Abstract
OBJECTIVE
Both food insecurity (limited food access owing to cost) and living in areas with low physical access to nutritious foods are public health concerns, but their relative contribution to diabetes management is poorly understood.
RESEARCH DESIGN AND METHODS
This was a prospective cohort study. A random sample of patients with diabetes in a primary care network completed food insecurity assessment in 2013. Low physical food access at the census tract level was defined as no supermarket within 1 mile in urban areas and 10 miles in rural areas. HbA1c measurements were obtained from electronic health records through November 2016. The relationship among food insecurity, low physical food access, and glycemic control (as defined by HbA1c) was analyzed using hierarchical linear mixed models.
RESULTS
Three hundred and ninety-one participants were followed for a mean of 37 months. Twenty percent of respondents reported food insecurity, and 31% resided in an area of low physical food access. In adjusted models, food insecurity was associated with higher HbA1c (difference of 0.6% [6.6 mmol/mol], 95% CI 0.4–0.8 [4.4–8.7], P < 0.0001), which did not improve over time (P = 0.50). Living in an area with low physical food access was not associated with a difference in HbA1c (difference 0.2% [2.2 mmol/mol], 95% CI −0.2 to 0.5 [−2.2 to 5.6], P = 0.33) or with change over time (P = 0.07).
CONCLUSIONS
Food insecurity is associated with higher HbA1c, but living in an area with low physical food access is not. Food insecurity screening and interventions may help improve glycemic control for vulnerable patients.
Introduction
Diabetes affects more than 30 million Americans and is both more common and more likely to lead to complications in those with lower socioeconomic status (1,2). Adherence to a healthy diet is a cornerstone of diabetes management, but those who are socioeconomically vulnerable face several important barriers to dietary adherence. First, food insecurity, the uncertain or limited availability of food owing to cost, is highly prevalent in the U.S., affecting ∼13% of American households overall, 20% of Americans with diabetes, and 25% of Americans with poor glycemic control (hemoglobin A1c [HbA1c] >9.0%) (3,4). In addition, area factors, such as having a place to purchase nutritious food, termed “physical food access” by the U.S. Department of Agriculture (USDA) (5), lack of transportation, and neighborhood deprivation, can also make following an appropriate diet more challenging.
A growing body of evidence has found associations between area-level factors and cardiometabolic health overall and diabetes in particular (6–13). However, there is a significant gap in our knowledge regarding how these factors may act to influence the management of diabetes. In particular, it is important to distinguish compositional factors (the individual-level features of the people who make up a given area) from contextual factors (features of the area itself). This distinction has significant implications for public health and health policy. The concept of food “deserts,” areas with low income and low physical access to food, has spurred major policy initiatives involving both the government and the private sector (14). Even as initiatives designed to address contextual factors have been implemented, many programs that target individual-level factors have been cut. For example, the Supplemental Nutrition Assistance Program (SNAP) (formerly the Food Stamp Program), which directly combats food insecurity, has seen reductions in benefit levels, along with proposals for restricting eligibility and additional deep financial cuts (15,16).
Given the implications for intervention programs, distinguishing the health impact of low physical food access from food insecurity is highly relevant for policy. In this study, we sought to determine the relative contribution of individual- and area-level factors to glycemic control, based on HbA1c, in patients with diabetes. From prior focus groups that we conducted, which discussed barriers to glycemic control in patients with diabetes, we hypothesized that food insecurity would be associated with worse glycemic control but that physical food access would not.
Research Design and Methods
Setting and Study Sample
Data collection methods for the study have previously been described (17). In brief, a randomly selected cohort of adult (aged ≥21 years) patients with diabetes (any type) was enrolled between June and October 2013 from four clinics in eastern Massachusetts (one academic primary care practice, two community health centers, and one specialty diabetes practice). Participants completed a questionnaire, in English or Spanish, consisting of validated items regarding diabetes history and social circumstances. Responses to this questionnaire were linked with electronic health record information from the participant’s primary care network. Participants were then followed, without recontact, through their electronic health records. The social circumstances survey items queried participants regarding their experience over the 12 months prior to instrument administration; accordingly, this study used medical record data beginning on 1 June 2012. Participants were passively followed until 30 November 2016 or their last interaction with the primary care network—a period of up to 54 months. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Partners HealthCare (18). The Human Subjects Research Committee at Partners HealthCare approved this protocol.
Assessment of Food Insecurity and Physical Food Access
Food insecurity was assessed using the six-item short form of the USDA Food Security Survey Module (19). An example item is, “‘The food that I bought just didn’t last, and I didn’t have money to get more.’ Was that often, sometimes, or never true for you in the last 12 months?” In accordance with standard scoring, participants who answered affirmatively (“often” or “sometimes” for items where these were response categories and “yes” for items where “yes” or “no” were the response categories) to two or more items were recorded as reporting food insecurity. As is common in the food insecurity and diabetes literature, we individualized the questions and treated food insecurity as an individual-level factor for analysis purposes, even though it is technically a household-level factor (4,17,20–22). Five participants (1% of total) did not have a complete food insecurity assessment.
Residence in an area of low physical food access was based on linkage of participants’ geocoded addresses to the census tract–level physical food access data from the USDA’s Food Access Research Atlas (5). Participants with addresses that could not be geocoded, most commonly as a result of having a post office box listed for the address, were excluded. Addresses were updated and regeocoded annually throughout the study period. It is important to distinguish lack of physical food access (i.e., having a place to purchase food in your area) from other limits to food access, such as food insecurity (being unable to afford food) or the inability to go out and purchase food owing to disability. There have been several definitions of low physical food access used in research. The most common, and the primary definition for this study, comes from the USDA’s Food Access Research Atlas (5). This defines an area of low physical food access as “a tract with at least 500 people or 33% of the population living >1 mile (urban areas) or >10 miles (rural areas) from the nearest supermarket, supercenter, or large grocery store.” We also considered an alternative definition contained in the Food Access Research Atlas that uses a half mile in urban areas and 10 miles in rural areas as the cut point. Two other factors are important for further determining whether a low physical food access census tract is a food “desert”: low income level and low access to motor vehicles (a census tract–level proxy indicator). To incorporate these other considerations, we included in our study three additional census tract–level variables: median family income, poverty rate, and an indicator of low vehicle access (a tract where ≥100 households do not have vehicle access and live more than half a mile from a supermarket). In the U.S. overall, of 28,541 low physical food access tracts, 8,959 are considered food “deserts” (5).
We also examined an alternate methodology for classifying food access: the Modified Retail Food Environment Index (mRFEI), created by the Division of Nutrition, Physical Activity, and Obesity at the Centers for Disease Control and Prevention (CDC) (23). This index is calculated as the number of healthy food retailers in the census tract, divided by the total number of food retailers in the census tract (23). The mRFEI ranges from 0 to 1 and helps one to understand both low physical food access (absence of healthy food retailers, mRFEI scores = 0) and high levels of unhealthy food retail, such as a predominance of fast food restaurants (mRFEI scores close to, but not exactly, 0), a situation sometimes termed living in a food “swamp.”
The data for census tract characteristics for this study were taken from the USDA’s Food Access Research Atlas Data Download 2015, the most recent version available, except for the mRFEI score, which came from the CDC. The variables in the Food Access Research Atlas were collected between 2010 and 2015 and were considered up to date at the time they were released. Data on neighborhood economic circumstances contained in the Food Access Research Atlas were collected by the U.S. Census. The mRFEI score data were released by the CDC in 2011.
Primary Outcome
The primary outcome for this study was HbA1c level, which was measured as part of routine care. Multiple measurements took place during the study period, and all measurements were included in analysis of the outcome as described below. Data were obtained from the electronic health record. All HbA1c assays in this care network are conducted at the same central laboratory, which is certified by the National Glycohemoglobin Standardization Program. All available HbA1c values measured during the study period were included in the analysis.
Covariates
We considered several covariates that may confound the relationship among food insecurity, food access, and HbA1c at both the individual and census tract level. With the exception of the census tract variables discussed above, all data came from the completed questionnaire or the electronic health record. We considered the sociodemographic factors of baseline age (in years), sex, race/ethnicity (categorized as non-Hispanic white, non-Hispanic black, Hispanic, and Asian/multiple ethnicities [multi]/other), educational attainment (less than high school diploma, high school diploma, or greater than high school diploma), and health insurance (private, Medicare, Medicaid [including Medicare-Medicaid “dual-eligibles”], or self-pay/uninsured). We also considered health literacy (17,24) and whether the questionnaire was completed in English or Spanish. Clinical characteristics considered included age at diabetes diagnosis, Charlson comorbidity score (25), the use of insulin (of any kind), and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor (“statin”) use. To account for access to and engagement in care, we also considered the frequency of outpatient care visits during the study period.
Statistical Analysis
We made unadjusted comparisons using t tests for continuous variables and χ2 tests for categorical variables. To analyze whether food insecurity or living in a low physical food access area was associated with greater HbA1c, we fit hierarchical linear mixed models (SAS PROC MIXED). To do this, we conceptualized HbA1c observations as being nested within individual participants and those participants being nested within census tracts. The census tract information was time varying to correspond with the census tract the participant was living in at the time of HbA1c measurement. We first calculated intraclass correlations, which quantify the variation explained at each level of the model: individual and census tract. We did this by fitting null (intercept only) models, following the method proposed by Bell et al. (26), and calculating the percentage of the variance explained at each level (by dividing the variance for a given level by the total variance). For our main analysis, we fit three-level models with random effects terms for the individual and census tract. We included both random intercept terms as well as random slope terms for food insecurity and time (at the individual level) and physical food access indicator (at the census tract level). This allows the HbA1c trajectory to vary between groups. We used an autoregressive covariance structure to model HbA1c levels, as measurements taken closer together in time are more likely to be correlated. We tested whether the HbA1c trajectory changed over time by specifying interaction terms. We used interaction terms to test whether the temporal trend in HbA1c varied by the level of food insecurity or physical food access. In our modeling, variables related to census tract of residence (low food access, median family income, poverty rate, and vehicle access) were time varying (based on what census tract an individual was living in at the time of the HbA1c assessment) and updated annually, while individual-level variables were time fixed and collected at baseline. After fitting regression models, we used least squares means to calculate predicted HbA1c values adjusted for the factors in the model.
As sensitivity analyses, we fit the same models as our main analysis but using alternative indicators for low physical food access. First, we used the CDC’s mRFEI as a continuous variable. Next, we categorized low physical food access using a different distance metric: no supermarkets within half a mile in urban areas and 10 miles in rural areas. Finally, we fit a model using a combined food desert indicator.
All statistical analyses were conducted in SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Four hundred and eleven participants completed the survey; 391 of these (95%) had baseline addresses that could be geocoded and were thus included in the study. The demographic and clinical features of those participants excluded because they could not be geocoded were very similar to the features of those who could be geocoded (Supplementary Table 1). The overall mean and median durations of follow-up were 37 and 41 months, respectively. The mean age of study participants was 62 years, and the mean duration of diabetes was 12 years.
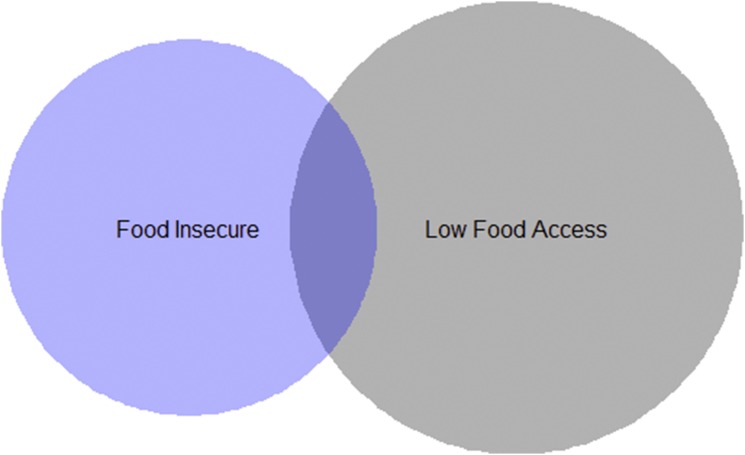
Twenty percent of respondents reported food insecurity. The duration of follow-up and number of HbA1c assessments for respondents with food insecurity did not differ from those for participants without food insecurity (Table 1). Thirty-one percent of respondents lived in an area with low physical food access at baseline. Fifteen percent of participants who reported food insecurity were living in an area with low physical food access, and 10% of participants living in an area with low physical food access were food insecure (P for comparison = 0.0009) (Fig. 1). Participants who reported food insecurity were more likely to be racial/ethnic minorities, have Medicaid insurance, and have low educational attainment compared with those who were food secure (Table 1). Participants living in an area with low physical food access were more likely to be non-Hispanic white. Only 2% of participants lived in a rural area. Participants with food insecurity had a greater number of outpatient visits during the follow-up period.
Table 1.
Demographics and clinical characteristics
| Overall | Food security status |
Food access |
|||||
|---|---|---|---|---|---|---|---|
| Food secure | Food insecure | P | Adequate access | Low access | P | ||
| N | 391 | 308 | 78 | 271 | 120 | ||
| Food insecure (at baseline) | 20.21 | n/a | n/a | 24.72 | 10.08 | 0.001 | |
| Baseline age, years | 61.94 (11.90) | 63.20 (11.88) | 56.72 (10.55) | <0.0001 | 61.21 (11.63) | 63.58 (12.38) | 0.08 |
| Female sex | 48.08 | 47.08 | 51.28 | 0.51 | 47.97 | 48.33 | 0.95 |
| Race/ethnicity | 0.008 | 0.002 | |||||
| Non-Hispanic white | 79.03 | 81.82 | 66.67 | 74.91 | 88.33 | ||
| Non-Hispanic black | 7.67 | 5.84 | 15.38 | 9.96 | 2.50 | ||
| Hispanic | 8.95 | 7.79 | 14.10 | 11.44 | 3.33 | ||
| Asian/other/multi | 4.35 | 4.55 | 3.85 | 3.69 | 5.83 | ||
| Education | 0.025 | 0.002 | |||||
| <High school diploma | 14.36 | 12.01 | 21.79 | 17.41 | 7.50 | ||
| High school diploma | 27.18 | 29.55 | 17.95 | 29.63 | 21.67 | ||
| >High school diploma | 58.46 | 58.44 | 60.26 | 52.96 | 70.83 | ||
| Insurance at baseline | 0.02 | 0.18 | |||||
| Private | 50.94 | 54.83 | 36.84 | 48.66 | 56.36 | ||
| Medicare | 26.42 | 25.17 | 30.26 | 25.67 | 28.18 | ||
| Medicaid | 13.48 | 11.03 | 22.37 | 15.71 | 8.18 | ||
| Uninsured/self-pay | 9.16 | 8.97 | 10.53 | 9.96 | 7.27 | ||
| Born outside U.S. | 20.00 | 17.86 | 29.49 | 0.02 | 22.96 | 13.33 | 0.03 |
| Low health literacy | 27.58 | 24.51 | 38.46 | 0.01 | 31.60 | 18.49 | 0.008 |
| Took survey in Spanish | 5.12 | 4.22 | 8.97 | 0.09 | 7.01 | 0.83 | 0.01 |
| Age at diabetes diagnosis, years | 49.74 (14.05) | 50.56 (13.92) | 46.19 (14.00) | 0.02 | 48.90 (14.06) | 51.62 (13.90) | 0.08 |
| Baseline Charlson score | 4.85 (2.98) | 4.74 (2.90) | 5.22 (3.34) | 0.25 | 4.89 (2.90) | 4.78 (3.17) | 0.74 |
| Baseline insulin use | 53.96 | 51.30 | 64.10 | 0.04 | 56.09 | 49.17 | 0.21 |
| Baseline statin use | 83.38 | 86.36 | 70.51 | 0.001 | 83.76 | 82.50 | 0.76 |
| Baseline ACEi/ARB use | 81.33 | 83.44 | 73.08 | 0.04 | 81.55 | 80.83 | 0.87 |
| Number of outpatient visits during study | 47.43 (37.54) | 44.60 (35.10) | 59.08 (45.22) | 0.01 | 50.13 (37.81) | 41.26 (36.31) | 0.03 |
| Number of HbA1c assessments during study | 10.00 (3.83) | 9.84 (3.78) | 10.48 (3.98) | 0.20 | 10.19 (3.87) | 9.45 (3.53) | 0.08 |
| Low food access, 1 and 10 miles | 30.69 | 34.74 | 15.38 | 0.001 | n/a | n/a | |
| Low food access, half a mile and 10 miles | 75.19 | 78.57 | 61.54 | 0.002 | n/a | n/a | |
| Low vehicle access | 33.50 | 32.79 | 35.90 | 0.60 | 27.68 | 46.67 | 0.0002 |
| mRFEI | 8.30 (7.21) | 8.17 (6.78) | 8.68 (8.55) | 0.69 | 7.70 (6.91) | 9.27 (7.62) | 0.09 |
| Median family income of census tract, $ | 91,921 (42,945) | 96,189 (42,746) | 76,104 (40,576) | 0.0002 | 85,808 (45,420) | 105,727 (32,958) | <0.0001 |
| Census tract poverty rate | 12.54 (9.14) | 11.34 (8.65) | 17.22 (9.69) | <0.0001 | 14.89 (9.34) | 7.24 (5.87) | <0.0001 |
Data are % or mean (SD) unless otherwise indicated. Food access defined using the 1 mile in urban areas and 10 miles in rural areas definition. Five (1% of total) participants did not have a complete food insecurity assessment. ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; n/a, not applicable.
Figure 1.
Proportional Venn diagram showing overlap between those with food insecurity and those living in a census tract with low physical food access (1 mile in urban area and 10 miles in rural area definition). Seventy-eight (20%) participants were food insecure, 120 (10%) lived in an area of low physical food access, and 12 (3%) were both food insecure and lived in an area of low physical food access.
Intraclass correlations showed that 62% of the variation in HbA1c was explained by individual-level factors (such as age or food insecurity), while only 4% of the variation in HbA1c was explained by census tract–level factors (such as poverty rates or living in an area with low physical food access).
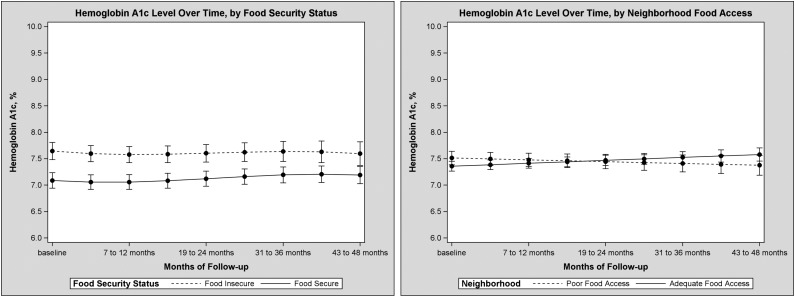
In unadjusted results, mean HbA1c was 7.86% (62 mmol/mol) in those who reported food insecurity versus 7.30% (56 mmol/mol) in those who did not (P = 0.001), and 7.21% (55 mmol/mol) in those who lived in a low physical food access area versus 7.49% (58 mmol/mol) in those who did not (P = 0.05). In hierarchical linear models, adjusted for age, sex, race/ethnicity, education, insurance, health literacy, survey language, age at diabetes diagnosis, Charlson comorbidity score, insulin use, statin use, outpatient visits, food access, vehicle access, median family income, and census tract poverty rate, food insecurity was associated with higher HbA1c (difference 0.6% [6.6 mmol/mol], 95% CI 0.4–0.8 [4.4–8.7], P = <0.0001) (Table 2). There was no evidence that the difference improved over time (estimated change in difference per month −0.003% [less than −0.1 mmol/mol], P = 0.50) (Fig. 2 and full model in Supplementary Table 2). In models adjusted for the same factors, living in an area with low physical food access was not associated with an overall difference in HbA1c compared with living in areas of adequate food access (difference 0.2% [2.2 mmol/mol], 95% CI −0.2 to 0.5 [−2.2 to 5.5], P = 0.33) and was not associated with change in HbA1c over time (change in difference per month −0.007% [less than −0.1 mmol/mol], P = 0.07) (full model in Supplementary Table 3).
Table 2.
HbA1c by food insecurity and food access
| Adjusted baseline HbA1c, % (95% CI) | Difference (95% CI) | P | Average slope [change per month in HbA1c, % (95% CI)] | Difference in change per month in HbA1c, % (95% CI) | P | |
|---|---|---|---|---|---|---|
| Food insecure | 7.6 (7.3–7.9) | 0.6 (0.4–0.8) | <0.0001 | 0.0002 (−0.01 to 0.01) | −0.003 (−0.01 to 0.01) | 0.50 |
| Food secure | 7.0 (6.8–7.3) | 0.003 (−0.001 to 0.007) | ||||
| Low food access | 7.4 (7.1–7.7) | 0.2 (−0.2 to 0.5) | 0.33 | −0.003 (−0.02 to 0.001) | −0.007 (−0.02 to 0.001) | 0.07 |
| Adequate food access | 7.3 (7.0–7.5) | 0.005 (0.0004–0.009) |
Data are means, and the difference in mean HbA1c was calculated using least squares means from the regression models presented in Supplementary Table 2 (for food security) and Supplementary Table 3 (for food access). Food access defined using the 1 mile in urban areas and 10 miles in rural areas definition. In addition to the exposures of interest, models were adjusted for age, age squared, sex, race/ethnicity, education, insurance, health literacy, language, age at diabetes diagnosis, baseline Charlson comorbidity score, insulin use, statin use, number of outpatient care visits, census tract median family income, census tract poverty rate, and census tract vehicle access.
Figure 2.
Predicted mean HbA1c values with SE bars from the models described in Supplementary Table 2 (left) and Supplementary Table 3 (right), by 6-month increments, comparing those who reported food insecurity and those who are food secure (left) and those who lived in neighborhoods with adequate and poor food access (right). In addition to the exposures of interest, models were adjusted for age, age squared, sex, race/ethnicity, education, insurance, health literacy, language, age at diabetes diagnosis, baseline Charlson comorbidity score, insulin use, statin use, number of outpatient care visits, census tract median family income, census tract poverty rate, and census tract vehicle access.
Sensitivity analyses using alternative definitions of neighborhood food access, which adjusted for the same covariates as the main analyses, yielded similar results. When physical food access was defined by the mRFEI, this contextual factor was still not associated with HbA1c (P = 0.56), while food insecurity was associated with 0.7% [7.7 mmol/mol] higher HbA1c (P < 0.0001) compared with food-secure participants (full model in Supplementary Table 4). When we used the half mile in urban areas and 10 miles in rural area definition, low physical food access was also not associated with HbA1c (P = 0.91) and food insecurity was still associated with increased HbA1c (0.5% [5.5 mmol/mol], P = <0.0001) (full model in Supplementary Table 5). Results were essentially unchanged when we fit a model with a combined indicator of low census tract income and physical food access (food “desert”). The difference for those reporting food insecurity compared with food security remained significant (0.5% [5.5 mmol/mol], P = <0.0001), while there was no significant difference in HbA1c for those living in food deserts versus those who did not (P = 0.35) (full model in Supplementary Table 6). Examining the distribution of outpatient visit frequency or number of HbA1c assessments did not suggest that differences in these factors between groups would explain the observed results (Supplementary Table 7). We did not find evidence of a time-by-education (an indicator of individual-level socioeconomic status) interaction that would confound the results reported (Supplementary Table 8). Sequential model building revealed that the results were robust across several progressively more complex modeling specifications (Supplementary Table 9).
Conclusions
In this longitudinal study of patients with diabetes, we found that individual-level factors, such as food insecurity, explained substantially more of the variation in HbA1c than census tract–level factors, such as low physical food access. Food insecurity was associated with ∼0.6% (6.6 mmol/mol) higher HbA1c. This difference did not improve over time despite higher use of clinical services by those with food insecurity. We did not find evidence that living in an area with low physical food access was associated with worse glycemic control.
These findings extend our understanding of food insecurity and food access among patients with diabetes. Prior cross-sectional studies have demonstrated an association between food insecurity and higher HbA1c (4,21,27,28), but there have been few longitudinal studies. A recent study in Illinois also found an association between food insecurity and greater HbA1c at baseline, with a slightly greater decrease in HbA1c over time for food-insecure, compared with food-secure, participants (29). However, the Illinois study examined a more limited set of potential confounders and did not examine area-level factors. There have been few previous studies of physical food access and HbA1c. One study in the San Francisco Bay Area found that losing or gaining a supermarket in a neighborhood was not associated with meaningful change in HbA1c (30). Prior studies of BMI and neighborhood food access in patients with diabetes also have generally not found an important association (31–33). We also think it is important to note that our findings regarding who resides in areas of low physical food access do not correlate well with common perceptions of who lives in these areas, as we found fewer racial/ethnic minorities and higher educational attainment in these areas. This is consistent with national data showing that low physical food access and low income census tracts do not have a high degree of overlap (5) and may challenge common perceptions of who lives in a low food access census tract. Further, while our study took place in an urban context, prior work in rural areas has also found that more deprived areas may, paradoxically, have closer food access (34). It is worth noting, however, that area poverty is associated with decreases in supermarket availability over time (35). Finally, this study may highlight a limitation of current, geographic information system–based methods for assessing the food environment. Measures that incorporate an individual’s subjective view of their food environment may correlate better with health outcomes.
Though the mechanism by which food insecurity may be related to higher HbA1c is not clear, proposed mechanisms supported by prior studies include lower dietary quality (36); increased trade-offs between food and medications (37,38), resulting in reduced medication adherence; and increased psychological distress (39) related to food insecurity. Longitudinal studies to examine these relationships may offer insight into how best to address food insecurity and improve management of diabetes.
Although this study did not find an association between glycemic control and physical food access, this should be considered within a larger context. Besides physical food access, there are other elements of the food environment that may impact self-management of diabetes and could be studied in future work. Other considerations in access to healthy foods include food affordability, food quality, and individual food preferences. We also think it is important to note that while physical food access did not have strong associations with glycemic control, this is only one element of diabetes self-management that physical food access interventions might try to address. Other health outcomes, such as weight and mental health, may have stronger associations with area food access. In addition, improving physical food access could indirectly lead to health benefits by driving economic growth, generating tax revenue to support public services, and improving neighborhood livability.
Given their potential broad impact on overall health and many other conditions besides diabetes per se, we should not eschew interventions to improve physical food access. Rather, combining food access interventions with interventions that directly address food insecurity may be more likely to improve diabetes outcomes specifically. Health care systems might consider routinely measuring food insecurity in those with suboptimal glycemic control and then using strategies to address food insecurity once it has been identified. Such strategies might include assistance with enrollment in SNAP, interventions that link patients to community resources (40), work with local food banks (41), or prescriptions for healthy food (42). Since these interventions rely on having community resources for patients to use, however, there is a clear interrelationship between individual- and area-level factors that should be considered. In highly vulnerable neighborhoods, addressing both compositional and contextual factors is likely needed (for example, both improving food access and reducing food insecurity to optimize an individual’s ability to manage diabetes). Cuts to Farm Bill–supported nutrition programs such as SNAP, as are currently proposed (16), would likely make addressing food insecurity more difficult for vulnerable patients.
The findings of this study should be interpreted in light of several limitations. Food security assessment occurred at a single time point, and so if the food security status of participants changed during the study period, this could lead to misclassification, which may bias results toward the null. Next, this study was conducted within a health care network in predominately urban eastern Massachusetts. While the occurrence of both food insecurity and low physical food access was similar to overall national averages (3–5), the results may not generalize to other areas. Next, because the study relied on self-report, there is the possibility of recall error. However, food insecurity is an inherently subjective concept, so there is no measurement alternative. Additionally, we used administrative records where possible (e.g., for laboratory values, number of clinic visits, and insurance information) to minimize reliance on self-reported information. Area-level data used in the study were the most recent available, but changes in neighborhood characteristics that were not captured in the data sets used may have resulted in misclassification. Finally, unmeasured confounding is always a threat to validity in observational studies such as this one. These limitations are balanced by several strengths, however. This was a longitudinal study, few patients were lost to follow-up, and there was detailed assessment of both individual- and area-level factors to permit adjustment for measured confounders.
Among patients with diabetes, food insecurity, but not physical food access, was associated with substantially worse glycemic control that did not improve despite higher frequency of outpatient visits. These findings suggest that interventions to improve glycemic control in vulnerable patients with diabetes should consider addressing food insecurity systematically and highlight the importance of distinguishing between compositional and contextual factors that may contribute to poor health.
Supplementary Material
Article Information
Funding. The role of S.A.B. in the research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K23-DK-109200). The role of S.A.A. was supported by T32DK007028-38/BAEDRC 5P30DK057521. The role of G.C.-S. was supported by the National Heart, Lung, and Blood Institute (2K24 HL105493-06-07 and 1R01HL120690-03). The role of H.K.S. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (P30-DK-092924).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A.B. and D.J.W. conceived of the study and drafted the manuscript. S.A.A. and L.S.B. assisted with collection and interpretation of data and revised the manuscript critically for intellectual content. A.J.K., G.C.-S., H.K.S., and S.J.A. assisted with analysis and interpretation of data and revised the manuscript critically for intellectual content. All authors gave approval of the manuscript version to be submitted. S.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Interim results of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1981/-/DC1.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017 [Internet]. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 10 August 2017
- 2.American Diabetes Association Promoting health and reducing disparities in populations. Sec. 1. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S6–S10 [DOI] [PubMed] [Google Scholar]
- 3.Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2015 [Internet], 2016. U.S. Department of Agriculture, Economic Research Service. Available from https://www.ers.usda.gov/webdocs/publications/79761/err-215.pdf?v=42636. Accessed 11 August 2017
- 4.Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care 2013;36:3093–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Agriculture, Economic Research Service Food Access Research Atlas: Documentation [Internet]. Available from https://www.ers.usda.gov/data-products/food-access-research-atlas/documentation/. Accessed 11 August 2017
- 6.Christine PJ, Auchincloss AH, Bertoni AG, et al. . Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med 2015;175:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zenk SN, Tarlov E, Wing C, et al. . Geographic accessibility of food outlets not associated with body mass index change among veterans, 2009-14. Health Aff (Millwood) 2017;36:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med 2009;36:74–81 [DOI] [PubMed] [Google Scholar]
- 9.Malambo P, Kengne AP, De Villiers A, Lambert EV, Puoane T. Built environment, selected risk factors and major cardiovascular disease outcomes: a systematic review. PLoS One 2016;11:e0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smalls BL, Gregory CM, Zoller JS, Egede LE. Assessing the relationship between neighborhood factors and diabetes related health outcomes and self-care behaviors. BMC Health Serv Res 2015;15:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger E, Diez-Roux AV, Lloyd-Jones DM, et al. . Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014;7:524–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubowitz T, Ghosh-Dastidar M, Eibner C, et al. . The Women’s Health Initiative: the food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring) 2012;20:862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujahid MS, Diez Roux AV, Morenoff JD, et al. . Neighborhood characteristics and hypertension. Epidemiology 2008;19:590–598 [DOI] [PubMed] [Google Scholar]
- 14.Fleischhacker SE, Flournoy R, Moore LV. Meaningful, measurable, and manageable approaches to evaluating healthy food financing initiatives: an overview of resources and approaches. J Public Health Manag Pract 2013;19:541–549 [DOI] [PubMed] [Google Scholar]
- 15.Center on Budget and Policy Priorities. More than 500,000 adults will lose SNAP benefits in 2016 as waivers expire [Internet], 2015. Available from https://www.cbpp.org/research/food-assistance/more-than-500000-adults-will-lose-snap-benefits-in-2016-as-waivers-expire. Accessed 1 February 2018
- 16.Center on Budget and Policy Priorities. President’s budget would radically restructure, cut SNAP [Internet], 2017. Available from https://www.cbpp.org/blog/presidents-budget-would-radically-restructure-cut-snap. Accessed 15 February 2018
- 17.Berkowitz SA, Meigs JB, DeWalt D, et al. . Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med 2015;175:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Agriculture, Economic Research Service Food security in the U.S.: Survey tools [Internet]. Available from: https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/survey-tools/#adult. Accessed 11 August 2017
- 20.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999-2002. J Gen Intern Med 2007;22:1018–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care 2012;35:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essien UR, Shahid NN, Berkowitz SA. Food insecurity and diabetes in developed societies. Curr Diab Rep 2016;16:79. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Census tract level state maps of the Modified Retail Food Environment Index (mRFEI) [Internet], 2011. Available from ftp://ftp.cdc.gov/pub/Publications/dnpao/census-tract-level-state-maps-mrfei_TAG508.pdf. Accessed 11 August 2017
- 24.Sarkar U, Schillinger D, López A, Sudore R. Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. J Gen Intern Med 2011;26:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008;61:1234–1240 [DOI] [PubMed] [Google Scholar]
- 26.Bell BA, Ene M, Smiley W, Schoeneberger JA. A multilevel model primer using SAS PROC MIXED [Internet], 2013. Available from http://support.sas.com/resources/papers/proceedings13/433-2013.pdf. Accessed 11 August 2017
- 27.Lyles CR, Wolf MS, Schillinger D, et al. . Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care 2013;36:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer VL, McDonough K, Seligman H, Mitra N, Long JA. Food insecurity, coping strategies and glucose control in low-income patients with diabetes. Public Health Nutr 2016;19:1103–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalowitz MU, Eng JS, McKinney CO, et al. . Food security is related to adult type 2 diabetes control over time in a United States safety net primary care clinic population. Nutr Diabetes 2017;7:e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YT, Mujahid MS, Laraia BA, et al. . Association between neighborhood supermarket presence and glycated hemoglobin levels among patients with type 2 diabetes mellitus. Am J Epidemiol 2017;185:1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laraia BA, Downing JM, Zhang YT, et al. . Food environment and weight change: does residential mobility matter? The Diabetes Study of Northern California (DISTANCE). Am J Epidemiol 2017;185:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YT, Laraia BA, Mujahid MS, et al. . Is a reduction in distance to nearest supermarket associated with BMI change among type 2 diabetes patients? Health Place 2016;40:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YT, Laraia BA, Mujahid MS, et al. . Does food vendor density mediate the association between neighborhood deprivation and BMI? A G-computation mediation analysis. Epidemiology 2015;26:344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharkey JR, Horel S. Neighborhood socioeconomic deprivation and minority composition are associated with better potential spatial access to the ground-truthed food environment in a large rural area. J Nutr 2008;138:620–627 [DOI] [PubMed] [Google Scholar]
- 35.Chen H-J, Wang Y. The changing food outlet distributions and local contextual factors in the United States. BMC Public Health 2014;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkowitz SA, Gao X, Tucker KL. Food-insecure dietary patterns are associated with poor longitudinal glycemic control in diabetes: results from the Boston Puerto Rican Health study. Diabetes Care 2014;37:2587–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med 2014;127:303–310.e3 [DOI] [PubMed] [Google Scholar]
- 38.Billimek J, Sorkin DH. Food insecurity, processes of care, and self-reported medication underuse in patients with type 2 diabetes: results from the California Health Interview Survey. Health Serv Res 2012;47:2159–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gucciardi E, Vogt JA, DeMelo M, Stewart DE. Exploration of the relationship between household food insecurity and diabetes in Canada. Diabetes Care 2009;32:2218–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berkowitz SA, Hulberg AC, Standish S, Reznor G, Atlas SJ. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med 2017;177:244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seligman HK, Lyles C, Marshall MB, et al. . A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Aff (Millwood) 2015;34:1956–1963 [DOI] [PubMed] [Google Scholar]
- 42.Goddu AP, Roberson TS, Raffel KE, Chin MH, Peek ME. Food Rx: a community-university partnership to prescribe healthy eating on the South Side of Chicago. J Prev Interv Community 2015;43:148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.