Abstract
OBJECTIVE
International studies on childhood type 1 diabetes (T1D) have focused on whole-country mean HbA1c levels, thereby concealing potential variations within countries. We aimed to explore the variations in HbA1c across and within eight high-income countries to best inform international benchmarking and policy recommendations.
RESEARCH DESIGN AND METHODS
Data were collected between 2013 and 2014 from 64,666 children with T1D who were <18 years of age across 528 centers in Germany, Austria, England, Wales, U.S., Sweden, Denmark, and Norway. We used fixed- and random-effects models adjusted for age, sex, diabetes duration, and minority status to describe differences between center means and to calculate the proportion of total variation in HbA1c levels that is attributable to between-center differences (intraclass correlation [ICC]). We also explored the association between within-center variation and children’s glycemic control.
RESULTS
Sweden had the lowest mean HbA1c (59 mmol/mol [7.6%]) and together with Norway and Denmark showed the lowest between-center variations (ICC ≤4%). Germany and Austria had the next lowest mean HbA1c (61–62 mmol/mol [7.7–7.8%]) but showed the largest center variations (ICC ∼15%). Centers in England, Wales, and the U.S. showed low-to-moderate variation around high mean values. In pooled analysis, differences between counties remained significant after adjustment for children characteristics and center effects (P value <0.001). Across all countries, children attending centers with more variable glycemic results had higher HbA1c levels (5.6 mmol/mol [0.5%] per 5 mmol/mol [0.5%] increase in center SD of HbA1c values of all children attending a specific center).
CONCLUSIONS
At similar average levels of HbA1c, countries display different levels of center variation. The distribution of glycemic achievement within countries should be considered in developing informed policies that drive quality improvement.
Introduction
For children with type 1 diabetes (T1D), achievement of optimal metabolic control, as measured by levels of glycated hemoglobin (HbA1c), is important in reducing the risk of vascular complications in later life (1). Guidelines from national and international organizations set specific standards of care and recommend a target HbA1c of <48–58 mmol/mol (6.5–7.5%) for most children with T1D (2–5). Despite the evidential and clinical consensus, many children with T1D in developed Western nations fail to achieve their target for glycemic control. Management of T1D requires ongoing patient education, access to appropriate treatment, and coordinated guidance from multidisciplinary teams, thus providing important insights into various elements of national health systems and their communication (6). Within-country studies have reported substantial differences in glycemic control across pediatric diabetes centers (7–9). Although some of these variations could be related to differences in patient case mix or preferences, some others may reflect differences in quality of, or access to, diabetes care. These unwarranted variations raise concerns about the equity of health care systems.
To date, analyses of between-center variations in childhood T1D outcomes typically have been conducted within individual countries, with existing international studies focusing on crude center comparisons (10) or on comparisons between selected centers that are not representative of their respective countries (11–13). Although this approach has provided national opportunities for improvement, it has been less informative about the performance of systems relative to other countries. At the same time, international comparisons of T1D have predominantly focused on whole-country mean or median HbA1c levels (14,15). Such comparisons are inherently limited, because they may conceal within-country variations. This represents a missed opportunity for cross-country learning. Each child with T1D should receive equal quality of care, regardless of the child’s country of residence or the center coordinating the child’s diabetes care within a specific country. Therefore, exactly how between-center variation in glycemic control differs across countries remains an important unanswered question. Similarly, variation within each center and country is of interest, because consistently good results are desired.
In the current study, we aimed to describe the extent of variation in glycemic control across and within eight high income countries, seven in Western Europe plus the U.S. Our specific objectives were as follows: to describe the variation in HbA1c values across countries and between centers within countries; to explore what proportion of the total variation in children’s glycemic control is attributable to differences between centers in each country; to examine cross-country differences in the association between within-center variation and children’s metabolic control; and finally to examine whether differences in country mean that HbA1c levels persist after adjusting for patient characteristics and center effects.
Research Design and Methods
Study Design and Participants
Anonymized data from six large registries/audits of children with T1D were used, representing eight countries: Germany and Austria from the Prospective Diabetes Follow-up Registry (DPV) (16), England and Wales from the National Pediatric Diabetes Audit (NPDA) (17), U.S. from the T1D Exchange (T1DX) (18), Sweden from the Swedish Pediatric Diabetes Quality Registry (SWEDIABKIDS) (8), Denmark from the Danish National Diabetes Registry (DanDiabKids) (19), and Norway from the Norwegian Childhood Diabetes Registry (NCDR) (20). All data sources were population-based registries or audits covering >80% of the national population of children with T1D, except for T1DX, which was a clinic-based registry (Table 1). Participants were included in the analysis if they had received a diagnosis of T1D at least 3 months before inclusion (since levels of HbA1c during the first 3 months after diagnosis are not reflective of ongoing diabetes care delivered by the center), were <18 years of age, and had at least one HbA1c measurement in 2013 (except for England and Wales, where data were collected between April 2013 and March 2014). We excluded children with missing information on risk adjustors and children who changed clinics during the study period. Finally, we excluded clinics with available data for <10 children for confidentiality reasons. The final sample consisted of 64,666 children with T1D across 528 centers (Supplementary Fig. 1). The study was approved by the individual registry/audits in each country with ethical approval to collect patient data.
Table 1.
Participant characteristics and data sources by country
| Country | Registry/audit | National coverage | HbA1c completeness, % | N of children | Male, % | Age, years* | Diabetes duration, years* | Minority status |
HbA1c* |
ISPAD target achievement, % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | % | mmol/mol | % | |||||||||
| Sweden | SWEDIABKIDS | ∼98% | ∼100 | 6,204 | 53 | 12.2 (4.0) | 4.7 (3.7) | Patient born outside of Sweden | 13 | 59 (13) | 7.6 (1.2) | 49 |
| Germany | DPV | ∼95% | 98 | 19,820 | 52 | 12.0 (3.9) | 4.6 (3.6) | Patient or at least one parent born outside of Germany/Austria | 20 | 61 (15) | 7.7 (1.4) | 46 |
| Austria | DPV | ∼80% | 99 | 1,571 | 55 | 11.9 (4.0) | 4.6 (3.7) | Patient or at least one parent born outside of Germany/Austria | 28 | 62 (16) | 7.8 (1.4) | 43 |
| Denmark | DanDiabKids | ∼100% | 91 | 1,877 | 51 | 12.7 (3.6) | 5.1 (3.6) | Both parents born outside of Denmark | 8 | 64 (16) | 8.0 (1.5) | 38 |
| Norway | NCDR | >95% | 96 | 2,315 | 52 | 12.7 (3.7) | 5.2 (3.5) | Mother born outside of the Nordic countries | 6 | 66 (14) | 8.2 (1.3) | 29 |
| England | NPDA | >95% | 95 | 20,751 | 52 | 12.4 (3.8) | 4.7 (3.7) | Any nonwhite ethnicity | 27 | 71 (18) | 8.6 (1.6) | 20 |
| U.S. | T1DX | N/A | 83 | 10,846 | 52 | 12.6 (3.5) | 5.7 (3.5) | Other than non-Hispanic white ethnicity | 22 | 72 (17) | 8.7 (1.6) | 18 |
| Wales | NPDA | >95% | 93 | 1,282 | 52 | 12.2 (3.7) | 4.7 (3.6) | Any nonwhite ethnicity | 5 | 72 (18) | 8.8 (1.6) | 17 |
ISPAD HbA1c target of <58 mmol/mol (7.5%). HbA1c completeness is defined as the proportion of eligible children in each country having a recorded HbA1c measurement during the study period.
*Data are shown as the mean (SD).
Outcome and Risk Adjustment
Glycemic control was assessed by levels of HbA1c. All registries reported HbA1c in mmol/mol in accordance with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) (21). Corresponding National Glycohemoglobin Standardization Program units (%) are given in parenthesis. The median HbA1c value over the study period was used for each child; however, two countries provided only a single HbA1c measurement for each child (first registered value during 2013 in Norway and the value closest to the child’s birthday in Denmark).
To ensure a fair comparison between centers, we adjusted our analyses for four clinically important glycemic determinants that are outside the control of the clinic, including children’s sex, age (<6 years, 6 to <12 years, and 12–18 years), duration of diabetes (<2 years, 2 to <5 years, and ≥5 years), and minority status (yes/no). We also allowed for the association between diabetes duration and HbA1c to vary across age categories by including age-duration interaction terms. Minority status was defined using the patient’s/parent’s country of birth or the patient’s ethnicity status (Table 1). Given the differences in the definition of minority status between countries, we repeated our analyses after excluding minority status from risk-adjusted models and observed any differences in center variations across countries.
Statistical Analysis
We first used country-specific, risk-adjusted, fixed-effects models to obtain estimates of mean HbA1c levels for each center following established methodology (22). Estimates derived from these models are akin to comparing centers in each country as if they had the same composition of children in terms of age, sex, diabetes duration, and minority status. We visualized variation between adjusted center means in each country by constructing boxplots with the distance between the top and the bottom of the box representing the middle 50% of centers. Given the traditional emphasis of international comparisons on mean HbA1c values, we presented center variations together with crude national mean values. To convey the absolute difference in glycemic control between centers with relatively low versus high HbA1c value within each country, we calculated the difference in adjusted glycemic levels between centers in the highest and lowest decile of the distribution in each country (i.e., the middle 80% range).
In addition to describing differences between center means, we further used risk-adjusted models with a random effect for center to calculate the proportion of total variation in glycemic control attributable to differences between centers in each country:
 |
(23). The ICC provides important information about how glycemic control is distributed across centers within a country and helps to determine the national scope for improvement that might be possible by reducing variation between centers (23). For example, large values of ICC suggest that children’s glycemic outcomes are heterogeneously distributed across centers and interventions targeting low-performing centers are likely to capture most of the poorly controlled children in the country. By contrast, a low ICC indicates that glycemic control is homogeneously achieved across centers and geographically targeted interventions aiming to only reduce variation between centers may have a limited influence on nationwide improvements. Therefore, this analysis could help a national health system or registry to target resources to most efficiently improve outcomes.
Additionally, we measured the variability in glycemic results within each center by calculating the SD of HbA1c values of all children attending a specific center (HbA1c-SD). The HbA1c-SD reflects the average deviation of a child from its center mean and provides an indicator of how consistent the glycemic performance of the center is. We extended the above country-specific, risk-adjusted models with a random effect for center by introducing HbA1c-SD as a center-level variable. Since center variability may be influenced by the number of children attending the center, we also adjusted all models for center volume. We extracted country-specific HbA1c-SD regression coefficients and pooled them by random-effects meta-analysis.
Finally, we conducted a pooled analysis of glycemic data including children from all countries to explore whether differences in mean HbA1c values between countries persist after removing center effects and differences in the risk profile of children across countries. In the pooled data set, we ran a risk-adjusted model with a random effect for center and introduced country as a fixed effect. Estimates of country means from the above model yield results similar to those from the comparison of countries as if they had the same composition of children and the same center characteristics. Hence, any differences can be fairly attributed to countries.
Parameters in random effects models were estimated using the maximum likelihood method. Model fit was examined by using the likelihood ratio test. The distribution of individual and center-level residuals was checked in all models and showed approximate normality. P values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and Stata version 13 (StataCorp, College Station, TX).
Results
Characteristics of children in each country are presented in Table 1. Children had a similar sex and age profile across all eight countries. The mean duration of diabetes was lowest in Germany and Austria (4.6 years) and highest in the U.S. (5.7 years). Minority status varied considerably from 5% in Wales to >26% in Austria and England. Achievement of the International Society for Pediatric and Adolescent Diabetes (ISPAD) HbA1c target of <58 mmol/mol (7.5%) ranged from 17% in Wales to 49% in Sweden. Characteristics of diabetes centers are presented in Supplementary Table 1.
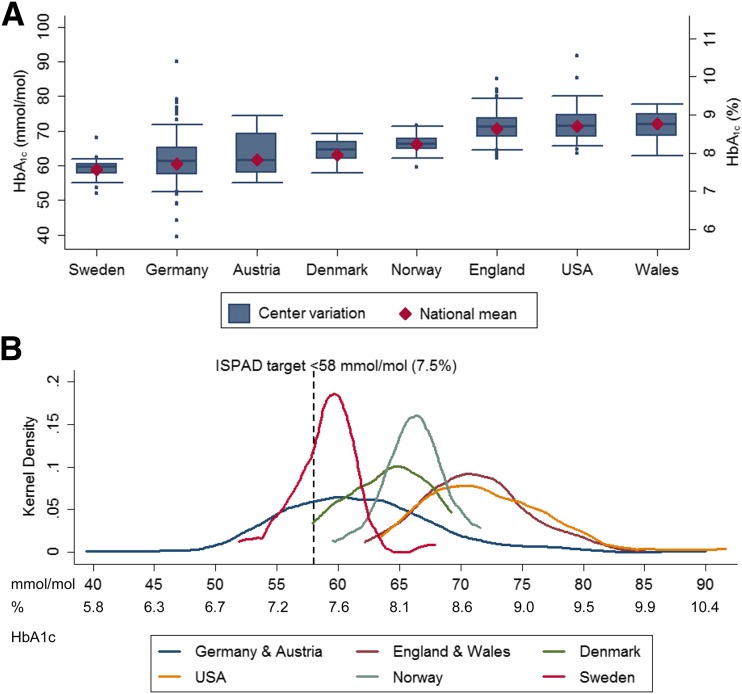
Figure 1A shows how adjusted center mean HbA1c levels vary around crude national mean values in each of the eight countries. Table 2 also shows the difference in mean HbA1c levels achieved between centers in the highest and lowest deciles of the distribution of their country. National mean levels of HbA1c showed a 1.2-fold variation across countries from 59 mmol/mol (7.6%) in Sweden to 72 mmol/mol (8.8%) in Wales. Sweden and Norway showed the lowest variation between centers; in both countries, the difference in risk-adjusted mean HbA1c between centers in the lowest and highest deciles was 6–7 mmol/mol (0.6%). Germany and Austria had the second and third lowest mean HbA1c values. However, they both showed the largest between-center variations, with centers in the highest decile having higher mean HbA1c levels by >14 mmol/mol (1.3%) compared with centers in the lowest decile. Figure 1B shows the distribution of adjusted center means by registry/audit against the ISPAD glycemic target.
Figure 1.
Between-center variation in HbA1c across countries. Center means derived from linear fixed-effects regression models adjusted for patient characteristics (sex, age, duration of diabetes, and minority status). A: Boxplots showing center variation in adjusted mean HbA1c across eight countries. The shaded box represents the interquartile range capturing the middle 50% of the centers. Whiskers extend to include centers within 1.5 times the interquartile range beyond the upper and lower quartiles; dots outside the whiskers represent outlying centers; crude national average HbA1c values are represented by diamonds. B: Kernel-smoothed distribution of adjusted center HbA1c means by registry/audit. The dashed vertical line represents the ISPAD glycemic target recommended for children with diabetes.
Table 2.
Absolute and relative measures of center variation in HbA1c by country after adjustment for patient characteristics
| Sweden | Germany | Austria | Denmark | Norway | England | U.S. | Wales | |
|---|---|---|---|---|---|---|---|---|
| HbA1c difference between centers in the highest and lowest decile, mmol/mol (%)* | 6.0 (0.6) | 14.5 (1.3) | 15.7 (1.4) | 9.8 (0.9) | 6.6 (0.6) | 11.0 (1.1) | 12.8 (1.2) | 12.3 (1.1) |
| Proportion of total variance in HbA1c attributable to differences between centers (ICC)† | 4.0% | 16.8% | 13.9% | 4.0% | 1.8% | 5.5% | 7.9% | 4.7% |
All analyses conducted separately in each country and were adjusted for patient characteristics with regard to individual sex, age, duration of diabetes, and minority status.
*Fixed-effects models.
†Models with a random effect for center.
Table 2 shows the share of the total variation in HbA1c that is attributable to differences between centers in each country after controlling for characteristics of the children. Adjusted ICC values in most countries were low, indicating that centers accounted for only a small proportion of the total variation in children’s glycemic control. However, adjusted ICC values varied considerably across countries, ranging from ≤4% in Nordic countries to ∼15% in Germany and Austria. The exclusion of minority status from risk adjustment only marginally affected center differences and ICCs except for the U.S., where the exclusion of minority status resulted in a substantial reduction in ICC from 7.9% to 6.6%.
We also looked at how the association between center HbA1c-SD and children’s glycemic outcomes varies across the eight countries. Across all countries, children who attended centers with larger variation in their glycemic performance (i.e., higher center HbA1c-SD values) had, on average, higher HbA1c values. Overall, there was a deterioration in glycemic control by 5.6 mmol/mol (0.5%) per 5 mmol/mol (0.5%) increase in center HbA1c-SD values; however, this varied from 2.8 mmol/mol (0.3%) in Norway to 7.2 mmol/mol (0.7%) in Austria (Supplementary Fig. 2).
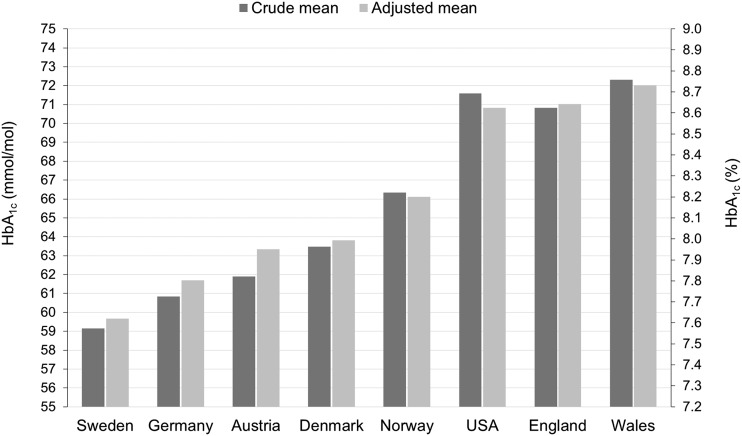
In the pooled analysis, differences between country mean HbA1c values were slightly attenuated after controlling for cross-country differences in patient characteristics and center effects (Fig. 2). However, the addition of country to the risk-adjusted random-effects model showed that the country where a child received care was a significant determinant of glycemic control regardless of center and children characteristics (P value of likelihood ratio test <0.001).
Figure 2.
Country mean HbA1c before and after adjustment for cross-country differences in the characteristics of children (age, sex, diabetes duration, and minority status) and center effects. Estimates of adjusted country means derived from a two-level model with a random effect for center including data from all eight countries.
Conclusions
We described variations in glycemic control between and within eight high-income countries using data from multicenter registries/audits for children with T1D. We found that the crude mean HbA1c level varied by 1.2-fold across countries. However, in some countries the variations between centers was even larger than these cross-country differences. We also calculated the proportion of the total variation in HbA1c which is attributable to differences between centers, and we found this to vary from ≤4% in Nordic countries to ∼15% in Germany and Austria. Across all countries, children who attended centers with larger variability in their glycemic performance had poorer glycemic control. Finally, differences between country mean HbA1c levels remained significant even after controlling for differences in patient and center characteristics.
We found that Sweden had the lowest mean HbA1c value and together with the other Nordic countries demonstrated small center variations indicating that low levels of glycemic control are homogeneously achieved by most children regardless of the clinic they attend. In Nordic countries, the establishment of collaboration between quality registries has been a major effort in promoting performance improvement in pediatric diabetes (10). Sweden has been particularly successful in establishing a nationwide program of continuous quality improvement in pediatric diabetes care that includes transparent public reporting of center performance, systematic monitoring of variations, the use of performance data as a clinical tool for professional development, and active participation of centers in quality improvement “collaboratives.” This system-wide approach probably accounts, at least in part, for the improved glycemic outcomes in Sweden (24).
Another important finding was that a lower national average glycemic control does not necessarily reflect homogenous distribution within a country. For example, large center variations were observed in Germany and Austria, countries with average HbA1c levels that are comparable to those of Sweden. In those countries, ∼15% of the total variation in HbA1c levels was located at the level of the center, which suggests that targeted interventions aiming to reduce center variability could have an appreciable impact on glycemic outcomes. Such large variations may be partly related to the structure of diabetes care. Unlike the U.K. and Nordic countries, where diabetes care is predominantly provided by hospital-based clinics normally treating children in their catchment areas, in Germany and Austria patients are free to choose their providers by a blend of hospital-based and private practices. This open competition might result in centers exhibiting variations in their discretionary policies. However, the magnitude of center variation is unlikely to be solely explained by uncaptured differences in patient mix or preferences.
In Germany and Austria, nationwide benchmarking has been provided to participating pediatric diabetes teams since 1995 in anonymized form. Analyses reporting quality indicators with each center openly identified have been available since 2000 for regional quality circles and since 2016 for all pediatric diabetes institutions in both countries. However, deanonymized reports are not openly available to the public (16). Benchmarking schemes were absent in the U.S. registry, where moderate center variations were observed. Public reporting of performance indicators in pediatric diabetes care has long been used as a core component of the accountability for quality improvement in Nordic countries and since 2012 in England and Wales. Evidence from other medical specialties shows that public disclosure of provider performance measures is linked to improved performance and has limited impact on patient movements (25). However, a climate of mutual trust needs to be created between clinicians and other stakeholders when implementing such policies to avoid defensive behaviors potentially leading to the discontinuing of information sharing.
Policies aiming to narrow center variation in pediatric diabetes care should be prioritized, yet such policies might not be sufficient to address cases where all centers in a nation are performing suboptimally. This might be the case in countries with high average HbA1c levels and low-to-moderate ICC values such as England, Wales, and the U.S. Some of the best clinics in those countries performed poorly when compared even with Swedish centers at the higher end of the distribution. This implies that quality improvement in those countries might best be achieved not only by targeting poor performers, but also by “shifting the curve” of overall pediatric diabetes practice toward higher quality levels. The recent changes toward tighter HbA1c targets for all children of <48 mmol/mol (6.5%) in the U.K. (2) and <58 mmol/mol (7.5%) in the U.S. (3) could help to achieve this goal. International experience has also shown that patient-centered policies might be effective in stimulating whole-system improvements (26). For example, the introduction of patient-reported experience measures for pediatric diabetes care in England and Wales in 2013 is considered an important step in informing local decision-making (27).
In all countries, children who attended centers with more variable glycemic results had, on average, higher HbA1c levels. This finding may reflect a range of factors related to goal setting, team cohesiveness, and organizational culture. Previous reports from the Hvidøre Study Group on Childhood Diabetes (28) demonstrated improved glycemic performance in centers where the team set consistent HbA1c targets. Achievement of higher consistency within a center also requires focusing attention on the management of challenging populations of children who are more likely to exhibit greater variability in their metabolic control (e.g., adolescents). Taken together, our findings suggest that, in addition to helping a higher percentage of their patients achieve target glycemic control, centers should also aim for lower variability in their glycemic performance.
We also found significant differences between countries’ glycemic levels over and above the characteristics of children and center differences. Several aspects of pediatric diabetes care could contribute to these differences, including the use of insulin pumps, patient education, lifestyle factors, the training of health care professionals, the impact of low socioeconomic status, and reimbursement schemes. However, the link with glycemic outcomes is not straightforward. For example, a previous study (29) showed that although pump use in children with T1D was much lower in England and Wales (14%) compared with Germany, Austria (41%), and the US (47%), country differences in glycemic control could not be adequately explained by differences in insulin delivery method. The results may have also been influenced by national HbA1c target levels. At the time of the study, these were ≤58 mmol/mol (7.5%) in Germany, Norway, England, and Wales; 52 mmol/mol (6.9%) in Sweden; 53 mmol/mol (7.0%) in Austria; 55 mmol/mol (7.2%) in Denmark; 69 mmol/mol (8.5%) for children <6 years of age; 64 mmol/mol (8.0%) for children 6–12 years of age; and 58 mmol/mol (7.5%) for children ≥13 years of age in the US. However, in our figures we presented the ISPAD HbA1c target of <58 mmol/mol (7.5%), which has been adopted by most countries in order to put country data in context by providing an internationally agreed upon target.
Our study should be interpreted within the context of its limitations. First, risk adjustment was restricted to the availability of comparable data. It is possible that unaccounted factors such as comorbidities and socioeconomic status might systematically vary between centers and therefore explain some of the observed variations. Second, in line with previous studies (14,29), we used the median HbA1c measurement for each child to avoid the effects that outliers can have on the mean. However, this approach may not accurately represent glycemic exposure over the observation period. Third, although all registries reported IFCC–aligned HbA1c values, it is likely that differences in laboratory methods across countries might have contributed to the observed variations. Fourth, we excluded centers with <10 children, which might have underestimated center variations in countries with many small practices (i.e., Germany). Fifth, differences in the definition of minority status across countries might have affected our comparisons. However, the exclusion of minority status from risk adjustment only minimally affected our results in most countries. In the U.S., larger center differences were masked by failing to adjust for minority status; such a result could occur, for example, when poorly performing centers have fewer minority children who tend to have poorer outcomes than non-Hispanic whites (30). Moreover, data from the U.S. were based on a selective group of diabetes clinics and might not be directly comparable with data from the European population-based registries. Finally, our analysis was a snapshot comparison of glycemic levels; a more dynamic comparison would be needed to address the link between quality improvement initiatives and glycemic performance.
In summary, our findings from this large international study showed considerable differences in mean HbA1c levels between and within countries. At similar average levels of glycemic control, countries displayed very different levels of center variation. This suggests that whole-country mean HbA1c levels are an inadequate summary of the glycemic performance of a country. The distribution of glycemic achievement across centers within countries should be considered, alongside national mean values, in developing informed policies that drive quality improvement.
Supplementary Material
Article Information
Acknowledgments. The authors thank all national pediatric diabetes groups, all participating centers, and all patients.
Funding. The University College London Children’s Policy Research Unit (CPRU) is funded by the England Department of Health Policy Research Programme (funding reference 10090001) and is supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children National Health Service Foundation Trust and University College London. The views expressed are not necessarily those of the Department of Health. NPDA is funded by National Health Service England and the Welsh Government. NCDR is funded by the South-Eastern Norway Regional Health Authority. DanDiabKids is funded by the Health Research Fund of Central Denmark Region. SWEDIABKIDS is supported by the Swedish Association of Local Authorities and Regions (SALAR). DPV is funded by the German Center for Diabetes Research (DZD), the German Diabetes Association (DDG), the European Foundation for the Study of Diabetes (EFSD), and the EU-IMI2 consortium INNODIA. T1DX is funded by the Helmsley Charitable Trust. M.C. receives funding from National Institute of Diabetes and Digestive and Kidney Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Helmsley Charitable Trust, and Jaeb Center for Health Research.
Funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the paper.
Duality of Interest. D.M.M. has received grants from Medtronic, Dexcom, Bigfoot Biomedical, Insulet, and Roche; has acted as a consultant for Abbott Diabetes Care; and has been an advisory board member for Insulet. T.St. has received grants from the Department of Health, England. N.F. has received funds through the T1D Exchange from the Helmsley Charitable Trust. M.C. has received funds from Medtronic, Caladrius, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Glooko, AstraZeneca, Janssen, and Jaeb Center for Health Research. Jaeb Center provides subcontract payments to Children’s Mercy for the conduct of the T1D Exchange Registry protocol and also contracts with Children’s Mercy to support 10% of the effort by M.C. as the pediatric chair for the T1D Exchange. The study was approved by all individual registry/audits in each country who have ethical approval to collect patient data. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.C. contributed to study conception, was involved in the design of the study, conducted the literature search, contributed to statistical analyses, wrote the first draft of the manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit the manuscript for publication. J.M.H. contributed to study conception, was involved in the design of the study, performed the statistical analyses, and was responsible for data cleaning and management. J.S. and S.F. were involved in the design of the study and contributed to data acquisition. T.St. had the general supervision of the study, contributed to study conception, and was involved in the design of the study. D.M.M., K.A., J.T.W., and L.H. contributed to study conception, were involved in the design of the study, and contributed to data acquisition. R.W.H. and R.H. had the general supervision of the study, contributed to study conception, were involved in the design of the study, and contributed to data acquisition. N.H.B. was involved in the design of the study and contributed to data acquisition. A.K.D. and A.-M.S. contributed to study conception, were involved in the design of the study, were responsible for data cleaning and management, and contributed to data acquisition. K.M.M., S.E.H., N.F., B.R.-M., K.D.-J., and M.C. contributed to data acquisition. T.Sk. contributed to study conception, was involved in the design of the study, was responsible for data cleaning and management, and contributed to data acquisition. S.J.K. was responsible for data cleaning and management and contributed to data acquisition. A.J. was involved in the design of the study, was responsible for data cleaning and management, and contributed to data acquisition. All authors provided substantial contributions to data interpretation and critically reviewed and commented on several drafts of the paper. J.M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 42nd Conference of the International Society for Pediatric and Adolescent Diabetes, ISPAD 2016, Valencia, Spain, 26–29 October 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2271/-/DC1.
References
- 1.Nathan DM, Bayless M, Cleary P, et al.; DCCT/EDIC Research Group . Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study at 30 years: advances and contributions. Diabetes 2013;62:3976–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NICE Clinical Guideline. Diabetes (type 1 and type 2) in children and young people: diagnosis and management [article online], 2015. Available from https://www.nice.org.uk/guidance/ng18/resources/diabetes-type-1-and-type-2-in-children-and-young-people-diagnosis-and-management-1837278149317. Accessed 15 February 2017
- 3.Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofer S, Bauer M, Lanzersdorfer R, Walser I. Diabetes mellitus bei Kindern und Jugendlichen. Pädiatrie & Pädologie 2010;(Suppl. 3)
- 5.Rewers MJ, Pillay K, de Beaufort C, et al.; International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014;15(Suppl. 20):102–114 [DOI] [PubMed] [Google Scholar]
- 6.Nolte E, Bain C, McKee M. Diabetes as a tracer condition in international benchmarking of health systems. Diabetes Care 2006;29:1007–1011 [DOI] [PubMed] [Google Scholar]
- 7.Gerstl EM, Rabl W, Rosenbauer J, et al. Metabolic control as reflected by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. Eur J Pediatr 2008;167:447–453 [DOI] [PubMed] [Google Scholar]
- 8.Hanberger L, Samuelsson U, Lindblad B, Ludvigsson J; Swedish Childhood Diabetes Registry SWEDIABKIDS . A1C in children and adolescents with diabetes in relation to certain clinical parameters: the Swedish Childhood Diabetes Registry SWEDIABKIDS. Diabetes Care 2008;31:927–929 [DOI] [PubMed] [Google Scholar]
- 9.Scottish Study Group for the Care of the Young Diabetic Factors influencing glycemic control in young people with type 1 diabetes in Scotland: a population-based study (DIABAUD2). Diabetes Care 2001;24:239–244 [DOI] [PubMed] [Google Scholar]
- 10.Hanberger L, Birkebaek N, Bjarnason R, et al. Childhood diabetes in the Nordic countries: a comparison of quality registries. J Diabetes Sci Technol 2014;8:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danne T, Mortensen HB, Hougaard P, et al.; Hvidøre Study Group on Childhood Diabetes . Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidøre Study Group. Diabetes Care 2001;24:1342–1347 [DOI] [PubMed] [Google Scholar]
- 12.de Beaufort CE, Lange K, Swift PG, et al.; Hvidoere Study Group . Metabolic outcomes in young children with type 1 diabetes differ between treatment centers: the Hvidoere Study in Young Children 2009. Pediatr Diabetes 2013;14:422–428 [DOI] [PubMed] [Google Scholar]
- 13.de Beaufort CE, Swift PG, Skinner CT, et al.; Hvidoere Study Group on Childhood Diabetes 2005 . Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care 2007;30:2245–2250 [DOI] [PubMed] [Google Scholar]
- 14.Maahs DM, Hermann JM, DuBose SN, et al.; DPV Initiative; T1D Exchange Clinic Network . Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia 2014;57:1578–1585 [DOI] [PubMed] [Google Scholar]
- 15.McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 2015;32:1036–1050 [DOI] [PubMed] [Google Scholar]
- 16.Hofer SE, Schwandt A, Holl RW; Austrian/German DPV Initiative . Standardized documentation in pediatric diabetology: experience from Austria and Germany. J Diabetes Sci Technol 2016;10:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner J, Morris S. The National Paediatric Diabetes Audit. Pract Diabetes 2014;31:257–261 [Google Scholar]
- 18.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 19.Svensson J, Cerqueira C, Kjærsgaard P, et al. Danish registry of childhood and adolescent diabetes. Clin Epidemiol 2016;8:679–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margeirsdottir HD, Larsen JR, Kummernes SJ, Brunborg C, Dahl-Jørgensen K. The establishment of a new national network leads to quality improvement in childhood diabetes: implementation of the ISPAD Guidelines. Pediatr Diabetes 2010;11:88–95 [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association; European Association for the Study of Diabetes; International Federation of Clinical Chemistry and Laboratory Medicine; International Diabetes Federation . Consensus statement on the worldwide standardisation of the HbA1c measurement. Diabetologia 2007;50:2042–2043 [DOI] [PubMed] [Google Scholar]
- 22.Department of Health. Patient Reported Outcome Measures (PROMs) in England. The case-mix adjustment methodology [article online], 2012. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216507/dh_133449.pdf. Accessed 22 February 2017
- 23.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006;60:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuelsson U, Åkesson K, Peterson A, Hanas R, Hanberger L. Continued improvement of metabolic control in Swedish pediatric diabetes care. Pediatr Diabetes 2018;19:150–157 [DOI] [PubMed] [Google Scholar]
- 25.Fung CH, Lim YW, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med 2008;148:111–123 [DOI] [PubMed] [Google Scholar]
- 26.Smith PC, Mossialos E, Papanicolas I, Leatherman S. Performance Measurement for Health System Improvement: Experiences, Challenges and Prospects. Cambridge, UK, Cambridge University Press, 2010 [Google Scholar]
- 27.Girling I, Day E, Fazakerley K, et al. ; Royal College of Paediatrics Child Health the National Paediatric Diabetes Audit Patient Reported Experience Measure Working Group. What young people want from their diabetes team: developing a patient reported experience measure (PREM) for young people with type 1 diabetes. Pract Diabetes 2015;32:142–147a [Google Scholar]
- 28.Swift PG, Skinner TC, de Beaufort CE, et al.; Hvidoere Study Group on Childhood Diabetes . Target setting in intensive insulin management is associated with metabolic control: the Hvidoere childhood diabetes study group centre differences study 2005. Pediatr Diabetes 2010;11:271–278 [DOI] [PubMed] [Google Scholar]
- 29.Sherr JL, Hermann JM, Campbell F, et al.; T1D Exchange Clinic Network; the DPV Initiative; and the National Paediatric Diabetes Audit and the Royal College of Paediatrics and Child Health Registries . Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87–91 [DOI] [PubMed] [Google Scholar]
- 30.Willi SM, Miller KM, DiMeglio LA, et al.; T1D Exchange Clinic Network . Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.