Abstract
The West Africa Ebola epidemic stimulated rapid implementation of Ebola vaccine trials in the 3 highly affected countries. In Sierra Leone, we studied the recombinant vesicular stomatitis virus Ebola vaccine (rVSV∆G-ZEBOV-GP) safety and efficacy. The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) was a randomized, unblinded Phase 2/3 trial with phased vaccine introduction, no placebo, and concurrent evaluation of vaccine safety and efficacy. Healthcare and frontline response workers in 5 districts were randomized to immediate or deferred (18–24 weeks later) vaccination and followed for 6 months postvaccination. We enrolled 8651 participants from April through August 2015; 7998 were vaccinated. No participants developed Ebola virus disease so an efficacy assessment was not possible. Overall, 132 (1.5%) participants experienced serious adverse events (SAEs); none were vaccine-related. In a detailed safety substudy (N = 436), vaccinated participants reported significantly more systemic adverse events (AEs) within 7 days than unvaccinated participants including fever higher than 38°C (20.5% vs 3.9%), headache (71.2% vs 22.1%), fatigue (50.7% vs 10.4%), and joint pain (31.7% vs 6.5%); most AEs were mild to moderate severity and resolved within 5 days. During days 5-28, vaccinated participants more commonly reported joint pain (17.0% vs 4.8%) and rash (7.8% vs 1.7%) (P<.05 for both comparisons). Vaccinated participants also more commonly reported skin vesicles (2.0% vs 0%) and mouth ulcers (2.0% vs 0%) but only during days 8-14 (P<.05 for both comparisons). Among almost 8000 high-risk workers vaccinated during the Sierra Leone Ebola epidemic, rVSV∆G-ZEBOV-GP was generally well tolerated with no vaccine-related SAEs. Reported joint pain, rash, skin vesicles, and mouth ulcers postvaccination are consistent with conditions associated with transient viral replication described among participants in other trials.
Clinical Trials Registration
ClinicalTrials.gov [NCT02378753] and Pan African Clinical Trials Registry [PACTR201502001037220].
Keywords: clinical trial, Ebola, safety, serious adverse events, vaccine
The 2014–2016 Ebola virus disease (Ebola) epidemic in West Africa, with 14112 reported cases and 4806 deaths in Sierra Leone alone, was unprecedented in magnitude and complexity and threatened global health security [1]. By October 2014, the Sierra Leone epidemic had reached most districts, and cases were increasing exponentially in urban areas [2]. Healthcare workers experienced 100-fold higher Ebola risk than other adults [3]. The public health community, industry, regulatory bodies, and others began planning to rapidly initiate Ebola vaccine trials [4].
Early formulations of the experimental recombinant vesicular stomatitis virus Ebola vaccine (rVSVΔG-ZEBOV-GP) (Merck & Co., Inc, Kenilworth, NJ) in nonhuman primates provided excellent mortality protection up to 4 weeks postvaccination [5]. Phase 1 results in humans documented good immunogenicity, but safety data were limited, and researchers temporarily halted one European trial in December 2014 because of postvaccination arthritis [6]. Given the urgent situation, 3 Phase 2/3 trials using this vaccine began in West Africa in early 2015, using different study designs to increase the likelihood of collecting robust efficacy and safety data. In August 2015, the World Health Organization (WHO)-led vaccine trial in Guinea reported promising efficacy [7].
The Centers for Disease Control and Prevention (CDC) sponsored the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) and conducted it in collaboration with the College of Medicine and Allied Health Sciences, University of Sierra Leone, and the Ministry of Health and Sanitation. We designed STRIVE to accelerate availability of a potentially efficacious vaccine to a high-risk population—healthcare and frontline response workers—while simultaneously evaluating safety and efficacy [8]. Because safety data were limited, especially in African populations, we included a detailed safety substudy. We launched STRIVE after aggressive Ebola control efforts were implemented and after the epidemic peaked; no STRIVE participants developed Ebola so an efficacy assessment was not possible. This report describes safety results from STRIVE, the largest cohort vaccinated with rVSVΔG-ZEBOV-GP reported to date.
METHODS
Study Design and Participants
The STRIVE study was an individually randomized, unblinded Phase 2/3 trial with phased vaccine introduction, no placebo, and concurrent evaluation of vaccine safety and efficacy. Participants were randomized to immediate (within 7 days of enrollment) or deferred (18–24 weeks after enrollment) vaccination (Figure 1); these groups were to be compared for vaccine safety and efficacy. Once vaccinated, deferred participants were termed “crossover-vaccinated” and continued safety follow-up. We randomized participants separately in each of the 7 enrollment sites and minimized site-level imbalance using the Big Stick Design with a maximum imbalance of 3 [9, 10]. We used a group sequential design for efficacy measurement, specifying up to 67 Ebola events during 3 interim and 1 final data analyses dependent on crossing an early stopping boundary. This design had a cumulative power of 80% to reject the null hypothesis of 0% vaccine efficacy if the true efficacy was ≥50% vaccine efficacy or there was a 2-fold infection risk increase with a 2-sided 5% level test.
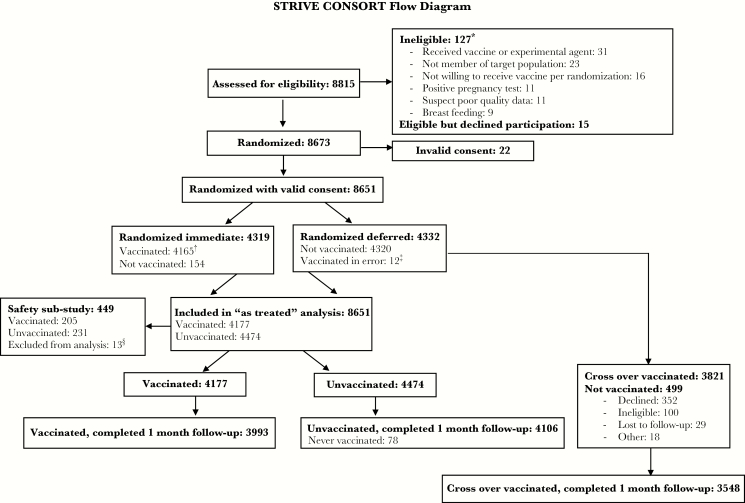
Figure 1.
Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) consort flow diagram. *More than 1 reason for ineligibility could be selected; the 6 most common reasons are listed. †A total of 4123 participants were vaccinated within 7 days; 42 were vaccinated after 7 days. ǂIncluded in the vaccinated group. §10 with invalid substudy consent; 3 other.
The study started in April 2015 in 5 districts with high Ebola incidence. Participants were healthcare and frontline Ebola response workers ≥18 years old. Exclusion criteria included history of Ebola, current pregnancy (pregnancy testing was required for all women <50 years old), breastfeeding, and self-reported human immunodeficiency virus infection or clinically important immunodeficiency (Appendix). Before crossover vaccination, we repeated screening procedures, including pregnancy testing.
The protocol was approved by the Sierra Leone Ethics and Scientific Review Committee, CDC Institutional Review Board, Pharmacy Board of Sierra Leone, and US Food and Drug Administration (FDA). Participants provided written informed consent. A scientific steering committee provided study design and implementation guidance. An independent data safety monitoring board reviewed study data approximately weekly for the first 4 months, monthly until deferred vaccination was complete, and then every 2 months. When preliminary data from the WHO trial in Guinea trial reported efficacy of rVSVΔG-ZEBOV-GP in August 2015 [7], we amended our study protocol to allow early vaccination of deferred participants who were not-yet-vaccinated if they were at increased risk of an Ebola exposure.
Vaccine
The rVSVΔG-ZEBOV-GP is a replication-competent recombinant vaccine [11]. Details on vaccine formulation, reconstitution, and storage are in the Appendix. Participants received a single 2 × 107 plaque-forming unit dose by intramuscular injection, were observed for 60 minutes, and counseled on Ebola prevention measures and the need to seek care for possible Ebola symptoms. Women were advised to avoid pregnancy for 2 months after vaccination [12].
Safety Monitoring
Main Study
From enrollment through 6 months after vaccination, the study staff contacted participants monthly to monitor for adverse events (AEs), suspected Ebola, and pregnancy; thus, deferred participants were monitored for up to 12 months. Safety
monitoring was conducted in accordance with FDA guidance [13]. The primary safety endpoint was serious adverse events (SAEs) within 6 months of vaccination; we report the 1- and 6-month results. Participants received a cell phone to access a toll-free hotline for nursing advice and medical referrals; home visits were conducted if participants were not reached by telephone for the monthly call. Women who became pregnant within 2 months of vaccination or enrollment were monitored for external congenital anomalies in their infants that were detectable at birth or during the neonatal period. Study physicians provided free medical care to participants with AEs of concern at designated clinics/hospitals. With the local principal investigator, the study physicians assessed SAEs for expectedness, severity, and causality according to FDA guidance. They attempted a full autopsy after deaths and, if that was not feasible, they conducted a verbal autopsy (a method of gathering health information about a deceased individual to determine his or her cause of death) using standard WHO methodology [13, 14] (Appendix).
Safety Substudy
We enrolled initial participants from one enrollment site (Western Rural) in the safety substudy, which included telephone assessments scheduled on days 1, 3, 7, 14, and 28 and a daily symptom diary card. Endpoints were solicited injection-site and systemic reactogenicity AEs on vaccination day and during the following 7 days, and solicited and unsolicited AEs through 28 days. Solicited AEs of particular interest included joint pain, joint swelling, rash, skin vesicles, and oral ulceration [6]. The SAEs and severe (Grade 3) AEs were assessed for causality.
Monitoring and Laboratory Testing for Ebola
To reduce the risk of unnecessary referral and exposure to an Ebola facility, STRIVE worked with national authorities to modify the Ebola case definition so participants with stable, vaccine-related AEs (eg, fever) within 48 hours of vaccination could be observed at home. Participants with any symptoms consistent with Ebola and not vaccination, any symptom of concern lasting > 24 hours, or any exposure to an Ebola patient were immediately referred for Ebola testing at national laboratories [15]. The CDC Ebola laboratory in Sierra Leone performed confirmatory testing (Appendix).
Statistical Analyses
The safety analysis included all randomized participants with at least 1 monthly follow-up assessment. We calculated the proportion of participants with SAEs overall and within Medical Dictionary for Regulatory Activities (MedDRA) v19.0 specific organ classes (SOC) and preferred terms. We compared groups by randomization status (immediate vs deferred) and vaccination status (vaccinated vs unvaccinated) using Barnard’s exact unconditional test. Data from crossover-vaccinated participants are described separately. Because the “intention to treat” (immediate versus deferred) and the “as-treated” (vaccinated versus unvaccinated) results were similar, we report the “as-treated” analyses. In the safety substudy, we similarly compared rates of solicited AEs within 7 and 28 days and during days 5–28, the latter interval chosen based on the timing of postvaccination arthritis and associated conditions reported in studies published after STRIVE initiation [6, 16]. For the safety analyses, we performed all comparisons at the 5% level; no adjustments were made for multiple comparisons.
RESULTS
Study Population
Between April 9 and August 15, 2015, we enrolled 8651 of 8815 eligibility-screened participants across 7 sites in the 5 districts (Figures 1 and 2). Eleven women were ineligible because of a positive pregnancy test. After enrollment, 4319 participants were randomized to immediate and 4332 to deferred vaccination. Vaccination for deferred participants occurred from September 19 to December 12, 2015. Overall, 7998 participants were vaccinated, including 96 deferred participants who were eligible for early vaccination in September 2015 due to risk of of Ebola exposure (median time to vaccination of 130 days; range, 94–151).
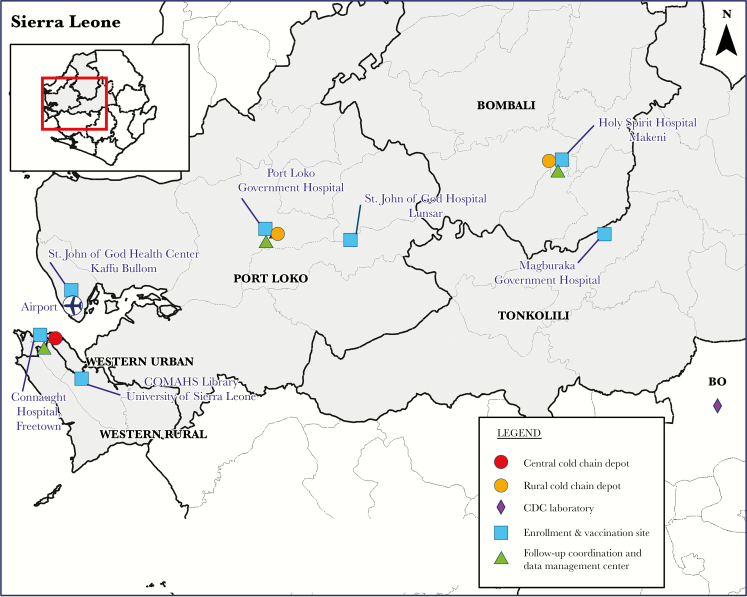
Figure 2.
Map of Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) study sites, Sierra Leone. Abbreviation: CDC, Centers for Disease Control and Prevention.
The median age of participants was 30.7 years (range, 18.0–79.5 years); 60.6% were male. Demographic characteristics and occupations were balanced across vaccination sites and study groups (Table 1, Supplementary Table 1). On enrollment, most participants were working as frontline response workers or nurses; 34.6% of participants worked in an Ebola facility and 39.8% worked in a hospital. Two thirds of participants reported a perceived high risk of Ebola infection.
Table 1.
Participant Demographics by Vaccination Status
| Study Variable | Unvaccinated (N = 4474) | Vaccinated (N = 4177) | Crossover Vaccinated (N = 3821) | All Vaccinated (N = 7998) | All Randomized (N = 8651) |
|---|---|---|---|---|---|
| Site (District)—N (%) | |||||
| COMAHS Library (Western Rural) | 993 (22.2) | 900 (21.5) | 849 (22.2) | 1749 (21.9) | 1893 (21.9) |
| Connaught Hospital (Western Urban) | 1707 (38.2) | 1546 (37.0) | 1376 (36.0) | 2922 (36.5) | 3253 (37.6) |
| Port Loko Government Hospital (Port Loko no. 1) | 439 (9.8) | 425 (10.2) | 400 (10.5) | 825 (10.3) | 864 (10.0) |
| Saint John of God Hospital, Lunsar (Port Loko no. 2) | 204 (4.6) | 193 (4.6) | 172 (4.5) | 365 (4.6) | 397 (4.6) |
| Saint John of God Health Center, Kaffu Bullom (Port Loko no. 3) | 151 (3.4) | 151 (3.6) | 139 (3.6) | 290 (3.6) | 302 (3.5) |
| Holy Spirit Hospital, Makeni (Bombali) | 612 (13.7) | 596 (14.3) | 546 (14.3) | 1142 (14.3) | 1208 (14.0) |
| Magburaka Government Hospital (Tonkolili) | 368 (8.2) | 366 (8.8) | 339 (8.9) | 705 (8.8) | 734 (8.5) |
| Age (years) | |||||
| Median [range] | 30.8 [18.0–79.5] | 30.6 [18.1–78.0] | 31.0 [18.0–79.5] | 30.8 [18.0– 79.5] | 30.7 [18.0– 79.5] |
| Gender—N (%) | |||||
| Female | 1813 (40.5) | 1594 (38.2) | 1360 (35.6) | 2954 (36.9) | 3407 (39.4) |
| Male | 2661 (59.5) | 2583 (61.8) | 2461 (64.4) | 5044 (63.1) | 5244 (60.6) |
| Primary Occupation—N (%) | |||||
| Nursea | 1527 (34.1) | 1350 (32.3) | 990 (25.9) | 2340 (29.3) | 2877 (33.3) |
| Allied health professionalsb | 77 (1.7) | 72 (1.7) | 58 (1.5) | 130 (1.6) | 149 (1.7) |
| Doctor | 12 (0.3) | 13 (0.3) | 7 (0.2) | 20 (0.3) | 25 (0.3) |
| Pharmacist | 20 (0.4) | 20 (0.5) | 20 (0.5) | 40 (0.5) | 40 (0.5) |
| Community health worker | 93 (2.1) | 85 (2.0) | 68 (1.8) | 153 (1.9) | 178 (2.1) |
| Laboratory worker | 143 (3.2) | 130 (3.1) | 141 (3.7) | 271 (3.4) | 273 (3.2) |
| Frontline Ebola response workerc | 2125 (47.5) | 2041 (48.9) | 1346 (35.2) | 3387 (42.3) | 4166 (48.2) |
| Surveillance worker | 258 (5.8) | 242 (5.8) | 170 (4.4) | 412 (5.2) | 500 (5.8) |
| Other/Not reported | 219 (4.9) | 224 (5.4) | 128 (3.3) | 352 (4.4) | 443 (5.1) |
| Not currently working in a health facility | 0 | 0 | 893 (23.4) | 893 (11.2) | 0 |
| Facility Type—N (%) | |||||
| Ebola Facility | 1553 (34.7) | 1436 (34.4) | 688 (18.0) | 2124 (26.6) | 2989 (34.6) |
| Hospital | 1784 (39.9) | 1659 (39.7) | 1369 (35.8) | 3028 (37.9) | 3443 (39.8) |
| Community/field/clinic | 809 (18.1) | 790 (18.9) | 842 (22.0) | 1632 (20.4) | 1599 (18.5) |
| Other/Not reported | 328 (7.3) | 292 (7.0) | 922 (24.1) | 1214 (15.2) | 620 (7.2) |
| Education—N (%) | |||||
| None | 369 (8.2) | 371 (8.9) | 340 (8.9) | 711 (8.9) | 740 (8.6) |
| Primary | 222 (5.0) | 229 (5.5) | 191 (5.0) | 420 (5.3) | 451 (5.2) |
| Secondary | 1977 (44.2) | 1846 (44.2) | 1778 (46.5) | 3624 (45.3) | 3823 (44.2) |
| Tertiary | 1884 (42.1) | 1711 (41.0) | 1504 (39.4) | 3215 (40.2) | 3595 (41.6) |
| Other/Not reported | 22 (0.5) | 20 (0.5) | 8 (0.2) | 28 (0.4) | 42 (0.5) |
| Perceived Risk of Ebola Infection—N (%) | |||||
| Highd | 2995 (66.9) | 2811 (67.3) | 927 (24.3) | 3738 (46.7) | 5806 (67.1) |
| Average | 773 (17.3) | 760 (18.2) | 724 (18.9) | 1484 (18.6) | 1533 (17.7) |
| Lowe | 705 (15.8) | 606 (14.5) | 2170 (56.8) | 2776 (34.7) | 1311 (15.2) |
| Not Reported | 1 (0.0) | 0 | 0 | 0 | 1 (0.0) |
Abbreviation: COMAHS, College of Medicine and Allied Health Sciences.
aIncludes nurse, community health nurse, maternal-child health aide, midwife, nurse aide, nursing student, vaccinator.
bIncludes dentist, medical counselor, nutritionist, physiotherapist.
cIncludes contact tracers, ambulance crew, burial workers, swabbers (took post mortem skin/mucosal swabs for Ebola testing on all deceased people).
dParticipants who responded “very high” or “high” are included in the high category.
eParticipants who responded “very low” or “low” are included in the low category.
Safety Monitoring
Main Study
Overall, among the 8651 participants, 132 (1.5%) reported 143 SAEs during follow-up; 54 (1.3%) vaccinated participants (median follow-up, 180 days), and 32 (0.7%) participants during their unvaccinated follow-up [median, 150 days] and 47 (1.2%) during their crossover vaccination follow-up (median, 180 days). No SAEs were considered vaccine-related (Supplementary Table 2). The most common SAEs were MedDRA SOC “infections and infestations”; malaria accounted for approximately half of these. Twenty-four (0.2%) study participants died during the 6-month safety follow-up, and 1 additional death occurred during pregnancy follow-up; deaths were balanced across study groups. Among 3993 vaccinated and 4106 unvaccinated participants who completed the 1-month follow-up, the proportion with reported SAEs within 1 month of vaccination or enrollment was significantly higher for vaccinated (0.5%) than unvaccinated participants (0.2%, P = .010) (Table 2).
Table 2.
Proportion of Participants With Serious Adverse Events by MedDRA System Organ Class and Preferred Term, by Vaccination Status Through Month 1 Postrandomization or Vaccination
| MedDRA System Organ Class/ Preferred Term | Vaccinated (N = 3993) | Unvaccinated (N = 4106) | Crossover Vaccinated (N = 3548) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | N (%) | 95% CI | Events | N (%) | 95% CI | Events | N (%) | 95% CI | P Valuea | |
| Blood and Lymphatic System Disorders (sickle cell anemia with crisis) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| Gastrointestinal Disorders | 8 | 8 (0.2) | (<0.1–0.4) | 1 | 1 (0.0) | (<0.1–0.1) | 3 | 3 (0.1) | (<0.1–0.2) | .018 |
| Enteritis | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | - |
| Inguinal hernia | 2 | 2 (0.1) | (<0.1–0.2) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | .153 |
| Inguinal hernia, obstructive | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | .314 |
| Peptic ulcer | 3 | 3 (0.1) | (<0.1–0.2) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .080 |
| Peptic ulcer perforation | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | .327 |
| Toothache | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| Umbilical hernia | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| General Disorders and Administration Site Conditions | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | .314 |
| Deathb | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | - |
| Herniac | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| Infections and Infestations | 9 | 8 (0.2) | (<0.1–0.4) | 3 | 2 (0.0) | (<0.1–0.2) | 3 | 3 (0.1) | (<0.1–0.2) | .053 |
| Appendicitis | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | .314 |
| Ludwig angina | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | .327 |
| Malaria | 7 | 7 (0.2) | (<0.1–0.4) | 1 | 1 (0.0) | (<0.1–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | .031 |
| Typhoid fever | 1 | 1 (0.0) | (<0.1–0.1) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 1.000 |
| Urinary tract infection | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | - |
| Injury, Poisoning, and Procedural Complications | 1 | 1 (0.0) | (<0.1–0.1) | 4 | 4 (0.1) | (<0.1–0.2) | 3 | 3 (0.1) | (<0.1–0.2) | .192 |
| Fractures, clavicled | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | .327 |
| Fractures, forearmd | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | - |
| Fractures, lower limbd | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | .314 |
| Joint dislocation | 0 | 0 | (0.0–0.1) | 2 | 2 (0.0) | (<0.1–0.2) | 0 | 0 | (0.0–0.1) | .165 |
| Laceration | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | .327 |
| Skeletal injurye | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | - |
| Metabolism and Nutrition Disorders (Diabetes mellitus) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| Psychiatric Disorders (Anxiety) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| Renal and Urinary Disorders (Ureterolithiasis) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | 1 | 1 (0.0) | (<0.1–0.2) | - |
| Vascular Disorders (Hypovolaemic shock) | 1 | 1 (0.0) | (<0.1–0.1) | 0 | 0 | (0.0–0.1) | 0 | 0 | (0.0–0.1) | .314 |
| Total Serious Adverse Eventsf | 23 | 20 (0.5) | (0.3–0.8) | 8 | 7 (0.2) | (<0.1–0.4) | 11 | 11 (0.3) | (0.2–0.6) | .010 |
Abbreviations: CI, confidence interval; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse events.
a P value for difference in proportion of vaccinated and unvaccinated participants by Barnard’s exact unconditional test.
bInsufficient information available to determine cause of death. See also Supplementary Table 2.
cSite of hernia not known.
dFor MedDRA codes for fractures, the order of the wording was reversed from “site of fracture, fractures” to “fractures, site of fracture” (eg, clavicle, fractures” to fractures, clavicle” so all fractures would be listed sequentially in the table.
eCar accident.
fTotals reflects the number of SAE events (42) and the number of participants reporting SAEs (38): 34 participants reported 1 SAE, and 4 participants reported 2 SAEs.
A total of 104 gestations in 103 women (43 vaccinated; 60 unvaccinated) occurred with estimated onset within 2 months after vaccination or enrollment. Birth outcomes were unknown for 3 (7.0%) vaccinated and 14 (23.3%) unvaccinated women. Among those with known birth outcomes, pregnancy losses occurred in 17 (42.5%) vaccinated (including 1 set of twins) and 20 (43.5%) unvaccinated women; 23 vaccinated and 26 unvaccinated women had 49 live births. No congenital anomalies were diagnosed among the 38 infants whose mothers consented to a 28-day follow-up nor in the neonatal death (1 of 2) where a maternal report was available.
Safety Substudy
The 436 substudy participants (205 vaccinated, 231 unvaccinated) had similar demographic characteristics to the overall study participants, except for a lower median age (27.6 vs 30.7 years; P < .001) (Supplementary Table 3). Among the vaccinated substudy participants, 91.2% reported systemic AEs within 7 days of vaccination compared with 35.5% of unvaccinated participants (P < .001); they were more likely to experience fever, feverishness, fatigue, feeling unwell, muscle pain, joint pain, chills, headache, nausea, abdominal pain, rash, and skin vesicles (Table 3). Most of these events occurred within 24–48 hours of vaccination and resolved within 5 days (Supplementary Figure 1). Five (2.4%) vaccinated participants reported severe systemic reactions, most commonly elevated temperature (≥39.0°C) with or without other symptoms (Table 3). Similar results were observed during the full safety substudy period (0–28 days postvaccination) (Supplementary Table 4). Two SAEs were reported in the substudy, malaria in a vaccinated participant, and Ludwig’s angina in an unvaccinated participant. One pregnancy was reported in each substudy group.
Table 3.
Local and Systemic Reactogenicity Through Day 7 by Vaccination Status: Safety Substudy
| Vaccinated (N = 205) | Unvaccinated (N = 231) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any | Severea | Any | Severea | Any | Severea | |||||
| Adverse Event | N (%) | (95% CI) | N (%) | (95% CI) | N (%) | (95% CI) | N (%) | (95% CI) | P Valueb | |
| Systemic Adverse Events | ||||||||||
| Elevated temperaturec | 42 (20.5) | (15.2–26.7) | 4 (2.0) | (0.5–4.9) | 9 (3.9) | (1.8–7.3) | 0 | (0.0–1.6) | <.001 | .034 |
| Feverishness | 104 (50.7) | (43.7–57.8) | 0 | (0.0–1.8) | 22 (9.5) | (6.1–14.1) | 0 | (0.0–1.6) | <.001 | - |
| Fatigue | 104 (50.7) | (43.7–57.8) | 1 (0.5) | (<0.1–2.7) | 24 (10.4) | (6.8–15.1) | 0 | (0.0–1.6) | <.001 | .360 |
| Feeling unwell | 58 (28.3) | (22.2–35.0) | 0 | (0.0–1.8) | 15 (6.5) | (3.7–10.5) | 0 | (0.0–1.6) | <.001 | - |
| Muscle pain | 59 (28.8) | (22.7–35.5) | 0 | (0.0–1.8) | 8 (3.5) | (1.5–6.7) | 0 | (0.0–1.6) | <.001 | - |
| Joint pain | 65 (31.7) | (25.4–38.6) | 0 | (0.0–1.8) | 15 (6.5) | (3.7–10.5) | 0 | (0.0–1.6) | <.001 | - |
| Joint swelling | 6 (2.9) | (1.1–6.3) | 0 | (0.0–1.8) | 2 (0.9) | (0.1–3.1) | 0 | (0.0–1.6) | .116 | - |
| Chills | 37 (18.0) | (13.0–24.0) | 0 | (0.0–1.8) | 10 (4.3) | (2.1–7.8) | 0 | (0.0–1.6) | <.001 | - |
| Headache | 146 (71.2) | (64.5–77.3) | 1 (0.5) | (<0.1–2.7) | 51 (22.1) | (16.9–28.0) | 0 | (0.0–1.6) | <.001 | .360 |
| Vomiting | 1 (0.5) | (<0.1–2.7) | 0 | (0.0–1.8) | 1 (0.4) | (<0.1–2.4) | 0 | (0.0–1.6) | .996 | - |
| Nausea | 10 (4.9) | (2.4–8.8) | 0 | (0.0–1.8) | 1 (0.4) | (<0.1–2.4) | 0 | (0.0–1.6) | .003 | - |
| Diarrhea | 4 (2.0) | (0.5–4.9) | 0 | (0.0–1.8) | 2 (0.9) | (0.1–3.1) | 0 | (0.0–1.6) | .371 | - |
| Abdominal pain | 23 (11.2) | (7.2–16.4) | 1 (0.5) | (<0.1–2.7) | 4 (1.7) | (0.5–4.4) | 0 | (0.0–1.6) | <.001 | .360 |
| Rash | 13 (6.3) | (3.4–10.6) | 0 | (0.0–1.8) | 1 (0.4) | (<0.1–2.4) | 0 | (0.0–1.6) | <.001 | - |
| Oral ulcers | 3 (1.5) | (0.3–4.2) | 0 | (0.0–1.8) | 1 (0.4) | (<0.1–2.4) | 0 | (0.0–1.6) | .303 | - |
| Skin vesicles | 7 (3.4) | (1.4–6.9) | 0 | (0.0–1.8) | 0 | (0.0–1.6) | 0 | (0.0–1.6) | .005 | - |
| Any systemic adverse eventsd | 187 (91.2) | (86.5–94.7) | 5 (2.4) | (0.8–5.6) | 82 (35.5) | (29.3–42.0) | 0 | (0.0–1.6) | <.001 | .017 |
| Local Adverse Events | ||||||||||
| Pain | 166 (81.0) | (74.9–86.1) | 0 | (0.0–1.8) | N/A | N/A | N/A | N/A | N/A | N/A |
| Redness (measurement grade) | 2 (1.0) | (0.1–3.5) | 0 | (0.0–1.8) | N/A | N/A | N/A | N/A | N/A | N/A |
| Swelling (measurement grade) | 6 (2.9) | (1.1–6.3) | 0 | (0.0–1.8) | N/A | N/A | N/A | N/A | N/A | N/A |
| Any local adverse eventsd | 166 (81.0) | (74.9–86.1) | 0 | (0.0–1.8) | N/A | N/A | N/A | N/A | N/A | N/A |
| Any Adverse Eventsd | 196 (95.6) | (91.8–98.0) | 5 (2.4) | (0.8–5.6) | 82 (35.5) | (29.3–42.0) | 0 | (0.0–1.6) | <.001 | .017 |
Abbreviations: CI, confidence interval; N/A, not applicable.
aMarked limitation in activity, some assistance usually required; medical intervention/therapy required, hospitalization possible.
b P value for difference in proportion of vaccinated and unvaccinated participants by Barnard’s exact unconditional test.
cElevated temperature defined as ≥38.0°C for any adverse event and ≥39.0°C for a severe adverse event.
dTotals reflect the number of participants with ≥1 adverse event.
For specific solicited AEs between 5 and 28 days postvaccination (N = 434) [6, 16], vaccinated participants were significantly more likely than unvaccinated participants to report joint pain (16.7% [median duration, 2 days; range, 1–21] vs 4.8% [median duration, 1 day; range, 1–3]) and rash (7.8% [median duration, 3 days; range, 1–21] vs 1.7% [median duration, 1 day; range, 1–14) overall and during each individual week (Table 4). One vaccinated participant reported severe joint pain during days 21–23 postvaccination. In addition, vaccinated participants were significantly more likely to report skin vesicles (2.0% versus 0%) and oral ulcers (2.0% versus 0%) during the second week postvaccination. Three vaccinated and 1 unvaccinated participant reported joint swelling (P = .303).
Table 4.
Percentage of Safety Substudy Participants Reporting Solicited Adverse Events by Time Period During Days 5–28 by Vaccination Status (Vaccinated n = 204; Unvaccinated n = 230)a
| Solicited Adverse Event | Days 5–7 | Days 8–14 | Days 15–21 | Days 22–28 | Total Days 5–28b,c |
|---|---|---|---|---|---|
| Joint Paind | |||||
| Vaccinated—N (%) | 8 (4.0) | 15 (7.4) | 17 (8.5) | 13 (6.5) | 34 (16.7) |
| Unvaccinated—N (%) | 4 (1.7) | 2 (0.9) | 3 (1.4) | 2 (0.9) | 11 (4.8) |
| P valuee | .177 | <.001 | <.001 | .002 | <.001 |
| Joint Swelling | |||||
| Vaccinated—N (%) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 0 | 3 (1.5) |
| Unvaccinated—N (%) | 1 (0.4) | 0 | 0 | 0 | 1 (0.4) |
| P value | .996 | .361 | .361 | - | .303 |
| Rash | |||||
| Vaccinated—N (%) | 2 (1.0) | 10 (5.0) | 7 (3.5) | 8 (4.0) | 16 (7.8) |
| Unvaccinated—N (%) | 0 (0) | 1 (0.4) | 2 (0.9) | 2 (0.9) | 4 (1.7) |
| P value | .144 | .003 | .066 | .038 | .003 |
| Skin Vesicles | |||||
| Vaccinated—N (%) | 3 (1.5) | 4 (2.0) | 3 (1.5) | 1 (0.5) | 5 (2.5) |
| Unvaccinated—N (%) | 0 | 0 | 1 (0.5) | 2 (0.9) | 2 (0.9) |
| P value | .068 | .035 | .305 | .720 | .228 |
| Oral Ulcers | |||||
| Vaccinated—N (%) | 1 (0.5) | 4 (2.0) | 1 (0.5) | 1 (0.5) | 5 (2.5) |
| Unvaccinated—N (%) | 0 | 0 | 1 (0.5) | 1 (0.5) | 2 (0.9) |
| P value | .360 | .035 | .998 | .998 | .228 |
aExcludes 2 of the 436 substudy participants who did not have follow-up on or after day 5; for each time period, N varied from 199 to 204 for vaccinated participants and 221 to 230 for unvaccinated participants.
bThe total may be greater than the sum of the time periods if a participant had the symptom during >1 time period.
cThe mean proportion of days with active symptoms among vaccinated versus unvaccinated participants reporting the symptom during days 5–28 was 2.9% versus 0.3% (P < .001) for joint pain, 0.1% versus 0.0% for joint swelling (P = .260), 2.6% versus 0.4% (P = .002) for rash, 1.0% versus 0.1% (P = .189) for skin vesicles, and 0.3% versus 0.0% (P = .189) for oral ulcers.
dOne vaccinated participant reported severe joint pain on days 21–23. This participant also reported an SAE of malaria and AE of vaginal discharge during this time period.
e P value for difference in proportion of vaccinated and unvaccinated participants by Barnard’s exact unconditional test.
Ebola Evaluations
During the efficacy follow-up period, 27 vaccinated and 17 unvaccinated participants had medical conditions that resulted in evaluations for Ebola; no cases were laboratory confirmed. Malaria was reported in 11 of these participants.
DISCUSSION
Responding to an urgent global need during the West Africa Ebola epidemic, we designed and implemented STRIVE in Sierra Leone. The study aimed to provide data on vaccine efficacy, safety, and immunogenicity, provide access to a candidate vaccine in closely monitored settings, and along with 2 other large vaccine trials initiated in the highly Ebola-affected countries, mitigate risk that a single trial would not provide sufficient data for licensure application [17–20]. In STRIVE, we vaccinated almost 8000 healthcare and frontline response workers, providing safety data from the largest cohort receiving rVSVΔG-ZEBOV-GP vaccine in any trial to date. The STRIVE immunogenicity specimens will be tested using a validated assay and the results reported separately.
In STRIVE, although 0.5% and 1.3% of immediate vaccinated participants reported SAEs within 1 and 6 months of follow-up, respectively, no SAEs were vaccine-related; nor were there any vaccine-related SAEs among crossover-vaccinated participants. The STRIVE’s SAE prevalence 1 month postvaccination was lower than in participants receiving rVSVΔG-ZEBOV-GP in the National Institutes of Health (NIH)-sponsored vaccine trial in Liberia, and it was lower 6 months postvaccination than subjects receiving the same VSVΔG-ZEBOV-GP dose in the Merck-sponsored lot consistency study; both of these studies conducted in-person follow-up [21]. This may have facilitated more complete SAE ascertainment than STRIVE’s telephone follow-up. Within 1 month of enrollment, we found more SAEs reported in the vaccinated than the contemporaneous unvaccinated group. We saw no clear pattern in the SAE reports, suggesting differential seeking of medical care or reporting bias. This is suggested by the fact that in our safety substudy, where events were more actively solicited, SAEs were similar between groups. Mild to moderate symptoms were common in the immediate vaccination period, but they were short-lived and generally well tolerated. Although STRIVE was not double-blind or placebo-controlled, systemic reactions among vaccinated and unvaccinated participants were similar in range to those described in placebo-controlled trials of rVSVΔG-ZEBOV-GP [16, 20–23]. Although the number of women who became pregnant within 2 months after vaccination was small, we provide the first reports on outcomes of such pregnancies with reassuring data that no congenital anomalies were detected in their infants. We consider the high rate of reported pregnancy loss in both vaccinated and unvaccinated women to be due to reporting bias. There are no published statistics on pregnancy loss in Sierra Leone. However, in a large cohort of US women, after excluding elective termination of pregnancy, reported pregnancy loss was approximately 20% [24]. Additional data on the safety of vaccination in pregnancy will help guide the development of Ebola vaccination recommendations.
Arthritis was reported in the second week after vaccination among a subset of older participants in the Geneva trial [16, 23], a finding recently confirmed in the Merck lot consistency study [21]. Therefore, we queried specifically for joint swelling in our safety substudy. Consistent with the other Phase 2/3 trials in West Africa [19, 20], we did not find an association of arthritis or joint swelling with vaccination, although the STRIVE substudy population was small for detecting rare events. The younger median age of our study participants, environmental or genetic differences, or differing methods for participant medical evaluation and follow-up may explain these differences [19, 20]. Nonetheless, we confirmed that vaccination was associated with some symptoms consistent with viral replication such as joint pain, rash, skin vesicles, and oral ulcers at various time intervals during 5 to 28 days postvaccination, most commonly during the second week postvaccination [16, 21, 23]. Although joint pain onset tracked closely with other systemic symptoms during the first 7 days postvaccination with rapid onset and short duration, vaccinated STRIVE participants reported joint pain more frequently than unvaccinated participants during every week from 0 to 28 days postvaccination. In the WHO trial of this vaccine in Guinea, joint pain was reported by 17.9% of participants 0–14 days postvaccination, most within the first 3 days; there was no comparison group [19]. In the NIH trial in Liberia, there was no difference in reported joint pain between the group that received rVSVΔG-ZEBOV-GP compared with the placebo group [20].
Fever and other systemic symptoms were common within 24–48 hours postvaccination and resolved quickly. Future use of this Ebola vaccine in outbreak responses will need to differentiate vaccine reactions from suspected Ebola to reduce the risk of unnecessary exposure to Ebola in treatment facilities while ensuring that true Ebola cases are properly identified. Our approach, which used a modified Ebola case definition, may be useful in future responses [15]. In the Guinea trial, where participants were given antipyretics for the prevention and management of postvaccination fever, only 0.1%–1.3% of vaccinated adults reported fever within 14 days [19]. They also reported less headache, myalgia, joint pain, and fatigue than STRIVE participants. Routine use of antipyretics to prevent postvaccination symptoms during an Ebola outbreak should be weighed against the possibility of masking Ebola, thereby delaying referral for diagnosis and treatment.
We faced major challenges conducting a large trial during an Ebola epidemic. A contemporaneously enrolled control group was important considering the epidemic dynamics and seasonality of febrile illnesses, including malaria [25]. It was critical not to hire staff away from the response, so STRIVE staff included many students or not-yet-employed graduates in nursing, pharmacy, and medicine; retired nurses; and part-time physicians. Because of the number of participants, large geographic area of the study, and complicated transportation logistics, we conducted routine follow-up by telephone; physicians evaluated participants with more serious illnesses.
CONCLUSIONS
Through strong commitments from global public-private partnerships, the rVSV∆G-ZEBOV-GP vaccine underwent rigorous testing for efficacy, safety, and immunogenicity during the 2014–2016 West African Ebola epidemic. Final results from the WHO trial in Guinea indicated high short-term efficacy for the vaccine [19]—long-term efficacy was not assessed. The NIH trial in Liberia reported immune responses elicited by rVSV∆G-ZEBOV-GP at 1 month; these responses were largely maintained through 12 months [20]. Our study contributes important safety information from approximately 8000 participants that complements safety, immunogenicity, and efficacy data from these and other vaccine trials; STRIVE immunogenicity data, when available, will provide additional information [16, 19–22]. The recent re-emergence of Ebola in the Democratic Republic of Congo highlights the ongoing challenges that Ebola will present and the importance of continued commitment to bring Ebola vaccines to licensure. This includes studies on the durability of the immune response and vaccine safety in children [26, 27]. Vaccination is likely to be a critical public health tool for controlling future Ebola outbreaks. Although recent recommendations and experience with use of this unlicensed vaccine cover outbreak response only under the Expanded Access framework [28], future policies need to consider whether vaccinating certain high-risk groups, such as healthcare and frontline workers, before an epidemic might also be beneficial [26, 29–32].
Supplementary Material
Notes
Acknowledgments. We thank the healthcare and frontline workers in Sierra Leone for their willingness to participate in the study. We also thank the hundreds of Centers for Disease Control and Prevention (CDC) STRIVE Study Team staff in Atlanta and Sierra Leone and the College of Medicine and Allied Health Sciences (COMAHS) STRIVE Study Team in Sierra Leone who worked tirelessly under challenging conditions to plan and implement this study. The rVSV∆G-ZEBOV-GP vaccine was provided by NewLink Genetics Corporation and Merck and Co., Inc. We are grateful for the advice and oversight provided by members of STRIVE’s scientific steering committee (Bailah Leigh, Kathleen Neuzil, Hazel Insip, George Risi, and Samba Sow) and data and safety monitoring board (Patricia Hibberd, William MacLeod, James Lavery, Tumani Corrah, and Aiah Gbakima). The STRIVE study would not have been possible without the support from the following organizations: eHealth Africa, The Emmes Corporation, FHI-360, Modality Solutions, and Technical Resource International. We also thank the following organizations and people for their significant contributions: Sierra Leone Ministry of Health and Sanitation; College of Medicine and Allied Health Sciences, University of Sierra Leone; US Department of Health and Human Services (including Biomedical Advanced Research and Development Authority [BARDA], US Food and Drug Administration, and National Institutes of Health, as well as the CDC); Ambassador John Hoover and the staff of the US Embassy Sierra Leone, US Department of State; Oliver Morgan and the staff of the CDC Ebola Response Team in Sierra Leone and the CDC Ebola Response Team in Atlanta; the World Health Organization in Geneva and Sierra Leone; and Global Good/Intellectual Ventures.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. The trial was funded by the Centers for Disease Control and Prevention, the Biomedical Advanced Research and Development Authority, and the National Institutes of Health, with additional support from the CDC Foundation.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interests. P. D., R. L., and C. R. P. report grants from BARDA during the conduct of the study. All authors have submitted the ICJME Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2016, New Orleans, Louisiana, October 26–30, 2016, Abstract 131; 65th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, Georgia, November 13–17, 2016, Abstract 1398.
Contributor Information
STRIVE Study Team:
Bailah Leigh, Kathleen Neuzil, Hazel Insip, George Risi, and Samba Sow
References
- 1. Gostin LO, Lucey D, Phelan A. The Ebola epidemic: a global health emergency. JAMA 2014; 312:1095–6. [DOI] [PubMed] [Google Scholar]
- 2. Incident Management System Ebola Epidemiology Team CDC, Guinea Interministerial Committee for Response Against the Ebola Virus, CDC Guinea Response Team et al. Update: Ebola virus disease outbreak--West Africa, October 2014. MMWR Morb Mortal Wkly Rep 2014; 63:978–81. [PMC free article] [PubMed] [Google Scholar]
- 3. Kilmarx PH, Clarke KR, Dietz PM, et al. Ebola virus disease in health care workers—Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1167–71. [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Meeting Summary of the WHO Consultation on Potential Ebola Therapies and Vaccines. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 5. Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis 2011; 204(Suppl 3):S1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 Trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374:1647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857–66. [DOI] [PubMed] [Google Scholar]
- 8. Widdowson MA, Schrag SJ, Carter RJ, et al. Implementing an Ebola Vaccine Study - Sierra Leone. MMWR Suppl 2016; 65:98–106. [DOI] [PubMed] [Google Scholar]
- 9. Soares JF, Wu CFJ. Some restricted randomization rules in sequential designs. Commun Stat Theory Methods 1983; 12:2017–34. [Google Scholar]
- 10. Zhao W, Weng Y, Wu Q, Palesch Y. Quantitative comparison of randomization designs in sequential clinical trials based on treatment balance and allocation randomness. Pharm Stat 2012; 11:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 2004; 78:5458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merck and Co., Inc. Investigator’s Brochure. V920. 4 December 2015. Edition 2. [Google Scholar]
- 13. Guidance for Industry and Investigators: Safety Reporting Requirements for INDs and BA/BE Studies. Silver Spring, MD: Food and Drug Administration, 2012. [Google Scholar]
- 14. World Health Organization. Verbal autopsy standards: the 2014 WHO verbal autopsy instrument. 2014. http://www.who.int/healthinfo/statistics/verbalau topsystandards/en/index1.html. Accessed 10 January 2018. [Google Scholar]
- 15. Conteh MA, Goldstein ST, Lisk DR, et al. Clinical surveillance and evaluation of suspected Ebola cases in a vaccine trial during an Ebola epidemic: the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE). J Infect Dis 2018; 217:s33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huttner A, Dayer JA, Yerly S, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase ½ trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kennedy SB, Neaton JD, Lane HC, et al. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: design, procedures, and challenges. Clin Trials 2016; 13:49–56. [DOI] [PubMed] [Google Scholar]
- 18. Ebola ça Suffit Ring Vaccination Trial Consortium. The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ 2015; 351:h3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy SB, Bolay F, Kieh M, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halperin SA, Arribas JR, Rupp R, et al. Six-month safety data of recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis 2017; 215:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 2017; 376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374:1647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossen LM, Ahrens KA, Branum AM. Trends in risk of pregnancy loss among US women, 1990–2011. Paediatr Perinat Epidemiol 2018; 32:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lane HC, Marston HD, Fauci AS. Conducting clinical trials in outbreak settings: points to consider. Clin Trials 2016; 13:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meeting of the Strategic Advisory Group of Experts on immunization, October 2015--conclusions and recommendations. Wkly Epidemiol Rec 2015; 90:681–700. [PubMed] [Google Scholar]
- 27. Agnandji ST, Fernandes JF, Bache EB, et al. Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: a phase I randomised trial. PLoS Med 2017; 14:e1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gsell PS, Camacho A, Kucharski AJ, et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect Dis 2017; 17:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coltart CE, Johnson AM, Whitty CJ. Role of healthcare workers in early epidemic spread of Ebola: policy implications of prophylactic compared to reactive vaccination policy in outbreak prevention and control. BMC Med 2015; 13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gulland A. Ebola vaccine will be made available for emergency use. BMJ 2016; 532:i386. [DOI] [PubMed] [Google Scholar]
- 31. Ebola virus disease (EVD) in West Africa: an extraordinary epidemic. Wkly Epidemiol Rec 2015; 90:89–96. [PubMed] [Google Scholar]
- 32. Meeting of the strategic advisory group of experts on immunization, April 2017 - conclusions and recommendations. Wkly Epidemiol Rec 2017; 92:301–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.