likelihood Estimation or Parametrical Estimation. 2012. 2012; 47:28. [Google Scholar]
- 49. Canty A, Ripley BD.. boot: Bootstrap R (S-Plus) Functions. 2017. Available at: http://CRAN.R-project.org/package=boot. [Google Scholar]
- 50. Wei R, Msamanga GI, Spiegelman D, et al. . Association between low birth weight and infant mortality in children born to human immunodeficiency virus 1-infected mothers in Tanzania. Pediatr Infect Dis J 2004; 23:530–5. [DOI] [PubMed] [Google Scholar]
- 51. Kuhn L, Kasonde P, Sinkala M, et al. . Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants?Clin Infect Dis 2005; 41:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Slyker JA, Patterson J, Ambler G, et al. . Correlates and outcomes of preterm birth, low birth weight, and small for gestational age in HIV-exposed uninfected infants. BMC Pregnancy Childbirth 2014; 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chikhungu LC, Newell ML, Rollins N. Under-five mortality according to maternal survival: a systematic review and meta-analysis. Bull World Health Organ 2017; 95:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. UNICEF. The state of the world’s children 2016: a fair chance for every child. New York: United Nations Children’s Fund, 2016. [Google Scholar]
- 55. Braddick MR, Kreiss JK, Embree JB, et al. . Impact of maternal HIV infection on obstetrical and early neonatal outcome. AIDS 1990; 4:1001–5. [DOI] [PubMed] [Google Scholar]
- 56. Bulterys M, Chao A, Munyemana S, et al. . Maternal human immunodeficiency virus 1 infection and intrauterine growth: a prospective cohort study in Butare, Rwanda. Pediatr Infect Dis J 1994; 13:94–100. [DOI] [PubMed] [Google Scholar]
- 57. Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS 2004; 18:2337–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.