Abstract
OBJECTIVE
We compared the ability of 1- and 2-h plasma glucose concentrations (1h-PG and 2h-PG, respectively), derived from a 75-g oral glucose tolerance test (OGTT), to predict retinopathy. 1h-PG and 2h-PG concentrations, measured in a longitudinal study of an American Indian community in the southwestern U.S., a population at high risk for type 2 diabetes, were analyzed to assess the usefulness of the 1h-PG to identify risk of diabetic retinopathy (DR).
RESEARCH DESIGN AND METHODS
Cross-sectional (n = 2,895) and longitudinal (n = 1,703) cohorts were assessed for the prevalence and incidence of DR, respectively, in relation to deciles of 1h-PG and 2h-PG concentrations. Areas under the receiver operating characteristic (ROC) curves for 1h-PG and 2h-PG were compared with regard to predicting DR, as assessed by direct ophthalmoscopy.
RESULTS
Prevalence and incidence of DR, based on direct ophthalmoscopy, changed in a similar manner across the distributions of 1h-PG and 2h-PG concentrations. ROC analysis showed that 1h-PG and 2h-PG were of similar value in identifying prevalent and incident DR using direct ophthalmoscopy. 1h-PG cut points of 230 and 173 mg/dL were comparable to 2h-PG cut points of 200 mg/dL (type 2 diabetes) and 140 mg/dL (impaired glucose tolerance), respectively.
CONCLUSIONS
1h-PG is a useful predictor of retinopathy risk, has a predictive value similar to that of 2h-PG, and may be considered as an alternative glucose time point during an OGTT.
Introduction
Hyperglycemia assessed by the oral glucose tolerance test (OGTT) is a well-accepted method for identifying individuals with type 2 diabetes and those at risk of developing type 2 diabetes (1). Current World Health Organization (WHO) (2) or American Diabetes Association (ADA) criteria (1) do not use 1-h plasma glucose (1h-PG) to identify those at elevated risk for a diagnosis of type 2 diabetes. However, the potential contribution of 1h-PG was previously appreciated, as shown by inclusion of 1h-PG in the 1979 National Diabetes Data Group criteria for identifying impaired glucose tolerance (IGT) and diabetes (3). Although some investigators preferred using 1h-PG over 2-h plasma glucose (2h-PG) for convenience and because of the high correlation between the two values (4), WHO criteria deemed it unnecessary in 1980, as did later ADA criteria that considered 2h-PG as the only postchallenge time point required (5,6). Though some evidence has indicated better reproducibility of the 2h-PG than the 1h-PG (4), the recommendation by WHO (5) was not based on evidence that the 1h-PG was inferior in predicting a relevant clinical end point such as diabetic retinopathy (DR), which may be a better judge of its value.
Four separate groups have previously reported that 1h-PG is a comparable or even better predictor of type 2 diabetes than 2h-PG (7–10), perhaps because it is more closely associated with insulin secretion (7,8,10), indicating that 1h-PG might also be a better predictor of DR than 2h-PG. In our report from a longitudinal study of American Indians living in the southwestern U.S., 1h-PG was more closely associated with insulin secretion, less closely associated with insulin action, but similarly able to predict future type 2 diabetes when compared with 2h-PG in individuals without type 2 diabetes (11). In a recently published Swedish longitudinal population study, 1h-PG was reported to be a better predictor of diabetes and a better predictor of complications of diabetes, including retinopathy, than 2h-PG (12). Many previous studies, including studies involving an American Indian community in the southwestern U.S., examined glycemic cutoffs for diabetes using fasting plasma glucose (FPG), 2h-PG, and hemoglobin A1c (A1C) and their associations with DR (13–16). To our knowledge, the ability of 1h-PG to predict DR has only been recently evaluated, and not in this closely followed American Indian cohort.
We sought to investigate the hypothesis that the ability of 1h-PG to predict DR is superior to that of 2h-PG. Accordingly, in an American Indian community in the southwestern U.S., the associations between postload glucose concentrations (1h-PG and 2h-PG) and DR were evaluated in both cross-sectional and prospective manners.
Research Design and Methods
Study Population
Briefly, participants were enrolled in a longitudinal epidemiological study that began in 1965 among an American Indian community in Arizona (17). Written informed consent was obtained from all participants, and the study was approved by the scientific director of the National Institute of Arthritis and Metabolic Diseases and, beginning in 1976, by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases. Individuals aged 5 years and older were invited, regardless of health, for standardized outpatient research examinations approximately every 2 years. These exams included a medical history, a physical examination, an OGTT, and an assessment of DR by direct ophthalmoscopy (4,14,18). BMI (kilograms per meter squared) was calculated based on measured height and weight with the volunteer wearing light clothing and no shoes.
Participants underwent a 75-g OGTT with venous plasma glucose measurements. Participants with visits before 1980 had both 1h-PG and 2h-PG (4); after that year 1h-PG was not collected. Plasma glucose concentrations were measured by the modified Hoffman method (Technicon Instruments Corporation, Tarrytown, NY), the hexokinase method (Ciba Corning Express 550 analyzer; Ciba Corning, Oberlin, OH), or the glucose oxidase method (Vital Diagnostics Envoy). Type 2 diabetes was diagnosed and classified according to the 2003 ADA criteria (19).
This study included only men and nonpregnant women (age ≥18 years) with baseline 1h-PG and 2h-PG measurements (milligrams per deciliter). Participants taking oral or injectable hypoglycemic medications (i.e., insulin) were excluded from the main analysis but included in the sensitivity analysis. The risk for DR in relation to 1h-PG and 2h-PG was evaluated in cross-sectional and longitudinal analyses. In cross-sectional analysis, the prevalence of retinopathy was determined from participants’ last outpatient exam with 1h-PG and 2h-PG measurements and at which DR was also assessed by dilated pupillary exam via direct ophthalmoscopy. For the longitudinal analysis, the first visit when both 1h-PG and 2h-PG were available and retinopathy was absent, as evaluated by direct ophthalmoscopy, was chosen as the baseline visit.
Direct Ophthalmoscopy
Direct fundoscopic examinations were preformed after pupil dilation by physicians who were unaware of each individual’s diabetes status. The physicians were not ophthalmologists by training, but all underwent an initial training period. On the basis of the worse eye at each exam, DR was classified into three groups: 1) normal, 2) nonsevere, and 3) severe. The normal group included eyes with hard exudates only, as this was previously shown to be a common finding in American Indians without type 2 diabetes (18). The nonsevere group involved at least a single microaneurysm in one eye in the absence of proliferative retinopathy. Those with proliferative changes or scars from prior laser therapy in at least one eye were considered to have severe DR.
Retinal Photography
Beginning in 1982, retinal photographs of two standard fields for both eyes were taken at the same visit using a Canon CR4–45NM fundus camera, as previously described (20). Therefore, we do not have retinal photography data from exams where 1h-PG was measured, but we have retinal photographs available for subsequent exams. As an additional objective assessment of DR, another analysis compared the association of 1h-PG and 2h-PG at the baseline visit (before 1980) with DR in a subgroup of participants who were later evaluated by fundus photography at a second visit (between 1982 and 2007). These photographs were graded in a standardized manner, without knowledge of clinical details, using the modified Airlie House classification system (20,21). DR was classified based on the worse eye. Similar to the direct ophthalmoscopy exams, “normal” eyes included isolated hard exudates but no other sign of retinopathy or retinal hemorrhage without microaneurysms, nonsevere DR included the presence of microaneurysms (level 20 or higher but below level 51), and severe DR was based on proliferative changes, hemorrhages, and/or intraretinal microvascular abnormalities present (level ≥51) (22).
Statistical Analyses
Statistical analyses were performed using SAS (version 9.4; SAS Institute, Inc., Cary, NC) and SPSS (version 23; IBM, Armonk, NY). In cross-sectional analysis, the prevalence of any DR on direct ophthalmoscopy was examined by 10ths of the distributions of 1h-PG and 2h-PG. In longitudinal analysis of participants without DR at baseline, participants were followed until DR developed, as observed on direct ophthalmoscopy. Cumulative incidence of DR on direct ophthalmoscopy was estimated using the Kaplan-Meier method and plotted by deciles of 1h-PG and 2h-PG. On the basis of predicted cumulative incidence of DR at 15 years (any DR on direct ophthalmoscopy) and 25 years (severe DR on direct ophthalmoscopy), receiver operating characteristic (ROC) curves were derived for 1h-PG and 2h-PG by modeling the survival probability with PROC PHREG in SAS software, using successive detection thresholds for 1h-PG and 2h-PG, in increments of 1 mg/dL, as previously described (23). Cutoffs to classify IGT and type 2 diabetes using 1h-PG values were identified based on the point on the ROC curve closest to the well-established 2h-PG cutoffs of 140 and 200 mg/dL. Kappa statistics (24) were used to assess agreement between 1h-PG and 2h-PG when categorizing normal glucose tolerance, IGT, and type 2 diabetes and to assess agreement between direct ophthalmoscopy and retinal photography for classifying DR.
Prediction models for DR, accounting for the time to event, enabled calculation of C-statistics to compare predictive abilities of 1h-PG, 2h-PG, and both 1h-PG and 2h-PG combined (25). C-statistics were calculated by the method described by Pencina and D’Agostino (26) and compared with the use of the method developed by DeLong et al. (27). For these models, nonnormally distributed data, such as 1h-PG and 2h-PG, underwent ranked-based inverse normalization via Blom calculation (28). As an additional analysis, these analyses were repeated with severe DR based on direct ophthalmoscopy.
For an additional supportive analysis, 1h-PG and 2h-PG ROC curves were developed using PROC LOGISTIC for DR diagnosed by retinal photographs taken after the baseline longitudinal visit. Those with DR level ≥20 on retinal photography—a level that includes both nonsevere and severe DR—were compared with those with DR level <20 (22).
Results
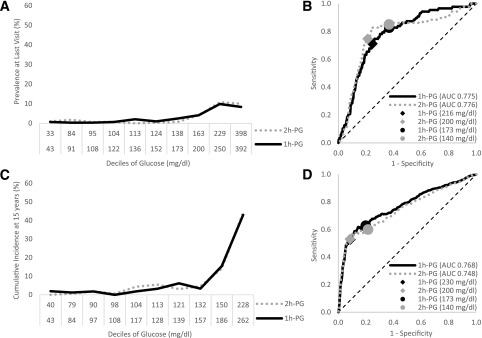
Baseline characteristics of participants included in the cross-sectional and longitudinal analyses are shown in Table 1. The cross-sectional analysis (n = 2,895) included 87 total cases of DR, of which 15 were severe. The prevalence of DR was examined by deciles of the distribution of 1h-PG and 2h-PG (Fig. 1A and Supplementary Table 1). The frequencies of DR were similar for each decile of 1h-PG and 2h-PG. The ROC curves for DR prevalence were also similar for 1h-PG and 2h-PG (Fig. 1B).
Table 1.
Participant characteristics at baseline examination
| Variables |
Cross-sectional cohort (n = 2,895) | Longitudinal cohort (n = 1,703) | ||
|---|---|---|---|---|
| Values |
Participants (n) |
Values |
Participants (n) |
|
| Age (years) | 31.7 (23.7, 46.3) | 2,895 | 26.3 (20.1, 37.4) | 1,703 |
| Male sex | 1,331 (46) | 2,895 | 648 (38) | 1,703 |
| American Indian | 2,895 (100) | 2,895 | 1,703 (100) | 1,703 |
| BMI (kg/m2) | 31.1 ± 6.8 | 2,895 | 30.7 ± 6.3 | 1,703 |
| Smoking | 946 (34) | 2,794 | 557 (38) | 1,471 |
| Blood pressure (mmHg) | ||||
| Systolic | 127 ± 21 | 2,889 | 126 ± 18 | 1,701 |
| Diastolic | 77 ± 13 | 2,888 | 75 ± 12 | 1,700 |
| Hypertension | 932 (32) | 2,895 | 404 (24) | 1,703 |
| Total cholesterol (mg/dL) | 174 ± 37 | 2,881 | 173 ± 38 | 1,687 |
| Serum creatinine (mg/dL) | 0.76 ± 0.44 | 2,880 | 0.69 ± 0.18 | 1,687 |
| FPG (mg/dL) | 97 (89, 115) | 1,509 | 92 (86, 102) | 436 |
| 1h-PG (mg/dL) | 152 (114, 220) | 2,895 | 127 (102, 169) | 1,703 |
| 2h-PG (mg/dL) | 123 (99, 185) | 2,895 | 112 (94, 139) | 1,703 |
| Type 2 diabetes | 663 (23) | 2,895 | 204 (12) | 1,703 |
| Diagnosis of diabetes at baseline examination | 227 (8) | 2,895 | 108 (6)¥ | 1,703 |
| Any retinopathy | 87 (3) | 2,895 | 498 (29) | 1,703 |
| Follow-up (years) | — | — | 22.7 (16.3, 28.8) | 1,703 |
| Severe retinopathy | 15 (0.5) | 2,895 | 148 (9) | 1,703 |
| Follow-up (years) | — | — | 24.6 (17.6, 29.9) | 1,703 |
Data are mean ± SD, median (interquartile range [25th, 75th percentiles]), or n (%) unless otherwise indicated.
¥Retinopathy at a follow-up (or censored) exam.
Figure 1.
Prevalence (A and B) and predicted incidence at 15 years (C and D) of any retinopathy based on direct ophthalmoscopy. Retinopathy in relation to glucose deciles (A and C) and ROC curves (B and D) of 1h-PG and 2h-PG concentrations. The glucose values shown in A and C are the lower bounds for each decile.
Longitudinal analysis (n = 1,703) included 498 incident cases of DR with a median follow-up of 22.7 years. When the 15-year cumulative incidence of DR was assessed by glucose decile, the curves for 1h-PG and 2h-PG were similar, showing a relative absence of retinopathy below the ninth decile (Fig. 1C). When the same data used for the graphs in Fig. 1A (prevalence) and Fig. 1C (15-year cumulative incidence) were alternatively plotted on a linear scale using the midpoint glucose concentration within each decile of 1h-PG and 2h-PG (Supplementary Fig. 4A and B, respectively), the prevalence and cumulative incidence appeared to increase more gradually, suggesting that the risk for retinopathy may gradually increase. The current 2h-PG threshold for type 2 diabetes (i.e., 200 mg/dL) falls within the ninth decile. The ninth decile range for 1h-PG was 186–261 mg/dL, indicating that an appropriate equivalent threshold for type 2 diabetes falls somewhere in this range. The 1h-PG concentration of 230 mg/dL has sensitivity and specificity for retinopathy almost identical to those of the 2h-PG concentration of 200 mg/dL (Fig. 1D), indicating that 1h-PG ≥230 mg/dL may be an equivalent threshold for type 2 diabetes diagnosis. Likewise, 1h-PG of 173 mg/dL has a sensitivity and specificity similar to those of the 2h-PG concentration of 140 mg/dL in analyses of both cumulative incidence (Fig. 1D) and prevalence (Fig. 1B), indicating that 173 mg/dL for 1h-PG may be equivalent for identifying those at intermediate risk for type 2 diabetes. Using this classification scheme for 1h-PG, three groups can be constructed that classify low, intermediate, and high risk for DR and provide similar prognostic information as the current 2h-PG classification (Table 2). The results did not change in cross-sectional and prospective analyses that included participants taking hypoglycemic medications (Supplementary Fig. 1). The results also did not change in additional analysis restricted to severe DR but excluding participants taking hypoglycemic medications at baseline (Supplementary Fig. 2; only prospective analysis because insufficient data were available for cross-sectional analysis). There was substantial agreement between 1h-PG and 2h-PG when categorizing into normal glucose tolerance, IGT, and type 2 diabetes when considering the cross-sectional (weighted κ 0.81; 95% CI 0.79–0.82) and longitudinal (weighted κ 0.77; 95% CI 0.74–0.80) groups (Supplementary Table 2).
Table 2.
Prevalence and cumulative incidence of DR by fundoscopy for risk groups based on 1h-PG and 2h-PG cutoffs for normal glucose tolerance, IGT, and type 2 diabetes
| Retinopathy | Normal glucose tolerance (%) | IGT (%)* | Type 2 diabetes (%)† |
|---|---|---|---|
| Prevalence, any retinopathy (n = 2,895) | |||
| 1h-PG | 0.9 | 3.1 | 8.6 |
| 2h-PG | 0.7 | 2.0 | 9.8 |
| 15-year c.i., any retinopathy (n = 1,703) | |||
| 1h-PG | 2.4 | 9.4 | 40.2 |
| 2h-PG | 2.5 | 9.1 | 40.0 |
| 25-year c.i., severe retinopathy (n = 1,703) | |||
| 1h-PG | 1.9 | 7.0 | 34.7 |
| 2h-PG | 2.1 | 5.6 | 34.6 |
c.i., cumulative incidence.
*IGT cutoffs for 1h-PG were ≥173 and <230 mg/dL, and for 2h-PG were ≥140 and <200 mg/dL.
†Type 2 diabetes cutoff for 1h-PG was ≥230 mg/dL and for 2h-PG was ≥200 mg/dL.
Supplementary Table 3 provides the C-statistics for risk prediction models that involved 1h-PG and 2h-PG and accounted for the time to event. The model with 1h-PG (C-statistic 0.778; 95% CI 0.755–0.801) predicted risk statistically significantly better (P = 0.04) than the model with 2h-PG (C-statistic 0.759; 95% CI 0.735–0.784), though the difference was small and likely not clinically meaningful. Additional sensitivity analysis restricting events to severe DR showed that 1h-PG did not differ significantly from 2h-PG (Supplementary Table 3).
In further supportive analyses, models were assessed involving DR diagnosed by retinal photography. Of the initial 2,895 participants, 1,361 (47%) had a later exam with a retinal photograph, and 360 of the 1,361 (26%) were diagnosed with any DR based on photography. With any DR diagnosed by retinal photography as the outcome measure, areas under the curve (AUCs) for 1h-PG (AUC 0.711; 95% CI 0.678–0.743) and 2h-PG (AUC 0.715; 95% CI 0.682–0.747) were similar (P = 0.72) (Supplementary Fig. 3A). Adjusting for follow-up time (median 23.0 years; interquartile range 18.0–27.4 years), the 1h-PG (AUC 0.741; 95% CI 0.717–0.764) and 2h-PG (AUC 0.725; 95% CI 0.700–0.748) were also similar (P = 0.08) (Supplementary Fig. 3B). Of the 1,361 participants with retinal photographs, 1,322 also had direct ophthalmoscopy performed during the same exam. There was moderate to substantial agreement between the two methods for evaluating any DR (unweighted κ 0.62; 95% CI 0.57–0.67) (Supplementary Table 4).
Conclusions
This study examined the prevalence and incidence of DR in relation to measurements of 1h-PG and 2h-PG concentrations during the OGTT. Complementary cross-sectional and longitudinal analyses indicated that 1h-PG and 2h-PG provide similar information about risk for DR. This study also identified that cutoffs for 1h-PG (≥230 mg/dL indicates type 2 diabetes; ≥173 and <230 mg/dL indicate IGT) provide information about DR risk similar to that provided by current 2h-PG criteria.
The relation between different glycemic measures (e.g., FPG, 2h-PG, and A1C) and retinopathy forms the basis for current diagnostic criteria for type 2 diabetes (2,29,30). The 2h-PG cutoff for type 2 diabetes (i.e., 2h-PG ≥200 mg/dL) was established to indicate a threshold above which subjects have a substantially elevated risk for type 2 diabetes–associated microvascular complications such as DR (30–32). The specific levels of A1C and FPG were adopted as diagnostic criteria for type 2 diabetes after 2h-PG, largely because they were shown to be equivalent to 2h-PG in predicting the development of DR (13). Although some groups previously favored 1h-PG for epidemiological studies (4), the routine clinical use of 1h-PG declined after the WHO recommendations in 1980 (5). Although at that time some evidence showed that 1h-PG was less reproducible than 2h-PG (4), investigators recognized that a superior arbiter between 1h-PG and 2h-PG should involve predictions of specific manifestations of type 2 diabetes, such as DR from longitudinal studies (4)—outcomes that had not been published to inform the 1980 WHO recommendations. Given the results of the current study, the choice of either 1h-PG or 2h-PG likely would have been satisfactory. Moreover, time and expense could be saved by choosing 1h-PG over 2h-PG.
Consistent with prior results for FPG and 2h-PG from this American Indian cohort (31) and other populations (30,32), 1h-PG also has an approximate threshold above which the prevalence and incidence of DR increase markedly, indicating that establishing a cutoff to define type 2 diabetes is appropriate. The prevalence and 15-year cumulative incidence of any DR below a 1h-PG cutoff of ∼230 mg/dL were low (<5%). Although direct ophthalmoscopy has been shown to have high specificity for DR, use of retinal photographs could have resulted in greater prevalence and incidence in these low 1h-PG or 2h-PG deciles, because direct ophthalmoscopy is less sensitive than retinal photography (22,33). As previously reported, these “missed” cases of retinopathy were most often microaneurysms without other lesions (34). Although retinal photographs may identify isolated lesions such as microaneurysms or small retinal hemorrhages that are more difficult to identify through the use of direct ophthalmoscopy, such lesions have not been clearly shown to predict future type 2 diabetes and may be nonspecific markers of hypertension as opposed to DR (35). In addition, the moderate to substantial agreement between direct ophthalmoscopy and retinal photography in the subgroup of participants with these measures available and in the cohort at large (22) suggests that it is unlikely that the results would have changed with regard to the finding that 1h-PG and 2h-PG are similar predictors of DR; cases diagnosed by direct ophthalmoscopy likely represent true DR.
We could not compare the predictive ability of 1h-PG with that of FPG or A1C because these tests were not generally performed during the research exams where 1h-PG was also measured. On the other hand, if the basis for FPG and A1C to classify type 2 diabetes is largely from equivalence with 2h-PG in the prediction of DR, it is unlikely that 1h-PG would be inferior to those glycemic parameters. Nevertheless, a longitudinal comparison of all four glucose indices is needed.
We studied a specific population of American Indians at high risk for type 2 diabetes, so it is unknown how results would differ from other racial or ethnic groups. However, the association between 1h-PG and DR is unlikely to change because the underlying pathophysiology of type 2 diabetes, and the factors associated with and thresholds for predicting DR, have been shown to be shared by both American Indian and other populations (22,36,37). Similar studies in other populations would be needed to determine whether these relationships are universal.
Strengths of this study include the long follow-up time and the large number of individuals developing DR, including severe cases. In addition, detailed clinical data permitted the exclusion of individuals taking hypoglycemic medications, an exclusion criterion not necessarily used in our own earlier and other studies (32,38). Individuals taking hypoglycemic medications may have lower 1h-PG or 2h-PG concentrations, and prevalent or incident cases of DR could potentially be misattributed to low 1h-PG or low 2h-PG concentrations if diabetes medications were not excluded.
In conclusion, the ability of 1h-PG and 2h-PG to predict the prevalence and incidence of retinopathy was similar. Given that 1h-PG can shorten the OGTT and presents logistic and economic advantages over 2h-PG, 1h-PG should be considered as an alternative postload glucose time point to identify those at elevated risk for DR.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants and all the staff of the Diabetes Epidemiology and Clinical Research Section of the Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, who participated in this study and collected data since 1965 under protocol OH76-DK-0256 under principal investigators Peter H. Bennett and W.C.K., the Diabetes Epidemiology and Clinical Research Section of the Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases. The authors also acknowledge Pivithra Vijayakumar and Robert L. Hanson, the Diabetes Epidemiology and Clinical Research Section of the Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, who developed the method and programmed the ROC curve analysis based on estimated incidence rates at specific follow-up times, and Peter H. Bennett, who contributed to the critical reading of the manuscript.
Funding. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.P. designed the study, analyzed data, and wrote the manuscript. H.C.L., P.P., W.C.K., J.K., and D.C.C. designed the study, analyzed data, and reviewed and edited the manuscript. H.C.L., W.C.K., and J.K. acquired data. D.C.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1900/-/DC1.
References
- 1.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes–2016. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Foundation ID: definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. In Report of a WHO/IDF Consultation. Geneva, Switzerland, World Health Organization, 2006 [Google Scholar]
- 3.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 4.Rushforth NB, Bennett PH, Steinberg AG, Miller M. Comparison of the value of the two- and one-hour glucose levels of the oral GTT in the diagnosis of diabetes in Pima Indians. Diabetes 1975;24:538–546 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. World Health Organization Expert Committee on Diabetes Mellitus: Second Report. Geneva, World Health Organization, 1980 (Tech. Rep. Ser., no. 646) [PubMed]
- 6.World Health Organization. Diabetes Mellitus. Report of a WHO Study Group. Geneva, World Health Organization, 1985 (Tech. Rep. Ser., no. 727) [PubMed]
- 7.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 2009;32:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alyass A, Almgren P, Akerlund M, et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia 2015;58:87–97 [DOI] [PubMed] [Google Scholar]
- 10.Oka R, Aizawa T, Miyamoto S, Yoneda T, Yamagishi M. One-hour plasma glucose as a predictor of the development of type 2 diabetes in Japanese adults. Diabet Med 2016;33:1399–1405 [DOI] [PubMed] [Google Scholar]
- 11.Paddock E, Hohenadel MG, Piaggi P, et al. One-hour and two-hour postload plasma glucose concentrations are comparable predictors of type 2 diabetes mellitus in Southwestern Native Americans. Diabetologia 2017;60:1704–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pareek M, Bhatt DL, Nielsen ML, et al. Enhanced predictive capability of a 1-hour oral glucose tolerance test: a prospective population-based cohort study. Diabetes Care 2018;41:171–177 [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Nandhini LP, Kamalanathan S, Sahoo J, Vivekanadan M. Evidence for current diagnostic criteria of diabetes mellitus. World J Diabetes 2016;7:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorf A, Ballintine EJ, Bennett PH, Miller M. Retinopathy in Pima Indians. Relationships to glucose level, duration of diabetes, age at diagnosis of diabetes, and age at examination in a population with a high prevalence of diabetes mellitus. Diabetes 1976;25:554–560 [DOI] [PubMed] [Google Scholar]
- 15.Rushforth NB, Miller M, Bennett PH. Fasting and two-hour post-load glucose levels for the diagnosis of diabetes. The relationship between glucose levels and complications of diabetes in the Pima Indians. Diabetologia 1979;16:373–379 [DOI] [PubMed] [Google Scholar]
- 16.Gabir MM, Hanson RL, Dabelea D, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care 2000;23:1113–1118 [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol 1978;108:497–505 [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Bennett PH, Ballintine EJ. Increased incidence of retinopathy in diabetics with elevated blood pressure. A six-year follow-up study in Pima Indians. N Engl J Med 1980;302:645–650 [DOI] [PubMed] [Google Scholar]
- 19.Genuth S, Alberti KG, Bennett P, et al.; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 20.Nagi DK, Pettitt DJ, Bennett PH, Klein R, Knowler WC. Diabetic retinopathy assessed by fundus photography in Pima Indians with impaired glucose tolerance and NIDDM. Diabet Med 1997;14:449–456 [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BEK, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology 1986;93:1183–1187 [DOI] [PubMed] [Google Scholar]
- 22.Looker HC, Krakoff J, Knowler WC, Bennett PH, Klein R, Hanson RL. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in Pima Indians. Diabetes Care 2003;26:320–326 [DOI] [PubMed] [Google Scholar]
- 23.Vijayakumar P, Nelson RG, Hanson RL, Knowler WC, Sinha M. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care 2017;40:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chmura Kraemer H, Periyakoil VS, Noda A. Kappa coefficients in medical research. Stat Med 2002;21:2109–2129 [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB Sr. Evaluating discrimination of risk prediction models: the C statistic. JAMA 2015;314:1063–1064 [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–2123 [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 28.Beasley TM, Erickson S, Allison DB. Rank-based inverse normal transformations are increasingly used, but are they merited? Behav Genet 2009;39:580–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K; DETECT-2 Collaboration Writing Group . Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes Diabetes Care 2011;34:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 31.McCance DR, Hanson RL, Charles M-A, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 1994;308:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelgau MM, Thompson TJ, Herman WH, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care 1997;20:785–791 [DOI] [PubMed] [Google Scholar]
- 33.Harding SP, Broadbent DM, Neoh C, White MC, Vora J. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ 1995;311:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care 2003;26:76–81 [DOI] [PubMed] [Google Scholar]
- 35.Mohamed Q, Evans A. Retinopathy, plasma glucose, and the diagnosis of diabetes. Lancet 2008;371:700–702 [DOI] [PubMed] [Google Scholar]
- 36.Tudor SM, Hamman RF, Baron A, Johnson DW, Shetterly SM. Incidence and progression of diabetic retinopathy in Hispanics and non-Hispanic whites with type 2 diabetes. San Luis Valley Diabetes Study, Colorado. Diabetes Care 1998;21:53–61 [DOI] [PubMed] [Google Scholar]
- 37.Agardh E, Agardh CD, Koul S, Torffvit O. A four-year follow-up study on the incidence of diabetic retinopathy in older onset diabetes mellitus. Diabet Med 1994;11:273–278 [DOI] [PubMed] [Google Scholar]
- 38.Wong TY, Liew G, Tapp RJ, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet 2008;371:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.