Abstract
Improved imaging and the evolution of surgical techniques have permitted a rapid growth in hip preservation surgery over the last few decades. The management of the painful borderline dysplastic hip however remains controversial. In this review, we will identify the pertinent issues and describe the patient assessment and treatment options. We will provide our own recommendations and also identify future areas for research.
INTRODUCTION
Improved knowledge about hip biomechanics and the evolution of surgical techniques have permitted a rapid growth in hip preservation surgery over the last few decades. The spectrum covers a wide range from hips with shallow acetabuli, which are unstable, to hips with deep acetabuli that are suffering from femoro-acetabular impingement (FAI). While there is a general agreement that the best treatment for the unstable dysplastic hip is a reorientation of the acetabulum to increase cover, there is equal agreement that the rim of the over-covering acetabulum has to be reduced to remove impingement. On all those hips a cam deformity may be present that needs to be addressed at the time of surgical correction [1]. At the far ends of the spectrum the requisite treatment is obvious. However, there is a transition zone where it is difficult to discriminate instability from FAI. In the past these hips were referred to as ‘borderline’ hips. Usually, this included hips with a lateral center edge (LCE) angle between 20° and 25° [2]. However, the term ‘borderline’ is problematic, because it is a radiographic definition and only addresses one of several parameters important to describe hip stability. Acetabular roof obliquity, anterior and posterior cover and femoral antetorsion are other factors that should be included into an analysis of hip stability.
The association of hip dysplasia with hip osteoarthritis is established [3, 4] and dysplastic hips with signs of instability degenerate at a higher rate [5]. A borderline hip can either be unstable, impinging or maybe both. The stability of the borderline is difficult to determine and subject to interpretation with a general tendency in the orthopaedic community to underestimate instability that then leads to inappropriate treatment. Recent studies suggest that arthroscopic hip surgery with labral repair and capsular plication in patients with borderline dysplasia (LCEA > 20°) may result in appropriate short-term improvements [3, 4]. However, there is evidence that a wrongly done previous hip arthroscopy has a negative impact on the outcome on the treatment of such hips [6].
Therefore, the management of the painful borderline dysplastic hip however remains an issue of great controversy. Borderline hip dysplasia is common in young adults with hip pain with a reported prevalence of 37.6% in selected patient cohorts [7]. In the borderline dysplastic hip there may be significant overlap with other causes of instability such as connective tissue laxity [8]. However, the fundamental issue is the difficulty in correctly classifying the underlying patho-biomechanics.
DEFINITION
The first problem lies in the definition. The Lateral Centre Edge Angle of Wiberg as measured on an Antero-posterior pelvic radiograph [9] (LCEA) has traditionally been used to classify hips as normal (LCEA >25°), dysplastic (LCEA <20°) or borderline (LCEA 20–25°) although these defining values vary widely in the literature [3, 10]. However, the use of the LCEA has two problems.
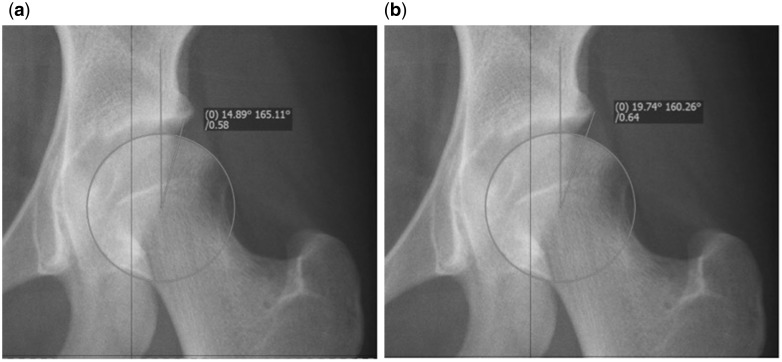
Firstly the method by which it should be measured. To measure the LCEA the center of the femoral head is first defined by a circle fitting the contour of the femoral head. The first branch of the angle runs perpendicular through the center of rotation. The second branch is defined by the center of the femoral head and the most lateral point of the sourcil (Fig. 1a). It is important not to use the most lateral point of the acetabulum (Fig. 1b), because this does not follow the definition of Wiberg, and will give false high values [11].
Fig. 1.
(a) Correct measurement of the LCEA using the edge of the sourcil, indicating moderate dysplasia. (b) Incorrect measurement of the LCEA in the same hip. Using this value would falsely classify this hip as borderline.
Secondly the actual term ‘Borderline hip dysplasia’ was first introduced by Wiberg himself, including hips with a LCEA between 20° and 25° [2]. LCEA is a radiographic measure and per se cannot predict stability in the borderline dysplastic hip nor does fully describe femoral head coverage. Therefore the LCEA cannot direct surgical decision making [12–14]. Part of the reason is that LCEA alone does not encompass the precise location of dysplasia and disregards anterior and posterior femoral head coverage. Also other parameters such as acetabular index (AI) and femoral antetorsion are very relevant for stability of the hip. In the presence of a decreased LCEA AI may be normal in which case the stability of the hip is difficult to assess [15]. On the other hand, excessive femoral anteversion may potentiate anterior hip instability [16].
WHAT IS THE FUNDAMENTAL ISSUE?
In the painful borderline dysplastic hip it is difficult to characterize the pathological mechanism as impingement (stable) or dysplasia (unstable) by a two-dimensional radiographic measurement alone, especially one that is solely a function of the acetabulum and takes no account of the femur. This functional characterization of hip stability is of paramount importance to guide surgical decision-making. An unstable hip would logically benefit from acetabular reorientation osteotomy whilst a stable hip would benefit from impingement surgery such as femoral cam osteoplasty.
So what is known about the intra-articular pathology? How should these patients be assessed? What are the treatment options? What are the surgical outcomes? What are the potential pitfalls with this group of patients? What are the future directions? In this narrative review article we aim to address these questions and elucidate the management of this challenging group of patients.
WHAT IS THE UNDERLYING PATHOLOGY OF HIP DYSPLASIA AND UNSTABLE BORDERLINE HIPS?
In hip dysplasia, there are abnormally high articular contact pressures and relative bony uncovering of the femoral head. The acetabulum is typically shallow and anteverted with an often compensatory enlarged labrum, but there is also a high prevalence of concomitant acetabular retroversion [17]. The femur is classically in valgus with high antetorsion [10]. These abnormal anatomical features cause pathological hip biomechanics which manifest as labral tears, chondral lesions, and hip instability, which can easily be misinterpreted as impingement. As the osseous stability is compromised the importance of the soft tissue stabilisers, namely the fibrocartilaginous labrum and the hip capsule, is accentuated [18]. Once the soft tissue constraints fail then the hip becomes unstable. However, one has to understand that the principal underlying pathology is the lack of osseous stability, which leads to failure of the hip and not the failing soft tissue stability.
The natural history of the subluxing dysplastic hip is a very poor prognosis and invariably leads to joint degeneration [5]. The rate of deterioration is directly related to subluxation severity and patient age and usually about 10 years after onset of symptoms severe degenerative changes have developed [19]. The natural history in the absence of subluxation is more difficult to predict concerning the speed of degeneration. The same accounts for borderline dysplastic hips. A recent study highlights the importance of acetabular cover. In a large cohort of females, followed for 20 years, it was shown that each degree reduction in LCE below 28° is associated with 13% increased risk of radiographic OA [20]. Therefore, besides short-term relief of symptoms, the long-term possible evolution has to be kept in mind.
CLINICAL PRESENTATION
The clinical presentation of borderline acetabular dysplasia is very similar to that of other young active adult hip disorders, such as FAI syndrome [21] so a thorough history, physical examination, and radiographic evaluation are essential to properly diagnose these patients.
HISTORY
A focused history is taken. The primary symptom in patients with borderline hip dysplasia is pain. This is typically perceived in groin and lateral hip but can also be in the buttock. A full pain history is warranted. Particular symptoms of instability and ‘giving way’ are sought that may indicate that the limits of soft tissue compensation for a lack of osseous stability have been reached. Symptoms of clicking and catching are also common. Furthermore any indications that the patient has established hip arthritis, such as night pain, are asked for. The symptoms should be put into the context of the patient’s functional limitations and medical attention already received including physiotherapy, medications, other opinions and surgery.
EXAMINATION
A logical clinical examination of the hip should follow including apprehension and impingement tests. The patient will often display a ‘kneeing-in’ gait in association with an increased hip adductor moment and increased internal hip rotation consistent with increased femoral antetorsion. Hyperlordosis may be present in order to functionally increase anterior cover. Tenderness over the greater trochanter should be determined [22].
It is crucial to remember to examine the patient’s rotational profile, perform a neurovascular examination and to check for signs of generalized joint laxity and quantify this using Beighton’s score. Specific key aims include refuting the presence of (i) an advanced degenerative process manifest for example with fixed flexion deformity and decreased range of motion and (ii) alternative pathology such as pain referred from lumbar spondylosis or L5 radiculopathy.
INVESTIGATIONS
Diagnostic imaging should commence with standardized plain AP radiograph of the pelvis and a lateral femoral neck views (lateral cross table, Dunn view, false profile views) [23]. These images are scrutinized to measure the LCEA, AI, extrusion index, femoral neck-shaft angle and FEAR index (see below). The Tonnis grade of osteoarthritis should be determined along with whether there is cam morphology. Direct signs of instability should be scrutinized for and these comprise femoral head migration, recognized by an increased distance from the ilioischial line, a break in Shenton’s line and recentering of the femoral head on an AP view with the hip in abduction and Gadolinium in the posterior joint space when using MR-arthrography, that indicates anterior migration and thus instability of the femoral head. The FEAR index has a high association with instability (see below). The various parameters have to be measured precisely and recorded.
Cross-sectional imaging with three-dimensional computerized tomography (CT) for precise information on bony anatomy and location of dysplasia including the presence and location of periarticular cysts is warranted [24–26]. Furthermore CT should include estimation of femoral antetorsion which, if high may potentiate anterior hip instability. Magnetic resonance imaging (MR-arthrography) should follow a dedicated protocol for the examination of the hip, including radial image acquisition or reconstruction and intra-articular application of contrast [27] to examine for intra-articular structures and pathology of both labrum and articular cartilage. Other causes for similar symptoms such as avascular necrosis, trochanteric bursitis or gluteal pathology can be differentiated. Additional measurements include labral size [13, 28] and iliocapsularis volume [29]. In these patients, we also advocate non-traction MR arthrography to examine for a accumulation of gadolinium known as a ‘crescent sign’ which is a subtle sign of instability on the axial view [30].
WHAT IS THE VALUE OF THESE MEASUREMENTS?
On plain films those measurements that are direct signs of instability are femoral head migration with an increase of the distance from the ilioischial line, a break in Shenton’s line and recentering of the femoral head on the AP view with hips in abduction and the FEAR index. On MR-arthrography the presence of Gadolinium in the postero-inferior joint space indicates migration of the femoral head and thus instability. The AI, NSA, AT, high iliocapsularis volume and increased labral volume may be present but are not predictive of instability [30] (Table 1).
Table 1.
Overview of various parameters used to assess hip instability
| Parameters | Estimated predictive value in % | |
|---|---|---|
| Parameters proving hip instability | Break of Shenton's line | 100 |
| Increased distance from the ilioischial line | 100 | |
| Recentering of the femoral head on abduction view | 100 | |
| Posterior crescent sign on MR-arthrography | 90 | |
| FEAR index >2° | 92 | |
| Parameters highly indicative for hip instability | LCEA <20° | 70 |
| AI >10° | 70 | |
| NSA >135° | 55 | |
| Femoral antetorsion >25° | 70 | |
| Parameters often present in hip instability | Hypertrophy of labrum | 50 |
| Increased volume of M. iliocapsularis | 50 | |
| Lig. Teres tear | 70 |
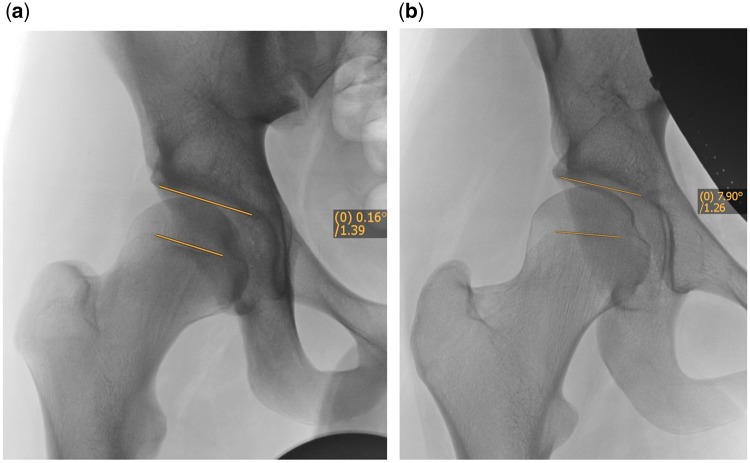
The FEAR index is a recently described parameter that seems to have a high value to predict stability of the hip [27]. It is formed by the angle between the acetabular roof and the central third of the femoral growth plate (Fig. 2). It is based on the fact that during growth the epiphyseal growth plate of the femur orients itself perpendicularly to the joint reacting forces of the hip. Growth and the orientation of the femoral neck are under the control of the subcapital growth plate [31]. Pauwels and Maquet [32] theorized that the resultant force acts from the center of the epiphyseal cartilage and that during growth, the epiphyseal plate orients itself perpendicular to the joint reaction force in accordance with the Heuter–Volkman principle. Pauwels and Maquet’s theory later was confirmed by Carter et al. [33] who studied the influence of hip loading by bi-dimensional finite element analysis. The angle of the closed epiphyseal plate indicates the balance of forces across the proximal femoral physis [34] and indicates how the transarticular forces acted in the past. Therefore, it is a functional parameter that reflects the joint reacting forces over a long period of time during growth of the hip. If the FEAR is <0° the hip is considered stable. Statistical analysis has shown that a cutoff value of 5° predicts stability with 80° probability. More recent work has shown that a cutoff value of 2° predicts stability with 90% probability (Batailler et al., in preparation). Case examples of using the FEAR index are shown in Fig. 3a and b.
Fig. 2.
The FEAR index. The angle is measured between a line connecting the most medial and lateral point of the sourcil and a line connecting the medial and lateral end of the straight part (usually central third) of the physeal scar of the femoral head. A negative FEAR index, with the angle opening medially as shown in Fig. 3a, indicates a stable hip.
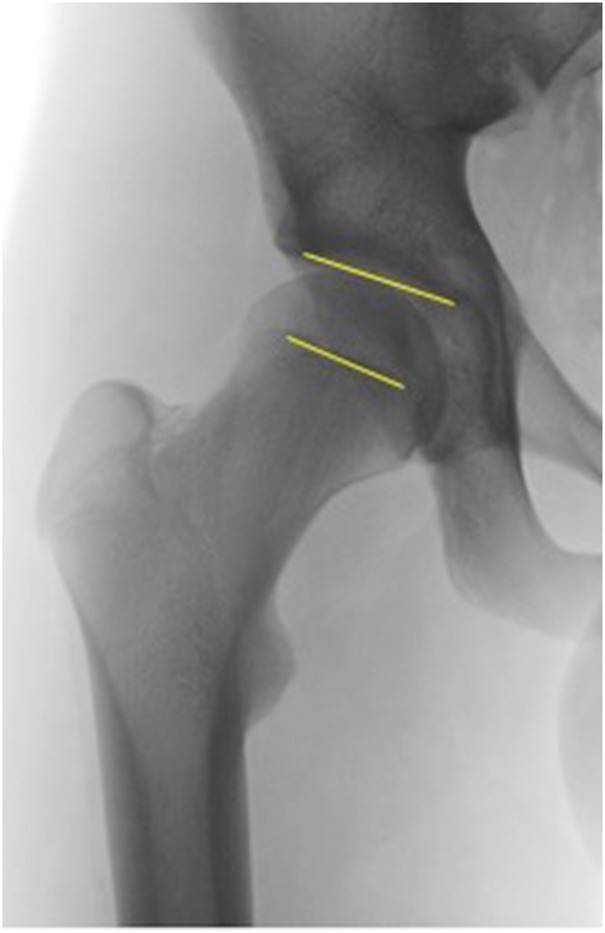
Fig. 3.
(a) Case examples using the FEAR index. 17-year-old male, LCEA 20°, FEAR 0°. Hip deemed therefore stable and patient managed with hip arthroscopy. (b) Case examples using the FEAR index. 17-year-old female, LCEA 20°, FEAR 8°. Hip deemed therefore unstable and patient managed with PAO.
WHAT ARE THE TREATMENT OPTIONS?
Treatment depends on the stability of the hip. The treatment alternatives for the painful borderline dysplastic hip include non-operative treatment, surgical treatment to address intra-articular impingement (FAI surgery by either hip arthroscopy or surgical hip dislocation) and surgical treatment to address instability (reorientation osteotomy with PAO and/or femoral osteotomy) (see Fig. 2). Non-operative management includes patient education, activity modification, simple analgesics, non-steroidal anti-inflammatory medications, and intra-articular injections [35]. Targeted physiotherapy can improve muscular conditioning, pain and proprioceptive control. The surgical treatment options for the borderline dysplastic hip which comprise arthroscopy and/or osteotomy will be discussed in the following paragraphs.
WHAT ARE THE RESULTS OF HIP ARTHROSCOPY IN THIS GROUP OF PATIENTS?
With the recent evolution in hip arthroscopy many surgeons are using this to address borderline dysplastic hips, not least because of perceived higher risks and longer post-operative recovery associated with alternative techniques such as periacetabular osteotomy. Hip arthroscopy in borderline dysplastic hips permits the surgeon to address intra-articular pathology such as a labral tear or femoral cam deformity [3, 12, 36]. If PAO is being considered to address the inadequate bony stability then arthroscopy may give the surgeon valuable insights not only into the intra-articular status of the hip but also how the patient is likely to fare with a much larger subsequent operation [37]. However, there is little published literature on hip arthroscopy in borderline dysplastic hips and what there is limited by short-term follow-up.
In the systematic review by Jo et al., 13 studies looking at arthroscopy in dysplastic hips were identified [10]. The studies were heterogeneous and all studies were case series. Only six studies reported on subjective and/or objective outcomes. The surgical indications for arthroscopy were ambiguous and patients had received variable non-operative management a priori. Furthermore the precise definition of borderline hip dysplasia varied and only two studies used the definition of Byrd and Jones [36]. Three studies reported on hip arthroscopy as an adjuvant tool and three as a stand-alone treatment. Labral tears had an overall prevalence of 77.3% and these were mostly located in the anterior or anterosuperior portion of the acetabular rim. Acetabular chondral lesions were more common than femoral lesions (59–75.2% versus 11–32%) and located adjacent to that of the labral pathology.
There were only two studies that examined the outcomes of arthroscopy in borderline hip dysplastic cases (LCEA 20–25°) of which only one described patient reported outcome measures. The latter, a prospective clinical case series by Byrd and Jones [36], had 66% of hips (32 hips) with borderline dysplasia. The mean modified Harris Hip score improved from 50 (poor) to 77 (fair) following arthroscopy. The authors concluded that the treatment response is likely a function of addressing the intra-articular pathology rather than the radiographic evidence of dysplasia.
WHAT ARE THE DANGERS WITH DOING HIP ARTHROSCOPY IN BORDERLINE DYSPLASTIC HIPS?
Arthroscopic labral resection and removal of lateral acetabular rim in borderline hip dysplasia can lead to fulminant joint instability [38]. Even if the labrum is repaired it is imperative to preserve the iliofemoral ligament and other static stabilizers of the hip to prevent the irreversible consequences or rendering the hip unstable [39–41]. There is no conclusive literature to support capsular repair in these cases but this seems a safe and sensible practice [42]. Capsular reduction techniques to improve stability have been described in borderline dysplastic hips [12]. If the hip is sufficiently unstable pre-operatively then addressing the intra-articular pathology alone by hip arthroscopy will be insufficient and the patient will require a PAO [43, 44]. One has to bear in mind that stability of the hip first line depends on the osseous geometry. In subtle instability (borderline dysplasia) stability may be secured by secondary soft tissue structures. Once these fail due to micro- or macrotrauma the hip becomes unstable. Restoring soft tissue stability may improve hip stability for a short period of time only, but it is likely that the soft tissues wear out again. Therefore the underlying osseous pathology has to be addressed first to achieve good long-term results.
A recent report showed an inferior hip specific functional outcome of PAO after failed hip arthroscopy in hip dysplasia [6]. Hip arthroscopy alone in this group of patients should be therefore approached with caution. However, it may have a role in those patients who are either unsuitable for PAO either because their hips are unfavourable (i.e. have a normal AI and normal femoral anteversion) or because their advanced age (i.e. >40 years).
WHAT ARE THE RESULTS OF REORIENTING PERIACETABULAR OSTEOTOMY IN THIS GROUP OF PATIENTS?
Acetabular reorientation via the periacetabular osteotomy has become the most common treatment for acetabular dysplasia with good outcomes reported at over 20 years postoperatively. Traditionally intra-articular pathology was addressed at the time of PAO by performing an anterior arthrotomy. However with the development of minimally invasive techniques for PAO this is no longer necessarily the case. Less invasive PAO techniques have decreased the time to postoperative recovery [45].
A recent study showed modifiable factors such as higher physical activity and higher BMI greater than 30 kg/m2 lead to a decreased age of presentation for PAO [46]. Furthermore patients also presented earlier for PAO with worse degrees of dysplasia: the LCEA was independently predictive of age at surgery, i.e. patients with a lower LCEA tended to require PAO surgery at an earlier age. However, there was no difference in outcomes following PAO between mild and moderate dysplasia. In this study mild dysplasia was classified as 15–25° which encompasses our definition of borderline hip dysplasia. A recent multicenter prospective cohort study that examined patient-reported outcome measures of PAO showed that, although overall results were good, improvements in borderline hip dysplastics and males were less than in those patients who had more severe dysplasia [47]. The authors discussed this with the danger of a small correction that may lead to overcorrection and iatrogenic FAI, increased femoral antetorsion and soft tissue laxity.
RECOMMENDATIONS AND FUTURE DIRECTIONS
In borderline hips the crucial step is to define stability. Regarding the stability of the hip there are only two conditions: The hip is either stable or unstable. There is nothing in between. If this concept is accepted, the treatment gets comparably simple. Instability may be combined with other pathologies like FAI or overload/overuse and cartilage disease which need concomitant treatment. If the hip is unstable, acetabular reorientation is necessary. Addressing only worn out secondary stabilizers does not solve the underlying biomechanic problem and at best will yield satisfactory short term results. In stable hips, open or arthroscopic joint preserving surgery may be performed. However, we have to keep in mind that each degree decrease of the LCE angle below 28° is associated with a 13% increase of osteoarthrosis [20]. Therefore, if in doubt, in order to maximize the chance of good long-term results, we would advocate for an acetabular reorientation operation.
It is important to identify the areas where we lack knowledge in order to guide further research. Longer-term follow-up studies comparing acetabular reorientation and hip arthroscopy in these patients, ideally in which all imaging parameters and Beighton scores are recorded would be performed. In addition patient-reported outcome measures and time to recovery and resumption of activities including sport should be attained.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Albers CE, Steppacher SD, Ganz R. et al. Impingement adversely affects 10-year survivorship after periacetabular osteotomy for DDH. Clin Orthop Relat Res 2013; 471: 1602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fredensborg N. The CE Angle of Normal Hips. Acta Orthop Scand 1976; 47: 403–5. [DOI] [PubMed] [Google Scholar]
- 3. Fukui K, Trindade CAC, Briggs KK. et al. Arthroscopy of the hip for patients with mild to moderate developmental dysplasia of the hip and femoroacetabular impingement. Bone Joint J 2015; 97-B: 1316–21. [DOI] [PubMed] [Google Scholar]
- 4. Nawabi DH, Degen RM, Fields KG. et al. Outcomes after arthroscopic treatment of femoroacetabular impingement for patients with borderline hip dysplasia. Am J Sports Med 2016; 44: 1017–23. [DOI] [PubMed] [Google Scholar]
- 5. Cooperman DR, Wallensten R, Stulberg SD.. Acetabular dysplasia in the adult. Clin Orthop Relat Res 1983; 175: 79–85. [PubMed] [Google Scholar]
- 6. Ricciardi BF, Fields KG, Wentzel C. et al. Early functional outcomes of periacetabular osteotomy after failed hip arthroscopic surgery for symptomatic acetabular dysplasia. Am J Sports Med 2017; 45: 2460–7. [DOI] [PubMed] [Google Scholar]
- 7. Paliobeis CP, Villar RN.. The prevalence of dysplasia in femoroacetabular impingement. Hip Int 2011; 21: 141–5. [DOI] [PubMed] [Google Scholar]
- 8. Kalisvaart MM, Safran MR.. Microinstability of the hip–it does exist: etiology, diagnosis and treatment. J. Dance Med Sci 2016; 20: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint: with special reference to the complication of osteoarthritis. Acta Chir Scand 1939; 83: 53–68. [Google Scholar]
- 10. Jo S, Lee SH, Wang SI. et al. The role of arthroscopy in the dysplastic hip—a systematic review of the intra-articular findings, and the outcomes utilizing hip arthroscopic surgery. J Hip Preserv Surg 2016; 3: 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omeroglu H, Biçimoglu A, Aguş H. et al. Measurement of center-edge angle in developmental dysplasia of the hip: a comparison of two methods in patients under 20 years of age. Skeletal Radiol 2002; 31: 25–9. [DOI] [PubMed] [Google Scholar]
- 12. Domb BG, Stake CE, Lindner D. et al. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia. Am J Sports Med 2013; 41: 2591–8. [DOI] [PubMed] [Google Scholar]
- 13. Garabekyan T, Ashwell Z, Chadayammuri V. et al. Lateral acetabular coverage predicts the size of the hip labrum. Am J Sports Med 2016; 44: 1582–9. [DOI] [PubMed] [Google Scholar]
- 14. Zaltz I, Kelly BT, Larson CM. et al. Surgical treatment of femoroacetabular impingement: what are the limits of hip arthroscopy? Arthroscopy 2014; 30: 99–110. [DOI] [PubMed] [Google Scholar]
- 15. Pereira F, Giles A, Wood G. et al. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int 2014. [DOI] [PubMed] [Google Scholar]
- 16. Sankar WN, Neubuerger CO, Moseley CF.. Femoral anteversion in developmental dysplasia of the hip. J Pediatr Orthopaedics 2009; 29: 885–8. [DOI] [PubMed] [Google Scholar]
- 17. Li PLS, Ganz R.. Morphologic features of congenital acetabular dysplasia. Clin Orthop Relat Res 2003; 416: 245–53. [DOI] [PubMed] [Google Scholar]
- 18. Henak CR, Ellis BJ, Harris MD. et al. Role of the acetabular labrum in load support across the hip joint. J Biomech 2011; 44: 2201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wedge JH, Wasylenko MJ.. The natural history of congenital dislocation of the hip: a critical review. Clin Orthop Relat Res 1978; 137: 154. [PubMed] [Google Scholar]
- 20. Thomas GER, Palmer AJR, Batra RN. et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage 2014; 22: 1504–10. [DOI] [PubMed] [Google Scholar]
- 21. Griffin DR, Dickenson EJ, O'Donnell J. et al. The Warwick agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med 2016; 50: 1179–76. [DOI] [PubMed] [Google Scholar]
- 22. Cronin MDJ, Bankes MJK.. Clinical diagnosis in hip disease In: The Young Adult Hip in Sport. London: Springer London, 2014, pp. 27–36. [Google Scholar]
- 23. Tannast M, Siebenrock KA, Anderson SE.. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. Am J Roentgenol 2007; 188: 1540–52. [DOI] [PubMed] [Google Scholar]
- 24. Stubbs AJ, Anz AW, Frino J. et al. Classic measures of hip dysplasia do not correlate with three-dimensional computer tomographic measures and indices. Hip Int 2011; 21: 549–58. [DOI] [PubMed] [Google Scholar]
- 25. Dandachli W, Kannan V, Richards R. et al. Analysis of cover of the femoral head in normal and dysplastic hips. Bone Joint J 2008; 90-B: 1428–34. [DOI] [PubMed] [Google Scholar]
- 26. Dandachli W, Ul Islam S, Tippett R. et al. Analysis of acetabular version in the native hip: comparison between 2D axial CT and 3D CT measurements. Skeletal Radiol 2011; 40: 877–83. [DOI] [PubMed] [Google Scholar]
- 27. Sutter R, Dietrich TJ, Zingg PO. et al. Femoral antetorsion: comparing asymptomatic volunteers and patients with femoroacetabular impingement. Radiology 2012; 263: 475–83. [DOI] [PubMed] [Google Scholar]
- 28. Leunig M, Podeszwa D, Beck M. et al. Magnetic resonance arthrography of labral disorders in hips with dysplasia and impingement. Clin Orthop Relat Res 2004; 418: 74–80. [DOI] [PubMed] [Google Scholar]
- 29. Babst D, Steppacher SD, Ganz R. et al. The iliocapsularis muscle: an important stabilizer in the dysplastic hip. Clin Orthop Relat Res 2011; 469: 1728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyatt M, Weidner J, Pfluger D. et al. The femoro-epiphyseal acetabular roof (FEAR) index: a new measurement associated with instability in borderline hip dysplasia? Clin Orthop Relat Res 2017; 475: 861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech 1994; 28: 505–19. [DOI] [PubMed] [Google Scholar]
- 32. Pauwels F, Maquet P. Biomécanique de l'appareil moteur: contributions à l'étude de l”anatomie fonctionnelle: traduit de l”allemand, entièrement revu et complété, comprenant… . 1979.
- 33. Carter DR, Orr TE, Fyhrie DP. et al. Influences of mechanical stress on prenatal and postnatal skeletal development. Clin Orthop Relat Res 1987; 237 − 50.. [PubMed] [Google Scholar]
- 34. Fabeck L, Tolley M, Rooze M. et al. Theoretical study of the decrease in the femoral neck anteversion during growth. Cells Tissues Organs 2002; 171: 269–75. [DOI] [PubMed] [Google Scholar]
- 35. Wall PDH, Fernandez M, Griffin DR. et al. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM&R 2013; 5: 418–26. [DOI] [PubMed] [Google Scholar]
- 36. Byrd JWT, Jones KS.. Hip arthroscopy in the presence of dysplasia. Arthroscopy 2003; 19: 1055–60. [DOI] [PubMed] [Google Scholar]
- 37. Nepple JJ, Clohisy JC.. The dysplastic and unstable hip: a responsible balance of arthroscopic and open approaches. Sports Med Arthrosc 2015; 23: 180–6. [DOI] [PubMed] [Google Scholar]
- 38. Benali Y, Katthagen BD.. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy 2009; 25: 405–7. [DOI] [PubMed] [Google Scholar]
- 39. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy 2009; 25: 400–4. [DOI] [PubMed] [Google Scholar]
- 40. Mei-Dan O, McConkey MO, Brick M.. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy 2012; 28: 440–5. [DOI] [PubMed] [Google Scholar]
- 41. Kain MS, Novais EN, Vallim C. et al. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg 2011; 93: 57–61. [DOI] [PubMed] [Google Scholar]
- 42. Nepple JJ, Smith MV.. Biomechanics of the hip capsule and capsule management strategies in hip arthroscopy. Sports Med Arthrosc 2015; 23: 164–8. [DOI] [PubMed] [Google Scholar]
- 43. Parvizi J, Bican O, Bender B. et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty 2009; 24: 110–3. [DOI] [PubMed] [Google Scholar]
- 44. Ross JR, Zaltz I, Nepple JJ. et al. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med 2011; 39: 72–78S. [DOI] [PubMed] [Google Scholar]
- 45. Khan OH, Malviya A, Subramanian P. et al. Minimally invasive periacetabular osteotomy using a modified Smith-Petersen approach. Bone Joint J 2017; 99-B: 22–8. [DOI] [PubMed] [Google Scholar]
- 46.Matheney T, Zaltz I, Kim Y-J. et al. Activity level and severity of dysplasia predict age at bernese periacetabular osteotomy for symptomatic hip dysplasia. J Bone Joint Surg 2016; 98: 665–71. [DOI] [PubMed] [Google Scholar]
- 47. Clohisy JC, Ackerman J, Baca G. et al. Patient-reported outcomes of periacetabular osteotomy from the prospective ANCHOR Cohort Study. J Bone Joint Surg 2017; 99: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]