Abstract
OBJECTIVE
The implementation of the Chronic Care Model (CCM) improves health care quality. We examined the sustained effectiveness of multicomponent integrated care in type 2 diabetes.
RESEARCH DESIGN AND METHODS
We searched PubMed and Ovid MEDLINE (January 2000–August 2016) and identified randomized controlled trials comprising two or more quality improvement strategies from two or more domains (health system, health care providers, or patients) lasting ≥12 months with one or more clinical outcomes. Two reviewers extracted data and appraised the reporting quality.
RESULTS
In a meta-analysis of 181 trials (N = 135,112), random-effects modeling revealed pooled mean differences in HbA1c of −0.28% (95% CI −0.35 to −0.21) (−3.1 mmol/mol [−3.9 to −2.3]), in systolic blood pressure (SBP) of −2.3 mmHg (−3.1 to −1.4), in diastolic blood pressure (DBP) of −1.1 mmHg (−1.5 to −0.6), and in LDL cholesterol (LDL-C) of −0.14 mmol/L (−0.21 to −0.07), with greater effects in patients with LDL-C ≥3.4 mmol/L (−0.31 vs. −0.10 mmol/L for <3.4 mmol/L; Pdifference = 0.013), studies from Asia (HbA1c −0.51% vs. −0.23% for North America [−5.5 vs. −2.5 mmol/mol]; Pdifference = 0.046), and studies lasting >12 months (SBP −3.4 vs. −1.4 mmHg, Pdifference = 0.034; DBP −1.7 vs. −0.7 mmHg, Pdifference = 0.047; LDL-C −0.21 vs. −0.07 mmol/L for 12-month studies, Pdifference = 0.049). Patients with median age <60 years had greater HbA1c reduction (−0.35% vs. −0.18% for ≥60 years [−3.8 vs. −2.0 mmol/mol]; Pdifference = 0.029). Team change, patient education/self-management, and improved patient-provider communication had the largest effect sizes (0.28–0.36% [3.0–3.9 mmol/mol]).
CONCLUSIONS
Despite the small effect size of multicomponent integrated care (in part attenuated by good background care), team-based care with better information flow may improve patient-provider communication and self-management in patients who are young, with suboptimal control, and in low-resource settings.
Introduction
Urgent measures are needed to reduce the growing burden of diabetes, especially in low- and middle-income countries (LMICs) (1,2). The aging population and rising prevalence of young-onset diabetes carry onerous implications on health care expenditure and societal productivity (1,3). The complex clinical course and pluralistic needs of people with diabetes call for an integrated care approach to identify needs and implement timely solutions (4,5). In controlled settings, type 2 diabetes is preventable and treatable, although in real-world practice, fewer than one in four adults underwent annual complication screening or attained composite cardiometabolic targets especially in LMICs (6,7). Insufficient care coordination and lack of communication between patients and health care providers (HCPs) can lead to delayed intervention, psychosocial stress, treatment nonadherence, and poor clinical outcomes (8,9). Here, quality improvement (QI) programs contain multiple components targeting patients, HCPs, or systems to enable periodic evaluation, identify treatment gaps, and promote information sharing for decision making (10,11). Previous meta-analyses concluded that team change, case management, and promotion of self-management had the largest effect sizes in reducing HbA1c (12,13). In this meta-analysis, we included only type 2 diabetes studies lasting at least 12 months in order to examine the sustained effects of multicomponent integrated care on surrogate clinical outcomes in different populations and settings.
Research Design and Methods
Data Sources and Searches
We performed literature searches of PubMed and Ovid MEDLINE for randomized controlled trials (RCTs) published between January 2000 and August 2016 that examined the effects of multicomponent integrated care in type 2 diabetes. The PubMed and Ovid MEDLINE search terms using Medical Subject Headings and text words are listed in Supplementary Table 1. We also searched references from original articles, clinical guidelines, narrative reviews, and previous systematic reviews/meta-analyses to identify additional eligible trials. We followed the PRISMA guidelines for conducting and reporting meta-analyses of RCTs.
Study Selection
Eligible studies were English-language peer-reviewed RCTs and their companion prospective follow-up studies. The 12 existing QI strategies were categorized into three domains, i.e., health system, HCPs, and patients (12,13). We updated their definitions and included electronic health (e-health) under the health system domain, bringing the total to 13 QI strategies (Supplementary Table 2) (14). We defined multicomponent integrated care models as those comprising at least two QI strategies from two of the three domains lasting at least 12 months and reporting at least one cardiometabolic or care process outcome (Supplementary Table 3). Trials with intervention(s) in a single domain or with fewer than 100 patients were excluded.
Data Extraction and Quality Assessment
Two independent reviewers (L.L.L. and E.S.H.L.) extracted data using standard templates, including authors, year of publication, geographic regions, national income levels, study settings, sample size, participants’ characteristics (age, sex, ethnicity, and duration of type 2 diabetes), QI strategies implemented, duration of intervention, and pre- and postinterventional cardiometabolic and care process outcomes. We appraised the quality of reporting using the Cochrane Effective Practice and Organisation of Care (EPOC) risk-of-bias tool (15,16). Each trial was assessed based on seven categories of biases, which were selection bias, attrition bias, performance bias, detection bias, reporting bias, contamination bias, and other risk of bias. Each bias was classified into “high risk,” “low risk,” or “unclear risk.” Trial authors were contacted for clarifications if required. Any disagreement between the two reviewers was resolved by senior investigators (A.P.S.K. and J.C.N.C.).
Data Synthesis and Analysis
We used random-effects modeling to pool the mean postinterventional differences in HbA1c, LDL cholesterol (LDL-C), systolic blood pressure (SBP), and diastolic blood pressure (DBP) levels between the intervention and control (usual care) groups. In some studies, some QI strategies were already part of usual care. Thus, the postinterventional estimates of individual QI strategies were the net differences of the number and total effects of QI strategies between the two groups. As these RCTs had different combinations of QI strategies and there were few trials with similar combinations for direct comparisons, we performed a mixed-effects meta-regression model to control for the confounding effects of cointerventions, adjusted for age, sex, and baseline cardiometabolic risk factors. Financial incentives strategies were excluded from the analysis as only two trials were involved.
In this meta-analysis, 28 RCTs adopted a trial design involving more than two arms. With these studies, we included data from the intervention arm with the largest number of QI strategies. We also compared the effects of these QI strategies stratified by geographic regions (North America, Europe, and Asia), national income levels (high vs. middle income), duration of intervention (>12 months vs. 12 months only), median age (<60 vs. ≥60 years), and cardiometabolic control at baseline, i.e., HbA1c (≥8% [≥64 mmol/mol] vs. <8% [<64 mmol/mol]), SBP (≥140 vs. <140 mmHg), DBP (≥80 vs. <80 mmHg), and LDL-C (≥3.4 vs. <3.4 mmol/L; to convert to mg/dL, multiply by 38.67). Subgroup analyses for SBP and LDL-C using 130 mmHg and 2.6 mmol/L, respectively, were not performed due to limited extractable data. Heterogeneity and publication bias were assessed by I2 statistic and funnel plots. All statistical analyses were performed using R software version 3.3.1 (17). A two-tailed P < 0.05 denoted statistical significance without multiplicity correction in all exploratory analyses.
Results
Study Characteristics
The search strategy yielded 15,458 citations, of which 972 full-text articles were assessed for eligibility. A total of 181 trials (including 12 companion prospective follow-up studies) involving 135,112 participants were included. The PRISMA flow diagram depicts the study selection (Supplementary Fig. 1). Most trials were conducted in high-income nations with only 10 from upper- and lower-middle income countries. The mean (SE) age was 59.6 (0.6) years and median (interquartile range) duration of intervention was 12 (12–24) months. The median (interquartile range) number of QI strategies in these multicomponent integrated care models was 4.5 (3–6), with patient education (91.2%), team change (56.9%), and clinician reminder/decision support (69.6%) as the top QI strategies targeted at patients, system, and HCPs, respectively, in addition to usual care (Supplementary Table 4).
The quality of reporting assessment is summarized in Supplementary Fig. 2. It was not feasible to blind the personnel/participants in 159 (88%) of these pragmatic RCTs. Their internal validity was mainly compromised by contamination bias (90 [50%]), lack of random sequence generation (94 [52%]), inadequate allocation concealment (98 [55%]), and attrition bias (77 [43%]).
Effects of Multicomponent Integrated Care on Cardiometabolic Outcomes
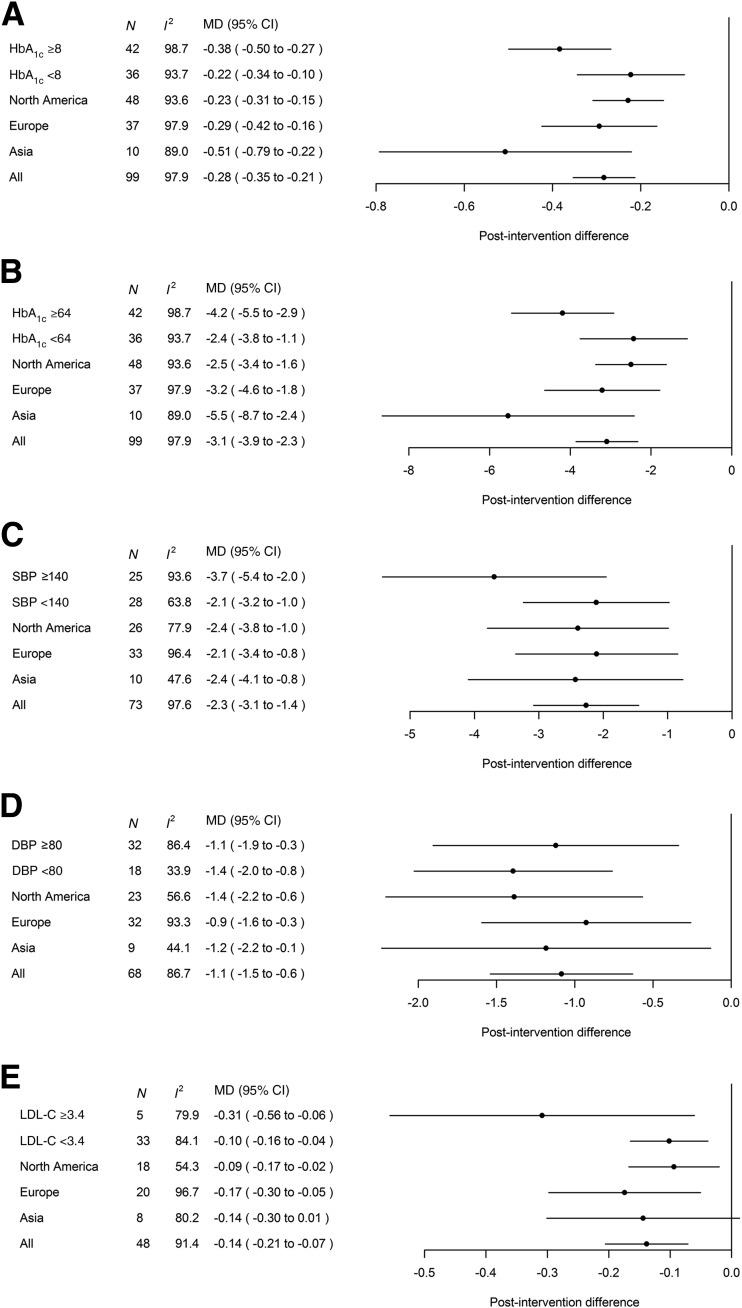
Supplementary Figs. 3–7 show the pooled mean differences of HbA1c, SBP, DBP, and LDL-C levels in all RCTs. Figure 1 summarizes the results of subgroup analyses stratified by different levels of baseline cardiometabolic control and geographic regions. Compared with usual care (e.g., clinical procedures, medical visits, and medications), multicomponent integrated care reduced HbA1c by 0.28% (3.1 mmol/mol) (95% CI −0.35 to −0.21 [−3.9 to −2.3]), with larger effect size in 1) patients with baseline HbA1c ≥8% (≥64 mmol/mol) (−0.38% [−4.2 mmol/mol] vs. −0.22% [−2.4 mmol/mol] for those with <8% [<64 mmol/mol]; Pdifference = 0.058); 2) studies in Asia (−0.51% [−5.5 mmol/mol] vs. −0.23% [−2.5 mmol/mol] in North America, Pdifference = 0.046, vs. −0.29% [−3.2 mmol/mol] in Europe, Pdifference = 0.100); 3) middle-income (−0.53% [95% CI −1.18 to 0.13], −5.8 mmol/mol [−12.9 to 1.4]; 3 trials) vs. high-income countries (−0.28% [−0.35 to −0.21], −3.1 mmol/mol [−3.9 to −2.3], 96 trials) (Pdifference = 0.275); and 4) studies lasting >12 months (−0.39% [−0.54 to −0.24], −4.3 mmol/mol [−5.9 to −2.6], 36 trials) vs. only 12 months (−0.22% [−0.30 to −0.15], −2.4 mmol/mol [−3.3 to −1.6], 62 trials) (Pdifference = 0.062) (data not shown).
Figure 1.
Meta-analyses results of the effects of multicomponent integrated care on HbA1c (%) (A), HbA1c (mmol/mol) (B), SBP (mmHg) (C), DBP (mmHg) (D), and LDL-C (mmol/L) (E), stratified by different levels of baseline cardiometabolic control and geographic regions. To convert LDL-C to mg/dL, multiply by 38.67. MD, mean difference; N, number of trials with analyzable data.
Multicomponent integrated care reduced SBP and DBP by 2.3 mmHg (95% CI −3.1 to −1.4) and 1.1 mmHg (−1.5 to −0.6), respectively (Fig. 1C and 1D). Patients with baseline SBP ≥140 mmHg tended to have greater reduction than those with <140 mmHg (−3.7 mmHg [−5.4 to −2.0] vs. −2.1 mmHg [−3.2 to −1.0]; Pdifference = 0.198), with similar effect sizes across the three regions. Studies from middle-income countries tended to have greater SBP (−4.4 mmHg [−6.4 to −2.4], 4 trials) and DBP (−2.2 mmHg [−3.7 to −0.8], 4 trials) reductions than high-income countries (−2.2 mmHg [−3.0 to −1.3], 69 trials; Pdifference = 0.319, and −1.0 mmHg [−1.5 to −0.6], 64 trials; Pdifference = 0.321, respectively). Studies lasting >12 months had greater improvement in SBP (−3.4 mmHg [−4.9 to −1.9], 29 trials) than 12-month trials (−1.4 mmHg [−2.2 to −0.6], 43 trials; Pdifference = 0.034). The corresponding data for DBP were −1.7 mmHg (−2.4 to −1.0, 27 trials) and −0.7 mmHg (−1.3 to −0.2, 40 trials) (Pdifference = 0.047).
Across 48 trials, multicomponent integrated care lowered LDL-C by 0.14 mmol/L (95% CI −0.21 to −0.07) compared with usual care, with greater effect size in patients with LDL-C ≥3.4 mmol/L (−0.31 mmol/L) versus those with <3.4 mmol/L (−0.10 mmol/L); Pdifference = 0.013 (Fig. 1E). Significant LDL-C reductions were reported in Europe and North America (−0.17 mmol/L vs. −0.09 mmol/L, respectively; Pdifference = 0.342) but not in Asia. The only trial from a middle-income country with analyzable LDL-C data showed a 0.18 mmol/L (−0.31 to −0.05) reduction compared with a 0.14 mmol/L reduction (−0.21 to −0.07, 47 trials) in high-income countries. Sustained LDL-C reduction was more likely in studies lasting >12 months (−0.21 mmol/L [−0.32 to −0.09], 21 trials) than those with only a 12-month duration (−0.07 mmol/L [−0.13 to 0.00], 26 trials) (Pdifference = 0.049).
When we stratified the analysis by median age at baseline (<60 vs. ≥60 years), improvement in HbA1c with multicomponent integrated care was greater in younger patients (−0.35% [−0.45 to −0.25], −3.8 mmol/mol [−4.9 to −2.8], 50 trials) than in older patients (−0.18% [−0.43 to 0.07], −2.0 mmol/mol [−4.7 to 0.7], 35 trials) (Pdifference = 0.029). There were no significant between-group differences for other cardiometabolic outcomes (Table 1). The majority of trials reported increased usage of organ-protective agents and uptake of complications screening with multicomponent integrated care (Supplementary Table 5). In 12 trials, hypoglycemia, either self-reported or objective measurements using glucometer/continuous glucose monitoring, was a study outcome. Nine trials indicated no between-group difference and two trials reported reduction in hypoglycemia with multicomponent integrated care. One trial reported increased nonsevere hypoglycemic events with intervention, albeit the rate was very low with no severe hypoglycemia. One sulfonylurea-treated patient in the control group had two severe hypoglycemia episodes (18).
Table 1.
Effects of multicomponent integrated care on cardiometabolic outcomes, stratified by median age at baseline
| Age <60 years (N = 65) |
Age ≥60 years (N = 54) |
Pdifference | |||
|---|---|---|---|---|---|
| N | MD (95% CI) | N | MD (95% CI) | ||
| HbA1c (%) |
50 |
−0.35 (−0.45 to −0.25) |
35 |
−0.18 (−0.43 to 0.07) |
0.029 |
| HbA1c (mmol/mol) |
50 |
−3.8 (−4.9 to −2.8) |
35 |
−2.0 (−4.7 to 0.7) |
0.029 |
| SBP (mmHg) |
32 |
−2.8 (−4.1 to −1.4) |
32 |
−2.4 (−5.6 to 0.7) |
0.728 |
| DBP (mmHg) |
30 |
−1.4 (−2.2 to −0.7) |
30 |
−1.1 (−2.8 to 0.7) |
0.461 |
| LDL-C (mmol/L) | 25 | −0.16 (−0.26 to −0.06) | 19 | −0.13 (−0.37 to 0.12) | 0.639 |
MD, mean difference; N, number of trials with analyzable data.
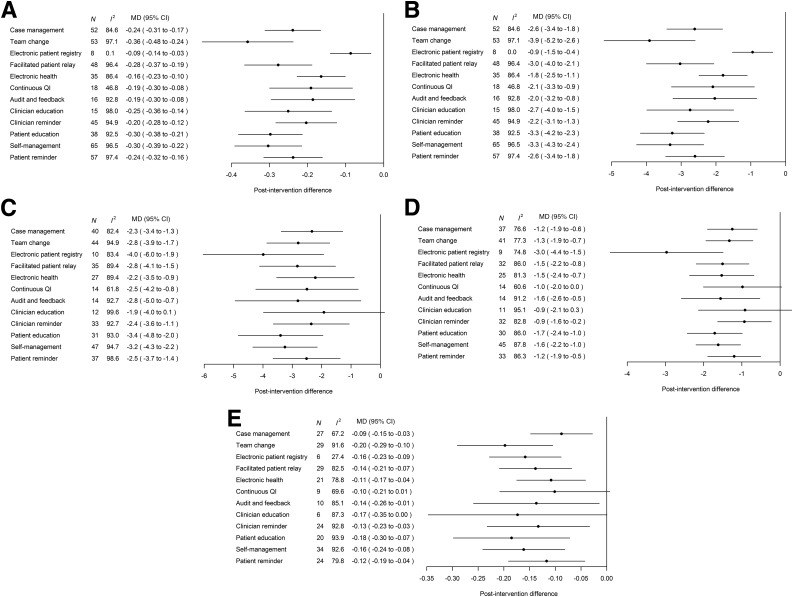
Effects of Individual QI Strategy on Cardiometabolic Outcomes
Most QI strategies were effective in improving cardiometabolic outcomes (Fig. 2 and Supplementary Table 6). System-targeted initiatives, e.g., team change and facilitated patient-provider relay, were most effective in reducing the respective HbA1c and LDL-C by 0.28–0.36% (3.0–3.9 mmol/mol) and 0.14–0.20 mmol/L, respectively, whereas electronic patient registry was the top QI strategy in lowering S/DBP (4/3 mmHg). Patient education and promotion of self-management were the most effective patient-targeted strategies. Other QI strategies targeted at HCPs, such as clinician reminder/decision support and audit and feedback, also improved all cardiometabolic risk factors, although the effect sizes were smaller compared with the aforementioned system- and patient-level interventions. Subgroup analyses indicated greater benefits of individual QI strategies in groups with suboptimal control, i.e., 0.29–0.50% (3.2–5.5 mmol/mol) for HbA1c ≥8% (≥64 mmol/mol) (vs. 0.07–0.37% for <8% [0.8–4.0 mmol/mol for <64 mmol/mol]), 2.6–5.2 mmHg for SBP ≥140 mmHg (vs. 2.3–4.5 mmHg for <140 mmHg), 1.3–3.3 mmHg for DBP ≥80 mmHg (vs. 1.1–2.8 mmHg for <80 mmHg), and 0.20–0.39 mmol/L for LDL-C ≥3.4 mmol/L (vs. 0.07–0.15 mmol/L for <3.4 mmol/L) (Table 2).
Figure 2.
Meta-analyses results of the effects of individual QI strategies on HbA1c (%) (A), HbA1c (mmol/mol) (B), SBP (mmHg) (C), DBP (mmHg) (D), and LDL-C (mmol/L) (E). To convert LDL-C to mg/dL, multiply by 38.67. MD, mean difference; N, number of trials with analyzable data.
Table 2.
Effects of individual QI strategies by HbA1c, blood pressure, and LDL-C subgroups
| QI strategy | HbA1c (NGSP) |
HbA1c (IFCC) |
||||||
|---|---|---|---|---|---|---|---|---|
| ≥8% |
<8% |
≥64 mmol/mol |
<64 mmol/mol |
|||||
| N | MD (95% CI) | N | MD (95% CI) | N | MD (95% CI) | N | MD (95% CI) | |
| Health system | ||||||||
| Case management |
23 |
−0.40 (−0.54 to −0.26) |
21 |
−0.08 (−0.13 to −0.03) |
23 |
−4.4 (−5.9 to −2.8) |
21 |
−0.9 (−1.4 to −0.3) |
| Team change |
24 |
−0.46 (−0.63 to −0.29) |
20 |
−0.37 (−0.59 to −0.15) |
24 |
−5.0 (−6.9 to −3.2) |
20 |
−4.0 (−6.4 to −1.6) |
| Electronic patient registry |
2 |
−0.43 (−0.68 to −0.19) |
5 |
−0.07 (−0.13 to −0.02) |
2 |
−4.7 (−7.4 to −2.1) |
5 |
−0.8 (−1.4 to −0.2) |
| Facilitated relay |
23 |
−0.36 (−0.53 to −0.20) |
13 |
−0.12 (−0.18 to −0.06) |
23 |
−3.9 (−5.8 to −2.2) |
13 |
−1.3 (−2.0 to −0.7) |
| E-health |
17 |
−0.29 (−0.41 to −0.17) |
12 |
−0.06 (−0.12 to 0.00) |
17 |
−3.2 (−4.5 to −1.9) |
12 |
−0.7 (−1.3 to 0.0) |
| Continuous QI |
10 |
−0.36 (−0.56 to −0.15) |
5 |
−0.09 (−0.14 to −0.03) |
10 |
−3.9 (−6.1 to −1.6) |
5 |
−1.0 (−1.5 to −0.3) |
| HCPs | ||||||||
| Audit and feedback |
4 |
−0.50 (−0.51 to −0.49) |
10 |
−0.04 (−0.10 to 0.02) |
4 |
−5.5 (−5.6 to −5.4) |
10 |
−0.4 (−1.1 to −0.2) |
| Clinician education |
6 |
−0.32 (−0.51 to −0.13) |
6 |
−0.15 (−0.28 to −0.02) |
6 |
−3.5 (−5.6 to −1.4) |
6 |
−1.6 (−3.1 to −0.2) |
| Clinician reminder |
22 |
−0.31 (−0.46 to −0.17) |
14 |
−0.07 (−0.14 to 0.00) |
22 |
−3.4 (−5.0 to −1.9) |
14 |
−0.8 (−1.5 to 0.0) |
| Patients | ||||||||
| Patient education |
16 |
−0.46 (−0.60 to −0.32) |
13 |
−0.12 (−0.18 to −0.07) |
16 |
−5.0 (−6.6 to −3.5) |
13 |
−1.3 (−2.0 to −0.8) |
| Promotion of self-management |
32 |
−0.39 (−0.51 to −0.26) |
20 |
−0.22 (−0.39 to −0.05) |
32 |
−4.3 (−5.6 to −2.8) |
20 |
−2.4 (−4.3 to −0.5) |
| Patient reminder system |
29 |
−0.36 (−0.50 to −0.22) |
16 |
−0.08 (−0.14 to −0.02) |
29 |
−3.9 (−5.5 to −2.4) |
16 |
−0.9 (−1.5 to −0.2) |
| SBP |
DBP |
|||||||
| ≥140 mmHg |
<140 mmHg |
≥80 mmHg |
<80 mmHg |
|||||
|
N |
MD (95% CI) |
N |
MD (95% CI) |
N |
MD (95% CI) |
N |
MD (95% CI) |
|
| Health system | ||||||||
| Case management |
14 |
−2.7 (−4.6 to −0.8) |
15 |
−2.9 (−4.7 to −1.0) |
15 |
−1.3 (−2.4 to −0.1) |
12 |
−1.6 (−2.6 to −0.7) |
| Team change |
14 |
−5.2 (−7.3 to −3.0) |
14 |
−2.3 (−3.6 to −1.0) |
23 |
−1.5 (−2.5 to −0.6) |
7 |
−1.1 (−1.3 to −1.0) |
| Electronic patient registry |
3 |
−4.9 (−7.6 to −2.2) |
4 |
−4.0 (−7.6 to −0.4) |
5 |
−3.3 (−5.0 to −1.6) |
3 |
−1.9 (−5.8 to 2.1) |
| Facilitated relay |
12 |
−4.4 (−7.1 to −1.8) |
15 |
−2.7 (−4.4 to −1.0) |
11 |
−1.6 (−3.1 to 0.0) |
10 |
−1.5 (−2.2 to −0.9) |
| E-health |
9 |
−2.6 (−5.0 to −0.2) |
10 |
−2.0 (−4.4 to 0.4) |
10 |
−1.7 (−3.3 to 0.0) |
8 |
−1.6 (−2.9 to −0.2) |
| Continuous QI |
6 |
−4.7 (−7.2 to −2.2) |
7 |
−1.1 (−2.8 to 0.6) |
5 |
−1.7 (−3.9 to 0.5) |
5 |
−0.9 (−2.2 to 0.5) |
| HCPs | ||||||||
| Audit and feedback |
9 |
−3.2 (−6.5 to 0.0) |
2 |
−2.9 (−7.0 to 1.3) |
6 |
−1.9 (−4.2 to 0.3) |
6 |
−1.3 (−1.8 to −0.7) |
| Clinician education |
5 |
−2.5 (−8.0 to 3.1) |
2 |
−4.5 (−7.4 to −1.5) |
6 |
−0.9 (−2.9 to 1.2) |
2 |
−1.2 (−2.8 to 0.4) |
| Clinician reminder |
12 |
−2.8 (−5.4 to −0.3) |
9 |
−4.3 (−6.5 to −2.0) |
15 |
−0.7 (−1.9 to 0.4) |
8 |
−1.4 (−3.0 to 0.1) |
| Patients | ||||||||
| Patient education |
15 |
−5.1 (−7.3 to −3.0) |
8 |
−2.9 (−5.9 to 0.1) |
17 |
−1.8 (−2.9 to −0.8) |
5 |
−2.8 (−4.6 to −0.9) |
| Promotion of self-management |
18 |
−4.7 (−6.7 to −2.7) |
20 |
−2.4 (−3.9 to −0.9) |
23 |
−1.7 (−2.6 to −0.8) |
13 |
−1.5 (−2.0 to −1.0) |
| Patient reminder system |
14 |
−3.2 (−5.6 to −0.8) |
11 |
−2.9 (−4.8 to −1.0) |
16 |
−1.5 (−2.8 to −0.3) |
9 |
−1.3 (−1.8 to −0.9) |
| LDL-C* |
||||||||
| ≥3.4 mmol/L |
<3.4 mmol/L |
|||||||
|
N |
MD (95% CI) |
N |
MD (95% CI) |
|||||
| Health system |
||||||||
| Case management |
2 |
−0.20 (−0.27 to −0.14) |
21 |
−0.07 (−0.14 to 0.00) |
||||
| Team change |
4 |
−0.31 (−0.60 to −0.02) |
18 |
−0.15 (−0.24 to −0.06) |
||||
| Electronic patient registry |
NA |
NA |
3 |
−0.13 (−0.26 to 0.01) |
||||
| Facilitated relay |
4 |
−0.39 (−0.62 to −0.17) |
20 |
−0.12 (−0.19 to −0.04) |
||||
| E-health |
NA |
NA |
16 |
−0.10 (−0.18 to −0.02) |
||||
| Continuous QI |
NA |
NA |
6 |
−0.10 (−0.25 to 0.04) |
||||
| HCPs |
||||||||
| Audit and feedback |
2 |
−0.33 (−0.62 to −0.04) |
6 |
−0.06 (−0.22 to 0.09) |
||||
| Clinician education |
NA |
NA |
5 |
−0.12 (−0.27 to 0.04) |
||||
| Clinician reminder |
NA |
NA |
17 |
−0.08 (−0.16 to 0.00) |
||||
| Patients |
||||||||
| Patient education |
4 |
−0.23 (−0.48 to 0.01) |
10 |
−0.11 (−0.21 to −0.02) |
||||
| Promotion of self-management |
4 |
−0.23 (−0.48 to 0.01) |
23 |
−0.13 (−0.20 to −0.06) |
||||
| Patient reminder system | 4 | −0.31 (−0.60 to −0.02) | 16 | −0.06 (−0.14 to 0.01) | ||||
IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; MD, mean difference; N, number of trials with analyzable data; NA, no available trials for analysis; NGSP, National Glycohemoglobin Standardization Program.
*To convert LDL-C to mg/dL, multiply by 38.67.
We performed meta-regression analysis to control for confounding effects among the 12 QI strategies with adjustment for age, sex, and baseline cardiometabolic risk factors (Supplementary Table 7). Using analyzable HbA1c data from 75 trials, patient education remained independently associated with HbA1c reduction (−0.15% [−0.28 to −0.02], −1.6 mmol/mol [−3.1 to −0.2]; P = 0.019) whereas team change (−0.12% [−0.25 to 0.02], −1.3 mmol/mol [−2.7 to 0.2]; P = 0.083) and facilitated patient-provider relay (−0.14% [−0.28 to 0.01], −1.5 mmol/mol [−3.1 to 0.1]; P = 0.059) tended toward significance. Other independent predictors included electronic patient registry for SBP (−4.4 mmHg [−8.0 to −0.8]; P = 0.016) and DBP reductions (−2.7 mmHg [−4.5 to −0.8]; P = 0.004) whereas the corresponding changes for promotion of self-management were −4.7 mmHg (−7.8 to −1.6) (P = 0.003) and −2.2 mmHg (−3.8 to −0.7) (P = 0.004). Overall, integrated care reduced HbA1c by 0.13% (−0.21 to −0.04) (1.4 mmol/mol [−2.3 to −0.4]) per 1% (11 mmol/mol) increase in baseline HbA1c. There was a significant trend of larger blood pressure reduction with an increasing number of QI strategies (Ptrend for SBP = 0.025 and for DBP = 0.007) but not for HbA1c and LDL-C changes.
Conclusions
In this meta-analysis, multicomponent integrated care lasting at least 12 months reduced HbA1c, SBP, DBP, and LDL-C levels by 0.28% (3.1 mmol/mol), 2.3 mmHg, 1.1 mmHg, and 0.14 mmol/L, respectively, on top of usual care (clinical procedures, medical visits, and drug therapy). Among the various QI strategies, team change, patient education, patient self-management, electronic registry, and using relay to promote patient-provider communication (e.g., personal reports, trained peers) had the largest independent effect sizes. These results extended that of a previous meta-analysis of 142 studies (12) to a diversity of settings and populations, with greater effect sizes in younger patients and those with suboptimal control and coming from Asia and middle-income countries.
Due to their phenotypic heterogeneity, patients with type 2 diabetes require individualized biomedical, cognitive, psychosocial, and behavioral interventions (19). However, effective delivery of personalized care requires a favorable practicing environment to nurture patient-provider relationship with adequate contact time in order to define unmet needs, reduce clinical inertia, and promote self-management. The use of a team to change workflow will enable HCPs to collect data, stratify risk, relay information, and improve patient-provider communication that will address these interlinking challenges (10).
Effective Patient-Provider Communication
In support of this notion, our analysis indicated that better information flow (e.g., issue of personal reports with treatment targets and decision support, e-health, trained peers, or community health workers) enabled treatment intensification, improved control of cardiometabolic risk factors, promoted self-care behaviors, and reduced hospitalizations, especially in individuals with suboptimal control (20,21). Similarly, interactive telemedicine enhanced patient-provider communication and problem-solving through facilitated learning and self-care monitoring with reduced health care utilization (22–24).
Approximately 20–30% of patients with type 2 diabetes had coexistent distress, anxiety, and/or depression (25), which could lead to suboptimal self-care (26), treatment nonadherence (27), and premature death (28,29). In several studies, trained peers and/or community health workers were used to support these difficult-to-treat or hard-to-reach patients, especially in low-resource settings. These trained nonclinical assistants could overcome the language and cultural communication barriers, alleviate negative emotions, exchange complex health information, and engage patients with linkage to the health care system, especially in those with frequent peer-to-peer contacts (30–33).
Patient Education and Self-management
The need to change lifestyle, self-manage, and adhere to lifelong treatment requires considerable commitment and self-discipline, especially when the disease is silent. Over 50% of patients with type 2 diabetes had major gaps in knowledge/skills, which could be improved by structured education programs with adequate contact time and reinforcement (34,35). In several studies, culturally relevant and patient-centered education improved negative emotions and health-coping behaviors (35–37). In other studies, if treatment nonadherence and default were reduced, there was also a reduction in cardiovascular events, all-cause death, hospitalizations, and treatment costs (38–41). In the 2016–2017 U.K. National Diabetes Audit Report, structured education was offered to 77% of newly diagnosed patients with type 2 diabetes, but the attendance rate was <10% (42). These low response rates call for more research to evaluate strategies to increase the reach and user-friendliness of these self-management and empowerment programs, especially for patients who cannot attend these sessions for reasons such as busy work schedule, social isolation, or physical disability.
Using Team-Based Integrated Care to Identify Unmet Needs
In clinical trial settings, use of additional nurses to implement protocols under medical supervision ensured care continuity and good clinical practice during the evaluation of novel therapies. In these settings, the event rates were considerably reduced compared with those observed in real-world practice (43). Some examples of multifaceted care such as the Steno-2 study (44–46) and Japan Diabetes Optimal Integrated Treatment Study for 3 Major Risk Factors of Cardiovascular Disease (J-DOIT3) (47) confirmed that optimal control of HbA1c, blood pressure, and LDL-C reduced micro- and macrovascular complications and death.
In the past two decades, the incidences of diabetes-related cardiovascular-renal complications and death have declined in high-income countries (48–51). However, this was less evident in young people (50–52) who often have competing priorities, different social values, and suboptimal psycho-behavioral health (7,53,54). In LMICs, where the health care system is less well developed and expensive treatment for advanced disease is least affordable, these team-based care models may be particularly relevant. Indeed, our results suggested the superior effects of integrated care in young people, those with suboptimal management, and in patients from Asia. Using Hong Kong as an example, the implementation of a structured assessment and education program using doctor-nurse teams in primary care and hospital settings has increased the attainment rate of HbA1c<7% (53 mmol/mol) from 33% to 50% during a 13-year period, with a significant decline in cardiovascular-renal events and death rates in patients with long disease duration (55).
Strengths and Limitations
Compared with a previous review (12) (Supplementary Table 8), we have included more patients who received complex interventions, including peer support and e-health, lasting at least 12 months and demonstrated the sustained effects, especially in patients who are young, with suboptimal control, and in low-resource settings. One of our limitations is that we have used protocols and criteria defined a priori to overcome search bias and to structuralize evaluation of the studies. The funnel plots showed slight asymmetry related to study heterogeneity, which had been adjusted in the random-effects meta-analysis modeling (Supplementary Figs. 8 and 9). Second, classification of these complex QI strategies is challenging and the presence of some QI strategies in usual care have attenuated the effect size of additional interventions. Third, despite our detailed classification of interventions and adjustment for confounding effects (e.g., patient characteristics, study design, settings, and co-interventions), there remained unknown or unmeasured confounders. Fourth, lack of access to patient-level data limited the robustness of our subgroup analyses. Here, socioeconomic/ education status, sex, ethnicity, disease duration, health care system (e.g., public, private, subsidized), and access to drugs can all influence the effectiveness of integrated care. Fifth, without access to patient-level data, we could not comment on the measurement variabilities of these trials and the possibility of regression to the mean. However, in these RCTs with low risk of selection bias, the mean differences of both intervention and control groups were equally affected by regression to the mean and thus should represent a true effect of the intervention. Last, although the greatest effects were observed in trials that came from LMICs, there were only 10 of them, which calls for more studies for validation.
Summary
By leveraging existing resources, changing the workflow, and using a team approach, doctors are in a better position to define the phenotypes and clinical needs of the patients for personalizing care that will improve control of cardiometabolic risk factors and clinical outcomes. These multicomponent integrated care models will particularly benefit those who are young, with suboptimal control, and in low-resource settings where patient volume is large and contact time with doctors is short. However, given the diversity of health care providing and financing policies, socioeconomic development, and expectations of payors, patients, providers, and regulators, context-relevant care models will need to be developed with economic analysis to confirm their acceptability and sustainability.
Supplementary Material
Article Information
Funding. L.L.L. is partially supported by the University of Malaya for pursuing a postgraduate study at The Chinese University of Hong Kong.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors’ Contributions. L.L.L. A.P.S.K., and J.C.N.C. conceptualized the study design. L.L.L. and E.S.H.L. performed the literature search, appraised the articles, and performed the analysis with support from J.C.N.C. and A.P.S.K. L.L.L. wrote the first draft and J.C.N.C. finalized the manuscript. All authors participated in the research methodology and data interpretation, revised the manuscript critically for important intellectual content, and approved the final version of the manuscript. J.C.N.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2010/-/DC1.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 8th edition [Internet]. Brussels, Belgium, International Diabetes Federation, 2017. Available from http://www.diabetesatlas.org. Accessed 15 December 2017
- 2.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bommer C, Heesemann E, Sagalova V, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol 2017;5:423–430 [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes [Internet]. Brussels, Belgium, International Diabetes Federation, 2012. Available from https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes. Accessed 5 April 2017
- 5.American Diabetes Association 3. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S28–S37 [DOI] [PubMed] [Google Scholar]
- 6.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 7.Chan JC, Gagliardino JJ, Baik SH, et al.; IDMPS Investigators . Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009;32:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronson R, Orzech N, Ye C, Brown RE, Goldenberg R, Brown V. Specialist-led diabetes registries and prevalence of poor glycemic control in type 2 diabetes: the Diabetes Registry Outcomes Project for A1C Reduction (DROP A1C). Diabetes Care 2016;39:1711–1717 [DOI] [PubMed] [Google Scholar]
- 9.Rushforth B, McCrorie C, Glidewell L, Midgley E, Foy R. Barriers to effective management of type 2 diabetes in primary care: qualitative systematic review. Br J Gen Pract 2016;66:e114–e127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78 [DOI] [PubMed] [Google Scholar]
- 11.Chan JC, Sui Y, Oldenburg B, et al.; JADE and PEARL Project Team . Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med 2014;174:972–981 [DOI] [PubMed] [Google Scholar]
- 12.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–2261 [DOI] [PubMed] [Google Scholar]
- 13.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–440 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. eHealth. Available from http://www.who.int/topics/ehealth/en/. Accessed 20 June 2016
- 15.Effective Practice and Organisation of Care (EPOC) Group. EPOC resources for review authors [Internet]. The Cochrane Collaboration. Available from http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed 3 December 2016
- 16.Higgins JPT, Green S, Eds. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.handbook.cochrane.org. Accessed 3 December 2016
- 17.The R Foundation. The R project for statistical computing Available from https://www.r-project.org/. Accessed 15 December 2016
- 18.Bosi E, Scavini M, Ceriello A, et al.; PRISMA Study Group . Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care 2013;36:2887–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J, Luk A, Wong R, et al. Regular mailing of personalized feedback reports improves glycemic control in diabetes: a randomized controlled trial. J Diabetes 2017;9:536–538 [DOI] [PubMed] [Google Scholar]
- 21.Tang TS, Funnell MM, Noorulla S, Oh M, Brown MB. Sustaining short-term improvements over the long-term: results from a 2-year diabetes self-management support (DSMS) intervention. Diabetes Res Clin Pract 2012;95:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2015;9:CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016;39:2089–2095 [DOI] [PubMed] [Google Scholar]
- 24.Jacques Rose K, Petrut C, L’Heveder R, de Sabata S. IDF Europe position on mobile applications in diabetes. Diabetes Res Clin Pract 2017;17:31356-6 [DOI] [PubMed] [Google Scholar]
- 25.Fisher EB, Chan JC, Nan H, Sartorius N, Oldenburg B. Co-occurrence of diabetes and depression: conceptual considerations for an emerging global health challenge. J Affect Disord 2012;142(Suppl.):S56–S66 [DOI] [PubMed] [Google Scholar]
- 26.Whitworth SR, Bruce DG, Starkstein SE, Davis WA, Davis TM, Bucks RS. Lifetime depression and anxiety increase prevalent psychological symptoms and worsen glycemic control in type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Res Clin Pract 2016;122:190–197 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Ting RZ, Yang W, et al.; China Depression in Chinese Patients with Type 2 Diabetes (DD2) Study Group . Depression in Chinese patients with type 2 diabetes: associations with hyperglycemia, hypoglycemia, and poor treatment adherence. J Diabetes 2015;7:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naicker K, Johnson JA, Skogen JC, et al. Type 2 diabetes and comorbid symptoms of depression and anxiety: longitudinal associations with mortality risk. Diabetes Care 2017;40:352–358 [DOI] [PubMed] [Google Scholar]
- 29.Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care 2017;40:1588–1596 [DOI] [PubMed] [Google Scholar]
- 30.Fisher EB, Coufal MM, Parada H, et al. Peer support in health care and prevention: cultural, organizational, and dissemination issues. Annu Rev Public Health 2014;35:363–383 [DOI] [PubMed] [Google Scholar]
- 31.Farzadfar F, Murray CJ, Gakidou E, et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: a nationally representative observational study. Lancet 2012;379:47–54 [DOI] [PubMed] [Google Scholar]
- 32.Carrasquillo O, Lebron C, Alonzo Y, Li H, Chang A, Kenya S. Effect of a community health worker intervention among Latinos with poorly controlled type 2 diabetes: the Miami Healthy Heart Initiative randomized clinical trial. JAMA Intern Med 2017;177:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patil SJ, Ruppar T, Koopman RJ, et al. Peer support interventions for adults with diabetes: a meta-analysis of hemoglobin A1c outcomes. Ann Fam Med 2016;14:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–1171 [DOI] [PubMed] [Google Scholar]
- 35.Creamer J, Attridge M, Ramsden M, Cannings-John R, Hawthorne K. Culturally appropriate health education for type 2 diabetes in ethnic minority groups: an updated Cochrane review of randomized controlled trials. Diabet Med 2016;33:169–183 [DOI] [PubMed] [Google Scholar]
- 36.Trento M, Gamba S, Gentile L, et al.; ROMEO Investigators . Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care 2010;33:745–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies MJ, Heller S, Skinner TC, et al.; Diabetes Education and Self Management for Ongoing and Newly Diagnosed Collaborative . Effectiveness of the Diabetes Education and Self Management for Ongoing and Newly Diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins JM, Thatcher GE, Webb DA, Valdmanis VG. Nutritionist visits, diabetes classes, and hospitalization rates and charges: the Urban Diabetes Study. Diabetes Care 2008;31:655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillett M, Dallosso HM, Dixon S, et al. Delivering the Diabetes Education and Self Management for Ongoing and Newly Diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341:c4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagliardino JJ, Lapertosa S, Pfirter G, et al.; PRODIACOR . Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR). Diabet Med 2013;30:1102–1111 [DOI] [PubMed] [Google Scholar]
- 41.Wong CK, Wong WC, Wan YF, et al. Patient empowerment programme in primary care reduced all-cause mortality and cardiovascular diseases in patients with type 2 diabetes mellitus: a population-based propensity-matched cohort study. Diabetes Obes Metab 2015;17:128–135 [DOI] [PubMed] [Google Scholar]
- 42.National Health Service. National diabetes audit report 1 care processes and treatment targets 2016-17 [Internet], 10 November 2017. Available from https://digital.nhs.uk/catalogue/PUB30142. Accessed 15 December 2017
- 43.Chan JC. What can we learn from the recent blood glucose lowering megatrials? J Diabetes Investig 2011;2:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 45.Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016;59:2298–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oellgaard J, Gæde P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int 2017;91:982–988 [DOI] [PubMed] [Google Scholar]
- 47.Ueki K, Sasako T, Okazaki Y, et al.; J-DOIT3 Study Group . Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:951–964 [DOI] [PubMed] [Google Scholar]
- 48.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 49.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418 [DOI] [PubMed] [Google Scholar]
- 50.Lind M, Garcia-Rodriguez LA, Booth GL, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia 2013;56:2601–2608 [DOI] [PubMed] [Google Scholar]
- 51.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 2016;39:1018–1026 [DOI] [PubMed] [Google Scholar]
- 52.Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol 2014;2:935–943 [DOI] [PubMed] [Google Scholar]
- 54.Steinarsson AO, Rawshani A, Gudbjörnsdottir S, Franzén S, Svensson AM, Sattar N. Short-term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: a longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia 2018;61:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luk AOY, Hui EMT, Sin MC, et al. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: the Hong Kong Diabetes Database. Diabetes Care 2017;40:928–935 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.