Abstract
OBJECTIVES
The objective of this study is to build a novel prognostic nomogram in non-small-cell lung cancer (NSCLC) incorporating pre-treatment peripheral blood markers beyond known pathoclinical predictors.
METHODS
We analysed 7158 patients with NSCLC diagnosed between 1 January 1997 and 31 December 2012 from a single institution with a uniform medical record and routine follow-up information. Besides common clinicopathological factors, we investigated the prognostic value of the neutrophil to lymphocyte ratio, monocytes and haemoglobin level in peripheral blood before treatment. Patients were randomly assigned to training (4772 patients, 66.7%) or validation cohorts (2386 patients, 33.3%). Cox proportional hazards models determined the effects of multiple factors on overall survival (OS). A nomogram was developed to predict median survival and 1-, 3-, 5- and 10-year OS for NSCLC. The performance of the nomogram was assessed by a concordance index and calibration curve.
RESULTS
In the training cohort, the multivariate Cox model identified the neutrophil to lymphocyte ratio, monocytes and haemoglobin level before treatment as significant prognostic factors for OS independent of patient age, gender, smoking history of intensity and cessation, performance status, disease stage, tumour cell type and differentiation grade and therapies. All the significant prognostic variables were incorporated into a nomogram. In the validation cohort, the nomogram showed notable accuracy in predicting OS, with a concordance index of 0.81, and was well calibrated for predictions of OS.
CONCLUSIONS
The proposed nomogram incorporating peripheral blood markers and known prognostic factors could accurately predict individualized survival probability of patients with NSCLC. It could be used in treatment planning and stratification in clinical trials.
Keywords: Nomogram, Non-small-cell lung cancer, Prediction model, Survival, Neutrophil to lymphocyte ratio
INTRODUCTION
Non-small-cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases in the USA. Although subgroups of patients with NSCLC have benefitted from targeted therapies against specific tumour mutations [1], the 5-year overall survival (OS) rate remains poor at 21% [2]. To better estimate patient outcomes, many prognostic factors and models have been established in NSCLC, such as tumour stage, patient age, gender, smoking status, smoking cessation, performance status (PS), tumour grade, histology and treatment type [3–5]. The identification of novel prognostic factors and the integration of all the prognostic factors into a model will enable better risk stratification for patients with NSCLC.
Statistical prediction models have been widely used for cancer outcome predictions. Among those models, nomograms are graphical interfaces for statistical models utilizing combined prognostic factors to precisely predict the outcome for a given patient. The estimation of individualized survival among cancer patients could be helpful for clinicians in making treatment decisions and choosing appropriate therapeutic approaches. Hoang et al. [6] reported the first NSCLC nomogram, based on 1436 patients, which could only be used for Stage IV patients who were treated with first-line chemotherapy. In that model, several known prognostic factors, such as age, gender, smoking status and smoking cessation, were not included.
Concurrent inflammation with cancer is a critical component in cancer progression [7]. The peripheral blood markers such as neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio have been identified as prognostic and predictive biomarkers in many types of cancers including NSCLC [8–10]. However, these studies included a limited number of cases and were mainly focused on the prognostic relevance of pretreatment NLR. To our knowledge, the prognostic values of monocytes, NLR at follow-up and NLR changes after treatment or progression have not yet been assessed in NSCLC.
Therefore, we sought to develop a prognostic nomogram that incorporates pretreatment peripheral blood markers and known pathoclinical predictors. We also investigated the prognostic relevance of peripheral blood markers at pretreatment in NSCLC.
MATERIALS AND METHODS
Study cohort and data collection
Starting from 1997, all patients with pathological results of primary lung cancer evaluated and managed at Mayo Clinic, Rochester, Minnesota, MN, USA, were prospectively enrolled and followed up for clinical outcome research. The protocols for recruitment were approved by the Mayo Clinic Institutional Review Board (IRB number: 225-99), and written informed consent was obtained from all participants included in the analysis. Detailed procedures of patient enrolment, diagnosis, data collection and follow-up have been described in our previous publications [11, 12]. The type of treatment followed in this study conforms to the NCCN guidelines. Between 1 January 1997 and 31 December 2012, a total of 11 702 patients with a pathologically confirmed diagnosis of NSCLC were enrolled. Complete blood count records (186 561 records) of all included patients were retrieved from 1992 to 2013. A total of 7158 NSCLC cases met our inclusion criteria because these patients had a complete blood count with leucocyte differential count performed before any treatment. Patients with leukaemia or lymphoma were excluded. As described in our previous study [12], a full medical record abstraction of each patient was conducted to obtain information on basic demographics, tobacco exposure history, pathological type, clinical staging and treatment strategy. All patients were actively followed up. Annual verification of patients’ status was completed through the Mayo Clinic’s electronic medical records and registration database, death certificates, next-of-kin reports and obituary documents filed in the patients’ medical records as well as through the Mayo Clinic Tumour Registry and Social Security Death Index website.
The peripheral blood markers were evaluated before treatment. Patients were divided into equal quartiles according to the 25th, 50th and 75th NLR, monocytes and haemoglobin percentile at baseline. For several laboratory values (NLR, monocytes and haemoglobin level), natural log transformations of continuous variables were performed to reduce their distribution skewness as described in our previous study [12].
Statistical analysis
Computer-generated random numbers were used to assign 4772 (66.7%) patients as a training cohort and 2386 (33.3%) patients as a validation cohort.
As described in previous studies [11–13], we used univariate and multicovariate Cox proportional hazards models to evaluate the effect of prognostic factors, including pretreatment haematological markers (continuous variables) and clinicopathological factors in the training cohort. Cox proportional hazards models estimated the effects of multiple factors for a nomogram, and among these, only the factors with a P-value <0.05 were incorporated into the nomogram. Continuous variables (i.e. age, NLR, monocytes and haemoglobin level) were fitted using restricted cubic splines to obtain flexible fit and permit non-linear relationships. A nomogram was developed to predict median survival and 1-, 3-, 5- and 10-year OS.
In the validation cohort, the performance of the nomogram was assessed by the concordance index (C-index) and calibration curve. The predictive accuracy for OS was estimated using the C-index for validation. As described in previous studies [11–13], the larger the C-index is, the more accurate the prognostic prediction will be. Five hundred bootstrap resamples were applied for validation of the accuracy estimates and to reduce overfit bias. Calibration refers to whether the predicted probabilities agree with the observed probabilities, which is generated by plotting the predicted survival probabilities against the observed outcome. In a well-calibrated model, the calibration curve should be close to 45°.
As described in our previous study [12], clinical data were reported as means ± standard deviation or median (full range). Cumulative survival was estimated with a Kaplan–Meier model and calculated using the time of diagnosis or progression as the starting point. Survival analyses, stratified by the NLR, monocytes and haemoglobin quartiles at baseline, were used to evaluate the predictive value of OS at pretreatment.
All statistical analyses were carried out with SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 3.0.2 (http://www.r-project.org/) using the rms and survival libraries. All P-values were 2-tailed, and a P-value <0.05 was considered significant.
RESULTS
Characteristics of patients
The demographics and clinical characteristics of the training and validation cohorts are listed in Table 1. Of the total 7158 patients, 5437 (76%) deaths were recorded with a median follow-up time of 1.3 years (7 days–17.2 years). For the surviving patients, the median follow-up time was 8.3 years (1.2 months–17.2 years). The median age of patients at the time of diagnosis was 68 years (range 18–97 years).
Table 1:
Demographics, clinical and pathological characteristics and pretreatment peripheral blood markers (n = 7158)
| Characteristics | Training cohort (n = 4772) | Validation cohort (n = 2386) | P-value |
|---|---|---|---|
| Age at diagnosis, median (range) | 68.0 (24.0–97.0) | 68.0 (18.0–93.0) | 0.38 |
| Gender, n (%) | |||
| Male | 2634 (55) | 1352 (57) | 0.24 |
| Female | 2138 (45) | 1034 (43) | |
| Cigarette smoking status, n (%) | |||
| Never | 708 (15) | 326 (14) | 0.21 |
| Former | 2310 (48) | 1203 (50) | |
| Current | 1754 (37) | 857 (36) | |
| Pack-year, n (%) | |||
| Missing | 567 | 329 | 0.80 |
| Never smoker | 708 (17) | 326 (16) | |
| 0–39 | 1291 (31) | 643 (31) | |
| 40–59 | 1032 (25) | 503 (25) | |
| ≥60 | 1174 (28) | 585 (28) | |
| Smoking cessation (years), n (%) | |||
| Quit ≥1 or never smoker | 2993 (63) | 1511 (63) | 0.75 |
| Quit at or after diagnosis | 904 (19) | 455 (19) | |
| Never quit | 875 (18) | 420 (18) | |
| Stage, n (%) | |||
| Ia | 825 (17) | 404 (17) | 0.79 |
| Ib | 434 (9) | 235 (10) | |
| IIa | 185 (4) | 88 (4) | |
| IIb | 298 (6) | 154 (7) | |
| IIIa | 651 (14) | 305 (13) | |
| IIIb | 571 (12) | 305 (13) | |
| IV | 1808 (38) | 895 (38) | |
| Pathological cell type, n (%) | |||
| Adenocarcinoma | 2533 (53) | 1258 (53) | 0.86 |
| Large cell | 99 (2) | 45 (2) | |
| Carcinoid | 160 (3) | 82 (3) | |
| Squamous | 1174 (25) | 612 (26) | |
| Sarcomatoid carcinoma | 48 (1) | 19 (1) | |
| Other NSCLC | 758 (16) | 370 (16) | |
| Tumour grade, n (%) | |||
| Well | 832 (17) | 426 (18) | 0.21 |
| Moderate | 1831 (38) | 932 (39) | |
| Poor | 1383 (29) | 710 (30) | |
| Non-gradable | 726 (15) | 318 (13) | |
| ECOG performance status, n (%) | |||
| <2 | 4101 (86) | 2043 (86) | 0.72 |
| ≥2 | 671 (14) | 343 (14) | |
| Therapy, n (%) | |||
| No treatment | 1016 (21) | 518 (22) | 0.86 |
| Surgery only | 1697 (36) | 861 (36) | |
| Surgery + chemotherapy | 155 (3) | 83 (4) | |
| Surgery + radiation | 70 (2) | 42 (2) | |
| Surgery + chemotherapy + radiation | 79 (2) | 35 (2) | |
| Chemotherapy only | 727 (15) | 368 (15) | |
| Radiation only | 381 (8) | 175 (7) | |
| Chemotherapy + radiation | 564 (12) | 270 (11) | |
| Surgery + neoadjuvant chemotherapy and/or radiation | 83 (2) | 34 (1) | |
| NLR, median (range) | 3.4 (0.1–131.8) | 3.5 (0.1–81.4) | 0.86 |
| Monocytes (109/l), median (range) | 0.6 (0.0–5.8) | 0.6 (0.0–2.4) | 0.83 |
| Haemoglobin (g/dl), median (range) | 13.4 (6.2–19.4) | 13.4 (6.1–18.6) | 0.64 |
| PLR, median (range) | 174.5 (2.3–3958.3) | 174.3 (4.9–3955.6) | 0.54 |
| RDW, median (range) | 13.4 (10.9–30.7) | 13.4 (11.0–25.7) | 0.73 |
| Lymphocytes (109/l), median (range) | 1.6 (0.1–50.9) | 1.5 (0.1–41.5) | 0.56 |
| Platelet count (109/l), median (range) | 269.0 (4.0–1120.0) | 266.0 (32.0–837.0) | 0.33 |
| Erythrocytes (1012/l), median (range) | 4.4 (2.0–6.8) | 4.4 (2.1–8.0) | 0.91 |
ECOG: Eastern Cooperative Oncology Group; NLR: neutrophil to lymphocyte ratio; NSCLC: non-small-cell lung cancer; PLR: platelet to lymphocyte ratio; RDW: red cell distribution width.
Nomogram development in the training cohort
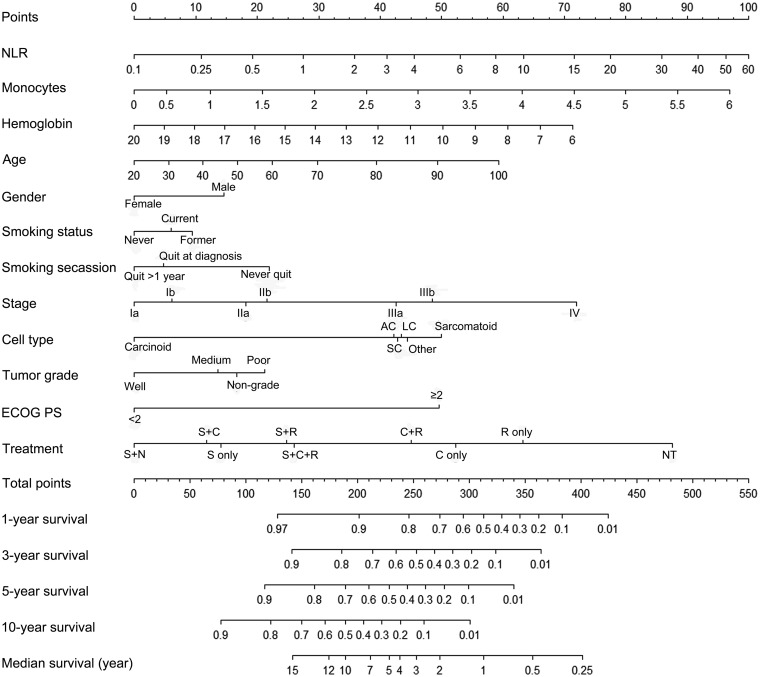
In the training cohort (n = 4772), the results of the univariate analysis are presented in Supplementary Material, Table S1. In the multivariate analysis, NLR, haemoglobin level, monocytes, age, gender, smoking status, smoking cessation, disease stage, cell type, tumour grade, Eastern Cooperative Oncology Group (ECOG) PS and therapy type were significantly associated with OS (Table 2). All the significant prognostic variables were used to build the nomogram (Fig. 1). The nomogram assigned points based on age, NLR, haemoglobin level and monocytes in a continuous but non-linear fashion. Outcomes were reported as 1-, 3-, 5-, 10-year OS and median survival.
Table 2:
Multicovariate Cox regression model for overall survival in NSCLC (n = 4772)
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Loge (NLR) | 1.41 | 1.31–1.52 | <0.0001 |
| Loge (RDW) | 0.77 | 0.55–1.08 | 0.13 |
| Log10 (PLR) | 0.86 | 0.70–1.07 | 0.18 |
| Haemoglobin | 0.91 | 0.89–0.93 | <0.0001 |
| Monocytes | 1.26 | 1.12–1.41 | 0.0001 |
| Lymphocytes | 1.01 | 0.97–1.05 | 0.77 |
| Age at diagnosis | 1.01 | 1.01–1.02 | <0.0001 |
| Gender (vs female) | |||
| Male | 1.28 | 1.19–1.37 | <0.0001 |
| Smoking status (vs never) | |||
| Former | 1.17 | 1.05–1.30 | 0.005 |
| Current | 1.10 | 0.88–1.39 | 0.39 |
| Smoking cessation (years) (vs never quit) | |||
| Quit ≥1 or never smoker | 0.69 | 0.55–0.86 | 0.0010 |
| Quit at or after diagnosis | 0.74 | 0.67–0.83 | 0.0010 |
| Stage (vs Ia) | |||
| Ib | 1.11 | 0.95–1.31 | 0.20 |
| IIa | 1.35 | 1.09–1.69 | 0.0075 |
| IIb | 1.44 | 1.20–1.72 | 0.0001 |
| IIIa | 2.06 | 1.76–2.42 | <0.0001 |
| IIIb | 2.27 | 1.93–2.67 | <0.0001 |
| IV | 3.39 | 2.91–3.94 | <0.0001 |
| Pathological cell type (vs carcinoid) | |||
| Adenocarcinoma | 2.02 | 1.48–2.76 | <0.0001 |
| Large cell | 2.06 | 1.40–3.03 | 0.0002 |
| Squamous | 2.05 | 1.48–2.83 | <0.0001 |
| Sarcomatoid carcinoma | 2.32 | 1.49–3.61 | 0.0002 |
| Other NSCLC | 2.10 | 1.52–2.91 | <0.0001 |
| Tumour grade (vs well) | |||
| Moderate | 1.26 | 1.12–1.41 | 0.0002 |
| Non-gradable | 1.32 | 1.15–1.52 | <0.0001 |
| Poor | 1.42 | 1.25–1.61 | <0.0001 |
| ECOG performance status (vs < 2) | |||
| ≥2 | 2.35 | 2.12–2.60 | <0.0001 |
| Therapy (vs no treatment) | |||
| Surgery only | 0.29 | 0.25–0.33 | <0.0001 |
| Surgery + chemotherapy | 0.27 | 0.22–0.35 | <0.0001 |
| Surgery + radiation | 0.35 | 0.27–0.46 | <0.0001 |
| Surgery + chemotherapy + radiation | 0.34 | 0.26–0.45 | <0.0001 |
| Chemotherapy only | 0.55 | 0.49–0.61 | <0.0001 |
| Radiation only | 0.66 | 0.58–0.75 | <0.0001 |
| Chemotherapy + radiation | 0.49 | 0.43–0.55 | <0.0001 |
| Surgery + neoadjuvant chemotherapy and/or radiation | 0.22 | 0.17–0.30 | <0.0001 |
CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; NLR: neutrophil to lymphocyte ratio; NSCLC: non-small-cell lung cancer; PLR: platelet to lymphocyte ratio; OR: odds ratio; RDW: red cell distribution width.
Figure 1:
Nomogram for 1-, 3-, 5- or 10-year survival and median survival in non-small-cell lung cancer. The nomogram is used by adding up the points identified on the points scale for each variable. For instance, locate the patient’s age and draw a line straight upward to the ‘Points’ axis to determine the score associated with that age. Repeat the process for other covariates of the patient, then sum the scores and locate this sum on the ‘Total Points’ axis. Draw a line straight down to the bottom scale to find the predicted probability. AC: adenocarcinoma; C: chemotherapy; ECOG: Eastern Cooperative Oncology Group; LC: large cell cancer; N: neoadjuvant therapy; NLR: neutrophil–lymphocyte ratio; NT: no treatment; Other: other non-small-cell lung cancer; R: radiation; S: surgery; SC: squamous cell cancer.
Nomogram validation in the validation cohort
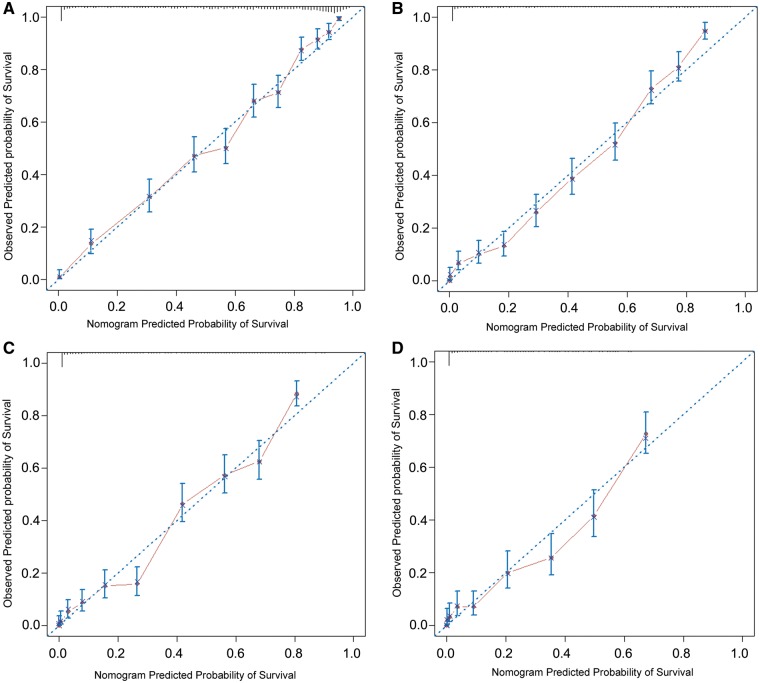
In the validation cohort (n = 2386), the nomogram-predicted OS was well calibrated with the Kaplan–Meier curves observed at 1-, 3-, 5- and 10-year OS (Fig. 2). The bootstrap C-index of the nomogram was 0.81.
Figure 2:
Calibrations of nomogram-predicted overall survival (OS) with observed OS are shown at (A) 1 year, (B) 3 years, (C) 5 years and (D) 10 years. On the calibration curve, the x-axis is the nomogram-predicted probability of OS, and the y-axis is the observed OS. The dotted line indicates the reference line on which an ideal nomogram would lie. The solid line indicates performance of the present nomogram. The vertical lines represent 95% confidence intervals.
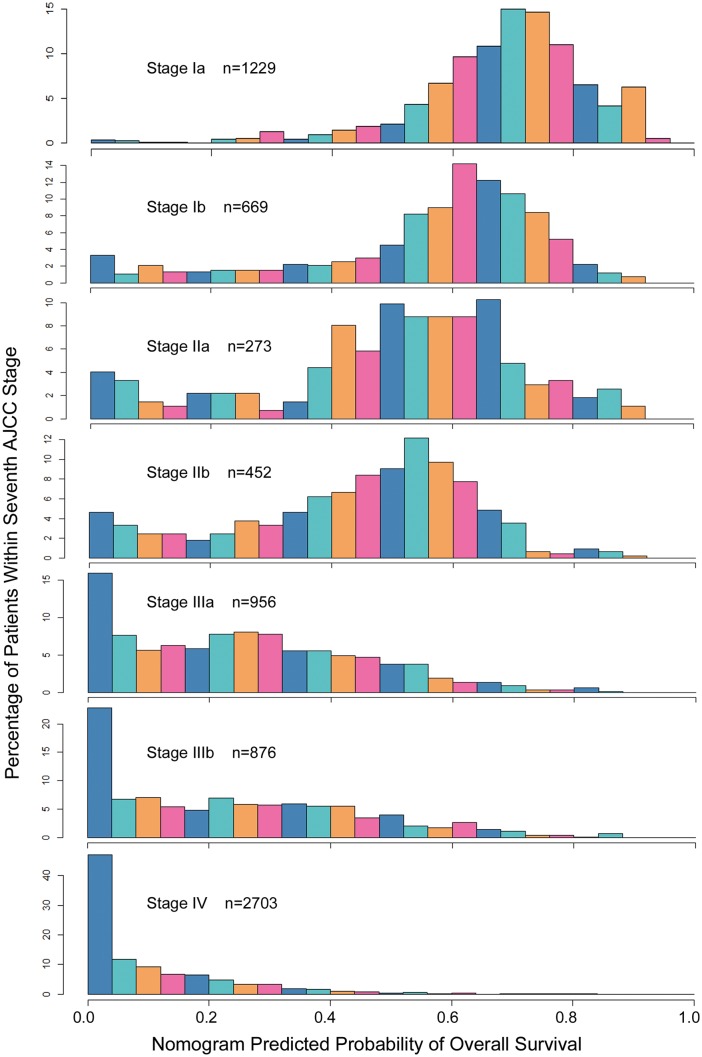
A histogram of nomogram-predicted 5-year survival probabilities in all patients (n = 7158) is shown in Fig. 3, which illustrates that patients with the same tumour, node and metastasis (TNM) stage have diverse survival rates.
Figure 3:
Comparisons of nomogram-predicted 5-year overall survival with the predictions of the AJCC stage groupings. The heterogeneity of the predicted probabilities of overall survival within each stage is shown. AJCC: American Joint Committee on Cancer.
Prognostic effect of neutrophil to lymphocyte ratio, monocytes and haemoglobin level in all patients
The Kaplan–Meier OS curves were plotted as 4 groups according to the 25th, 50th and 75th quartiles of the NLR, monocytes, and haemoglobin level at baseline (Supplementary Material, Fig. S1). Elevated monocytes at pretreatment were associated with poor prognosis (Supplementary Material, Fig. S1C, P < 0.0001). Elevated NLR (P < 0.0001) and low haemoglobin level (P < 0.0001) at pretreatment, post-treatment, progression and after 1- and 2-year diagnosis were all associated with poor prognosis (Supplementary Material, Figs S1A and B, S2 and S3).
DISCUSSION
We have developed and validated a nomogram that assigns predictions for OS based on the NLR, monocytes, haemoglobin level and other clinicopathological variables in a series of 7158 patients with NSCLC from a single institution. We hold the opinion that the nomogram provides individualized OS predictions and could be helpful for patients and clinicians in the treatment decision-making process. As described in our previous study [12], a predictive nomogram model was developed using peripheral blood markers for survival risk stratification in small-cell lung cancer patients. In addition, the nomograms have a high potential of estimating risk in clinical trial design. Such an individualized prognosis could be used for stratification in randomized studies.
NSCLC is very heterogeneous in its clinical presentation, histopathology, treatment response and disease prognosis. Patient prognosis is currently estimated on the basis of the seventh American Joint Committee on Cancer (AJCC) TNM staging system, not on other known prognostic factors such as age, gender, smoking status, smoking cessation, ECOG PS, histology, tumour grade, therapy and germ line and somatic markers. By integrating additional significant prognostic factors, a nomogram could be applied to more accurately estimate an individual patient’s survival. Based on the statistical model, our nomogram allows for individualized survival probability estimation for NSCLC, which discriminates better than the seventh AJCC TNM staging system (Fig. 3). Figure 3 shows the benefits of nomogram predictions: using the nomogram, patients within different AJCC TNM stages with heterogeneous prognosis are successfully discerned. In the Stage Ib group, for example, the nomogram predicted probability of OS, spanning the gamut of 0.0–0.9. Similarly, increased heterogeneity exists within the Stage IIa group, with some Stage IIa patients having better estimated OS outcomes than some Stage Ib patients. Hoang et al. [6] reported the first NSCLC prognostic nomogram, based on 1436 patients, which could only be used for Stage IV patients treated with first-line chemotherapy. In that model, 6 factors were identified, which include subcutaneous metastasis, decreased PS, loss of appetite, liver metastasis, 4 metastatic sites and no previous lung surgery. However, some known prognostic factors, such as age, gender, smoking status, smoking cessation, histology and tumour grade were not included in the model. Liang et al. [13] reported a prognostic nomogram in patients with resected NSCLC using a multi-institutional database. Compared with Liang et al.’s model, our nomogram further includes smoking status and smoking cessation [14], tumour grade [15], adjuvant therapy [16] and pretreatment blood markers [8, 17], and our model is based on a 17-year prospectively enrolled cohort with a uniform medical record and routine follow-up information [18].
In the training cohort, low haemoglobin level and elevated NLR and monocytes represent significant independent prognostic indicators in NSCLC (logeNLR: hazard ratio 1.41, 95% confidence interval 1.31–1.52, P < 0.0001; haemoglobin: hazard ratio 0.91, 95% confidence interval 0.89–0.93, P < 0.0001; and monocytes: hazard ratio 1.26, 95% confidence interval 1.12–1.41, P < 0.0001). Most of the previous studies used categorical variables of NLR, monocytes and haemoglobin in assessing the prognosis. However, in this study, we found that continuous variables of NLR, monocytes and haemoglobin are significant independent prognostic indicators (results not shown). When constructing a nomogram, we modelled NLR, monocytes and haemoglobin level as continuous variables, with restricted cubic splines to obtain a flexible fit, as their prognostic effects were not hypothesized to be the same in each part of the range.
In addition to NLR, monocytes and haemoglobin level, we identified that age, gender, smoking status, smoking cessation, ECOG PS, disease stage, tumour grade and therapy type were independent prognostic factors in NSCLC. These are consistent with previous reports [19, 20].
In the validation cohort, the performance of the nomogram was assessed by calibration and discrimination. The C-index reflects the predictive accuracy of a nomogram. In this study, internal validation demonstrated good discrimination power (C-index = 0.81). The nomogram was well-calibrated for predictions of OS (Fig. 2).
Elevated NLR [8–10, 17, 21] and monocytes [22] in the peripheral blood of cancer patients may reflect the extent of systemic inflammation elicited by the malignant cells, which have been identified as poor prognostic markers in many types of malignant tumours. However, these studies included a limited number of cases and solely focused on the prognostic relevance on pretreatment NLR. To our knowledge, the prognostic values of monocytes at baseline have not yet been elucidated. In our study, we identified that elevated monocytes, elevated NLR and low haemoglobin level before treatment were all associated with a poor prognosis.
As described in our previous study [12], peripheral blood markers are valuable in evaluating lung cancer patients when primary tumours are not available for extensive analyses due to tumour non-resectability. Assessment of the peripheral blood markers is easier and more cost-effective than the conventional tumour markers, such as serum neuron-specific enolase and carcinoembryonic antigen in clinical practice. Peripheral blood markers were included to build the nomogram, which could be readily available for validation in any other clinical settings.
Limitations
There are several limitations to this study. This model is developed based on a specific population managed at a single tertiary medical centre. It is established and validated internally, and thus it lacks external validation with a larger number of patients at multiple institutions. Targeted therapy is not included in our analyses, as such agents were not used as first-line therapy in majority of the patients. Cancer-specific survival or disease-free survival may be better than OS, especially when majority of the patients had early-stage NSCLC. The nomogram could also be constructed using cancer-specific survival or disease-free survival as the end-point in a future effort with information on all causes of death. An online tool with a user-friendly interactive nomogram implicated electronically could be further developed in the future, which might be useful in saving time for the clinician and enabling patient self-assessment. Finally, some known prognostic factors, such as the level of lactate dehydrogenase, albumin, carcinoembryonic antigen, adenocarcinoma subtype and tumour molecular and pharmacogenomic markers, were not included in our model because of the unavailability of complete information in our database. The addition of these markers in future studies may improve the predictive ability of the nomogram.
CONCLUSION
In summary, we have constructed a nomogram to accurately predict individualized survival probability of NSCLC. These models could assist clinicians and patients in clinical decision-making, targeted treatment and clinical trial design. These results could also be used to define proper stratification factors in future clinical trials. We have also identified that elevated monocytes at baseline, elevated NLR and low haemoglobin level at baseline and follow-up are predictors for poor OS.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Connie E. Edwards for her professional editing and technical assistance with the manuscript.
Funding
The work was supported by the US National Institutes of Health [R03CA77118, R01CA80127, R01CA84354 and R01CA115857], Mayo Clinic Foundation, Key Grant of Shanghai Municipal Commission for Major Disease [2013zyjb0401], Pujiang Project [15PJD034] and Clinical Guide Project of Shanghai science and Technology Commission [16411965800].
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr Federico Quaini (Parma, Italy): Is it possible to know whether these NLR data come from an increased neutrophil or a decreased lymphocyte number, because the ratio can be a result of both. So either you have lymphopenia or neutrophilia. Which is more important between these 2 parameters?
Dr Qiuyuan Li (Shanghai, China): Actually, I do not think this ratio can discriminate between elevation or the decrease on either side. I’m not participating in this study, so I’m not sure whether they have done the specific analysis of the scenario you’ve just mentioned. But based on the materials that I have these 2 situations were not differentiated.
Dr Eric Vallieres (Seattle, WA, USA): This is more of a suggestion than a question. There are a couple of other gene signatures out there that are trying to show the same thing, which is that within tumours of the same stage you may have different behaviours that identify different prognosis. It would be interesting to challenge your nomogram against one of the commercially available gene signatures for adenocarcinomas—as at this time the commercially available gene signatures, really out there right now, are only for adenocarcinomas—to see which one has the strongest prediction.
Dr Li: I think in the future we will incorporate gene markers into the nomogram model, so it will be more complete and integral.
REFERENCES
- 1. Bunn PA Jr, Doebele RC.. Genetic testing for lung cancer: reflex versus clinical selection. J Clin Oncol 2011;29:1943–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KD, Siegel R, Lin CC, Mariotto AB, Kramer JL, Rowland JH. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- 3. Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710–7. [DOI] [PubMed] [Google Scholar]
- 4. Komaki R, Cox JD, Hartz AJ, Byhardt RW, Perez-Tamayo C, Clowry L. et al. Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 1985;8:362–70. [DOI] [PubMed] [Google Scholar]
- 5. Cagini L, Monacelli M, Giustozzi G, Moggi L, Bellezza G, Sidoni A. et al. Biological prognostic factors for early stage completely resected non-small cell lung cancer. J Surg Oncol 2000;74:53–60. [DOI] [PubMed] [Google Scholar]
- 6. Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH.. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol 2005;23:175–83. [DOI] [PubMed] [Google Scholar]
- 7. Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M. et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E.. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425–8. [DOI] [PubMed] [Google Scholar]
- 9. Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B. et al. Neutrophil: lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20:650. [DOI] [PubMed] [Google Scholar]
- 10. Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH. et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z. et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188–95. [DOI] [PubMed] [Google Scholar]
- 12. Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI. et al. Nomograms predict overall survival for patients with small-cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol 2015;10:1213–20. [DOI] [PubMed] [Google Scholar]
- 13. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D. et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861–9. [DOI] [PubMed] [Google Scholar]
- 14. Ebbert JO, Williams BA, Sun Z, Aubry MC, Wampfler JA, Garces YI. et al. Duration of smoking abstinence as a predictor for non-small-cell lung cancer survival in women. Lung Cancer 2005;47:165–72. [DOI] [PubMed] [Google Scholar]
- 15. Sun Z, Aubry MC, Deschamps C, Marks RS, Okuno SH, Williams BA. et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg 2006;131:1014–20. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Sun Z, Cunningham JM, Aubry MC, Wampfler JA, Croghan GA. et al. Genetic variations in multiple drug action pathways and survival in advanced stage non-small cell lung cancer treated with chemotherapy. Clin Cancer Res 2011;17:3830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K. et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009;45:1950–8. [DOI] [PubMed] [Google Scholar]
- 18. Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES. et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest 2005;128:452–62. [DOI] [PubMed] [Google Scholar]
- 19. Birim O, Kappetein AP, Waleboer M, Puvimanasinghe JP, Eijkemans MJ, Steyerberg EW. et al. Long-term survival after non-small cell lung cancer surgery: development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg 2006;132:491–8. [DOI] [PubMed] [Google Scholar]
- 20. Birim O, Kappetein AP, van Klaveren RJ, Bogers AJ.. Prognostic factors in non-small cell lung cancer surgery. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2006;32:12–23. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi N, Usui S, Kikuchi S, Goto Y, Sakai M, Onizuka M. et al. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer 2012;75:223–7. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe R, Tomita N, Kishimoto K, Koyama S, Ogusa E, Ishii Y. et al. Absolute monocyte count in follicular lymphoma patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Res 2013;37:1208–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.