Abstract
OBJECTIVE
Patients undergoing noncardiac surgery frequently have diabetes mellitus (DM) and an elevated risk of cardiovascular disease. It is unknown whether temporal declines in the frequency of perioperative major adverse cardiovascular and cerebrovascular events (MACCEs) apply to patients with DM.
RESEARCH DESIGN AND METHODS
Patients ≥45 years of age who underwent noncardiac surgery from January 2004 to December 2013 were identified using the U.S. National Inpatient Sample. DM was identified using ICD-9 diagnosis codes. Perioperative MACCEs (in-hospital all-cause mortality, acute myocardial infarction, or acute ischemic stroke) by DM status were evaluated over time.
RESULTS
The final study sample consisted of 10,581,621 hospitalizations for major noncardiac surgery; DM was present in ∼23% of surgeries and increased over time (P for trend <0.001). Patients with DM experienced MACCEs in 3.3% of surgeries vs. 2.8% of surgeries for patients without DM (P < 0.001). From 2004 to 2013, the odds of perioperative MACCEs after multivariable adjustment increased by 6% (95% CI 2–9) for DM patients, compared with an 8% decrease (95% CI −10 to −6) for patients without DM (P for interaction <0.001). Trends for individual end points were all less favorable for patients with DM versus those without DM.
CONCLUSIONS
In an analysis of >10.5 million noncardiac surgeries from a large U.S. hospital admission database, perioperative MACCEs were more common among patients with DM versus those without DM. Perioperative MACCEs increased over time and individual end points were all less favorable for patients with DM. Our findings suggest that a substantial unmet need exists for strategies to reduce the risk of perioperative cardiovascular events among patients with DM.
Introduction
Patients with diabetes mellitus (DM) comprise an increasingly large proportion of the >300 million patients who annually undergo noncardiac surgery worldwide (1,2). In addition to increasing cardiovascular disease (CVD) risk (3), DM is often considered a risk factor for perioperative major adverse cardiovascular and cerebrovascular events (MACCEs), including acute myocardial infarction (AMI) and stroke (4). Moreover, patients with DM undergoing noncardiac surgery incur increased costs and experience longer lengths of hospital stay (5,6). The proportion of DM among inpatient hospitalizations is increasing, and it is estimated that by 2050, 1 in 3 individuals in the U.S. and 1 in 10 individuals worldwide will have DM (7–9).
Whereas increases in DM prevalence burden health care systems worldwide, overall CVD morbidity and mortality among patients with DM may be decreasing (10). Additionally, recent evidence from the U.S. National Inpatient Sample (NIS) of all inpatients undergoing noncardiac surgery suggests a decline in some, but not all, cardiovascular events (11). However, whether these trends apply to perioperative MACCEs among patients with DM has not been examined. To address this knowledge gap, we queried a large administrative database of U.S. hospital admissions to compare national trends in cardiovascular outcomes and mortality after noncardiac surgery among patients with and without DM. We also examined factors associated with in-hospital perioperative cardiovascular events among patients with DM.
Research Design and Methods
Study Population
Patients ≥45 years of age undergoing major noncardiac surgery from 2004 to 2013 were identified from the NIS of the Healthcare Cost and Utilization Project (HCUP), as previously described (11). The NIS is a national administrative database of discharge-level data from a 20% stratified sample of all U.S. hospitals. Patients were included if they had a principal ICD-9 procedure code for a major therapeutic operating room procedure (HCUP Procedure Class 4) during the hospital admission. Principal Clinical Classifications Software procedure codes, aggregates of related ICD-9 procedure codes, were used to stratify cases by surgical subtype. Patients who underwent cardiac procedures (n = 1,655,567), cardiac surgery and cardiac transplantation (n = 582,726), bone marrow transplantation (n = 18,151), ophthalmologic surgery (n = 13,342), radiation therapy (n = 9,817), dental surgery (n = 1,779), and non–operating room procedures (n = 386) were excluded from the analysis. Major noncardiac surgery Clinical Classifications Software procedure codes were clustered into the following 13 major surgical subtypes: endocrine, general, genitourinary, gynecologic, neurosurgery, obstetric, orthopedic, otolaryngology, skin and breast, thoracic, noncardiac solid organ transplant, and vascular surgery.
Outcomes
The primary outcome was MACCEs, defined as in-hospital all-cause death, AMI, or ischemic stroke. AMI was identified using ICD-9 diagnosis codes for acute ST-segment elevation myocardial infarction (410.01–410.61, 410.81, and 410.91) and non–ST-segment elevation myocardial infarction (410.71). Acute ischemic stroke was identified using ICD-9 diagnosis codes 433.x1, 434.x1, 436, and 437.1. DM was identified using ICD-9 diagnosis codes 250.x. DM was classified as type 1 or type 2 based on ICD-9 diagnosis codes, as listed in Supplementary Table 1, and was further classified as controlled (ICDM-9-CM codes 250.x0 and 250.x1, which code DM as “not stated as uncontrolled”) or uncontrolled (ICD-9-CM diagnosis codes 250.x2 and 250.x3) (Supplementary Table 3).
Sex-Specific Analysis
Several studies have observed sex-specific differences in the association between DM and CVD (12). We therefore also sought to investigate the association between DM and perioperative MACCEs when stratified by sex.
Statistical Analysis
Sampling weights were applied to calculate rates for trend analyses and to determine national incidence estimates according to HCUP guidance (13). Unweighted data were used in all other analyses, unless otherwise specified. To report adjusted odds ratios (ORs) for MACCEs, multivariable logistic regression models were generated with demographics, obesity, tobacco use, hypertension, hyperlipidemia, chronic kidney disease, end-stage renal disease, coronary artery disease, prior revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), peripheral arterial disease, valvular heart disease, congestive heart failure, prior venous thromboembolism, chronic lung disease, alcohol abuse, malignancy, anemia, elective hospitalization, noncardiac surgery subtype, and year of surgery as covariates. Additional adjustment was performed for atrial fibrillation and history of cerebrovascular accident, for socioeconomic status by insurance payer, and for quartile classification of the estimated median household income of residents in the patient’s zip code. Interaction tests for DM status and MACCEs over time were performed in the fully adjusted multivariable models. To facilitate data presentation in tables, the prevalence of DM and perioperative adverse events were reported in 2-year intervals, as follows: 2004–2005, 2006–2007, 2008–2009, 2010–2011, and 2012–2013. Statistical analyses were performed using SPSS 20 (IBM SPSS Statistics, Armonk, NY) and R (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests are two sided, and P values <0.05 were considered to be statistically significant.
Results
Study Population
From 2004 to 2013, 12,863,389 hospitalizations for major surgery were identified among patients ≥45 years of age. After excluding patients undergoing cardiac surgery, low-risk and nonoperative procedures, the final study sample consisted of 10,581,621 hospitalizations for major noncardiac surgery. Of this total, 2,438,204 hospitalizations for major surgery occurred among patients with DM, and 8,143,417 hospitalizations for patients without DM. After applying sampling weights, this corresponds to an estimated 11,645,711 surgical hospitalizations among patients with DM in the U.S. over this time period. Patients with DM undergoing major noncardiac surgery tended to be older, of nonwhite race, and of Hispanic ethnicity (Table 1). As expected, patients with DM were more frequently obese with concomitant cardiovascular risk factors and were more likely to undergo vascular surgery (Table 1). The proportion of patients with DM undergoing noncardiac surgery increased over time (20.3% in 2004% to 25.4% in 2013; P for trend <0.0001). Over this time period, there was a significant increase in the proportion of patients with type 2 DM, with a corresponding decrease in type 1 DM (Table 2).
Table 1.
Baseline characteristics of patients undergoing major noncardiac surgery
| Patient characteristics | No DM (n = 8,143,417) | DM (n = 2,438,204) |
|---|---|---|
| Age, years (±SD) | 65.44 (±12.6) | 66.72 (±11.2) |
| Female sex | 58.1 (4,716,623) | 51.7 (1,259,175) |
| Race/ethnicity | ||
| Non-Hispanic white | 66.8 (5,437,647) | 59.1 (1,441,419) |
| Non-Hispanic black | 6.7 (547,640) | 11.7 (285,680) |
| Hispanic | 4.8 (391,529) | 8.5 (207,028) |
| Other | 3.6 (293,191) | 4.6 (111,097) |
| Unknown | 18.1 (1,473,410) | 16.1 (392,980) |
| Obesity | 8.6 (697,195) | 18.8 (457,376) |
| Tobacco use | 20.9 (1,704,669) | 19.7 (480,091) |
| Hypertension | 51.8 (4,221,563) | 78.2 (1,906,909) |
| Hyperlipidemia | 24.5 (1,995,723) | 41.2 (1,003,336) |
| Chronic kidney disease | 4.9 (402,164) | 16.4 (398,751) |
| End-stage renal disease | 1.3 (107,836) | 6.1 (148,278) |
| Coronary artery disease | 14.3 (1,166,484) | 29.5 (719,029) |
| Prior PCI | 3.1 (249,641) | 5.7 (138,076) |
| Prior CABG | 3.6 (291,775) | 8.3 (203,314) |
| Peripheral arterial disease | 5.5 (449,900) | 11.9 (290,923) |
| Valvular heart disease | 4.2 (345,860) | 4.3 (104,844) |
| History of heart failure | 5.2 (423,534) | 11.4 (278,898) |
| History of venous thromboembolism | 2.5 (202,014) | 2.7 (66,852) |
| Chronic pulmonary disease | 16.3 (1,324,354) | 18.6 (454,288) |
| Alcohol abuse | 2.3 (183,818) | 1.5 (37,578) |
| Malignancy | 5.7 (467,405) | 4.6 (113,130) |
| Anemia | 13.7 (1,115,113) | 20.9 (509,488) |
| Elective surgery | 62.5 (5,075,736) | 54.1 (1,316,032) |
| Surgery type | ||
| Endocrine | 1.2 (99,209) | 1.0 (23,605) |
| General | 21.9 (1,783,831) | 19.6 (478,344) |
| GU | 7.6 (620,146) | 6.2 (150,150) |
| Neurosurgery | 5.9 (482,674) | 4.9 (119,455) |
| Ob/Gyn | 13.7 (1,118,770) | 5.7 (139,120) |
| Otolaryngology | 0.8 (63,448) | 0.6 (14,879) |
| Orthopedic | 40.0 (3,258,728) | 40.8 (994,493) |
| Thoracic | 2.3 (187,333) | 1.9 (45,595) |
| Transplant | 0.2 (20,269) | 0.5 (11,895) |
| Vascular | 9.3 (756,959) | 15.4 (376,469) |
| Skin and breast | 3.8 (311,435) | 6.3 (154,159) |
All values are percentage (n), unless otherwise indicated. GU, genitourinary; Ob/Gyn, obstetric and gynecologic; RCRI, revised cardiac risk index.
Table 2.
Trends in DM among patients undergoing major noncardiac surgery
| 2004–2005(N = 2,051,557) | 2006–2007(N = 2,117,047) | 2008–2009(N = 2,213,461) | 2010–2011(N = 2,254,360) | 2012–2013(N = 1,945,196) | Total(N = 10,581,621) | |
|---|---|---|---|---|---|---|
| No DM | 79.5 (1,631,311) | 78.1 (1,653,305) | 77.0 (1,704,807) | 75.5 (1,702,296) | 74.6 (1,451,698) | 77.0 (8,143,417) |
| DM | 20.5 (420,246) | 21.9 (463,742) | 23.0 (508,654) | 24.5 (552,064) | 25.4 (493,498) | 23.1 (2,438,204) |
| Type 1 | 6.6 (27,929) | 2.9 (13,327) | 2.2 (11,066) | 1.9 (10,611) | 1.9 (9,378) | 3.0 (72,311) |
| Type 2 | 93.4 (392,317) | 97.1 (450,415) | 97.8 (497,588) | 98.1 (541,453) | 98.1 (484,120) | 97.0 (2,365,893) |
All values are percentage (n).
MACCEs
MACCEs occurred in ∼3% (∼3,000 events per 100,000) of all major noncardiac surgeries.
MACCEs occurred in 80,632 major noncardiac surgeries (3,307 events per 100,000 major noncardiac surgeries [3.3%]) for patients with DM compared with 230,080 (2,825 events per 100,000 [2.8%]) for patients without DM. After applying sampling weights from 2004 to 2013, this corresponds to an estimated 383,970 in-hospital perioperative events for patients with DM, and 1,094,582 for patients without DM. When all patients with DM were compared with those without DM, the adjusted OR for perioperative MACCEs was 0.94 (95% CI 0.93–0.95). When comparing patients with uncontrolled DM to those without DM, the adjusted OR for MACCEs was 1.41 (95% CI 1.38–1.43). Patients with uncontrolled DM also had the greatest event rate of any MACCE over the study period (Supplementary Table 3).
Trends in MACCEs by DM Status
From 2004 to 2013, the number of perioperative MACCEs per 100,000 surgeries declined by 222 (95% CI 175–270; P for trend <0.001) for patients with DM versus 730 (95% CI 707–753; P for trend <0.001) for patients without DM (Fig. 1A). After multivariable adjustment, the odds of perioperative MACCE increased by 6% (95% CI 2–9) among patients with DM. In comparison, the adjusted odds of perioperative MACCEs decreased by 8% (95% CI −10 to −6) among patients without DM (P for interaction <0.001) (Fig. 2A). Similar trends in perioperative events for patients with and without DM were observed for major surgical subtypes (Supplementary Fig. 1). Adjustment for atrial fibrillation, history of cerebrovascular accident, insurance payer, and quartile classification of the estimated median household income of residents in the patient’s zip code did not alter the adjusted trends in MACCEs by DM status (data not shown).
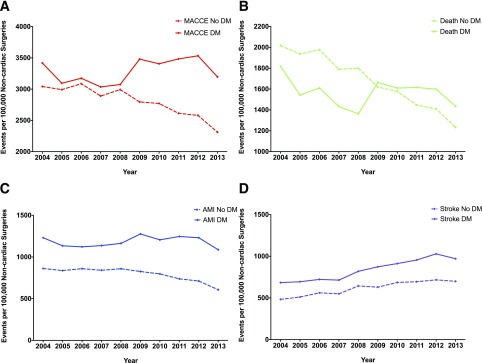
Figure 1.
Trends in rates by DM status of perioperative MACCEs. A: MACCEs. B: Death. C: AMI. D: Stroke.
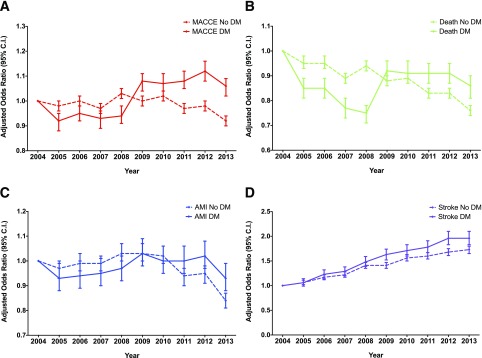
Figure 2.
Trends in adjusted odds of perioperative MACCEs by DM status over time. A: MACCEs. B: Death. C: AMI. D: Stroke. Multivariable models include age, sex, race/ethnicity, obesity, tobacco use, hypertension, hyperlipidemia, chronic kidney disease, end-stage renal disease, coronary artery disease, prior revascularization with either PCI or CABG, peripheral arterial disease, valvular heart disease, congestive heart failure, prior venous thromboembolism, chronic lung disease, alcohol abuse, malignancy, anemia, elective hospitalization, noncardiac surgery subtype, and year of surgery as covariates.
Supplementary Table 2 presents the results for the composite of MACCEs and individual end points over time for the population overall and stratified by DM status. From 2004 to 2013 the number of deaths per 100,000 surgeries declined by 383 (95% CI 349–417; P for trend <0.001) for patients with DM compared with 787 (95% CI 769–805; P for trend <0.001) for patients without DM (Fig. 1B). Over this time, the adjusted odds of perioperative mortality decreased by 14% (95% CI −19 to −10) among patients with DM compared with a 24% decrease (95% CI −26 to −22) among patients without DM (P for interaction <0.001) (Fig. 2B).
For the individual end point of perioperative AMI, the rate did not change significantly over time for patients with DM (P for trend = 0.122) (Fig. 1C). In contrast, among patients without DM, perioperative AMI per 100,000 surgeries decreased by 255 (95% CI 243–267; P for trend <0.001) (Fig. 1C). From 2004 to 2013, the adjusted odds of perioperative AMI decreased by 7% (95% CI −12 to −1) among patients with DM. In comparison, there was a 16% decrease (95% CI −19 to −13) in perioperative AMI among patients without DM (P for interaction <0.001) (Fig. 2C).
Similar to the noncardiac surgery population overall (11), the number of perioperative strokes per 100,000 surgeries increased by 286 (95% CI 262–310; P for trend <0.001) for patients with DM. The magnitude of increase in perioperative strokes was similar for patients without DM (216 [95% CI 205–227]; P for trend <0.001) (Fig. 1D). From 2004 to 2013, the adjusted odds of perioperative stroke increased by 96% (95% CI 83–110) among patients with DM. In comparison, there was a smaller 73% increase (95% CI 35–80) among patients without DM (P for interaction <0.001) (Fig. 2D).
Sex-Specific Analysis
For both sexes, the frequency of perioperative MACCEs was higher among patients with DM compared with without DM (3.0% vs. 2.4% among women, P < 0.001; 3.6% vs. 3.4% among men, P < 0.001). The association between DM and perioperative MACCEs was greater among women (OR 1.26; 95% CI 1.25–1.28) than among men (OR 1.06; 95% CI 1.05–1.07), P for interaction <0.001). From 2004 to 2013, the adjusted odds of perioperative MACCEs increased by 1% (95% CI −4 to 6) among women with DM compared with a 9% decrease (95% CI −12 to −6) among women without DM. In contrast, over this time period the adjusted odds of perioperative MACCEs increased by 10% (95% CI 5–16) among men with DM. In comparison, there was an 8% decrease (95% CI −10 to −5) in the adjusted odds of perioperative MACCEs among men without DM.
Conclusions
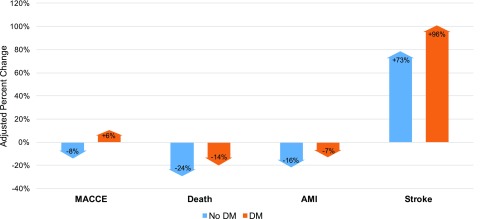
In this analysis of >10.5 million major noncardiac surgeries occurring from 2004 to 2013, MACCEs occurred in 3.3% of patients with DM compared with 2.8% of those without DM, corresponding to ∼25,000 excess perioperative events annually among patients with DM. Overall, DM was not associated with an increased risk of perioperative MACCEs after multivariable adjustment. However, uncontrolled DM was associated with adjusted odds of 1.41 (95% CI 1.38–1.43) for perioperative MACEs compared with those without DM. This indicates that covariates/confounders may drive excess perioperative MACCEs in patients with well-controlled DM, but uncontrolled DM is an independent risk factor for perioperative events. These findings are consistent with data from the revised cardiac risk index, demonstrating increased risk of perioperative MACCEs for insulin-requiring DM (14). In adjusted analyses, trends in perioperative MACCEs increased for patients with DM while they decreased for those without DM (Fig. 3). Trends for the individual the end points of death, MI and stroke were all less favorable in patients with versus without DM (Fig. 3). To our knowledge, this is the largest study to report on national trends in cardiovascular outcomes for patients with DM undergoing major noncardiac surgery. Our findings suggest that a substantial unmet need exists for strategies to reduce the risk of perioperative cardiovascular events among patients with DM.
Figure 3.
Adjusted percentage change in odds of perioperative MACCEs and individual end points, 2004–2013.
Patients with DM comprise an increasingly large proportion of the population undergoing major noncardiac surgery. The trends in MACCEs and individual end points among the growing proportion of patients with DM are discouraging and as yet unexplained. Surgical patients with DM have more comorbidities and cardiovascular risk factors than patients without DM. Observational data from the U.S. and Europe consistently show that patients with DM have worse surgical outcomes, including longer lengths of stay and increased costs (5,15–17). Some (14,18), but not all (19), risk score analyses suggest that DM confers an independent risk of perioperative cardiovascular events. In the most recent American College of Cardiology/American Heart Association Clinical Practice Guideline for the cardiovascular evaluation and management of patients undergoing noncardiac surgery (4), DM is included as an important factor in perioperative cardiovascular risk stratification and management.
In the nonoperative setting, trends in AMI and stroke among U.S. patients with DM appear to be improving (10). Improved cardiovascular risk factor control and adherence to preventive practices among U.S. outpatients with DM has also been reported (20). These trends contrast with the suboptimal trends in operative MACCEs and individual end points among patients with DM observed in the current study. Surgery is a period of physiologic stress including induction of anesthesia, surgical trauma, bleeding, anemia, hypoxia, and postoperative pain. These perturbations can cause catecholamine surges, platelet activation and a hypercoagulable state, inflammation, and bleeding (21). Patients with DM undergoing major noncardiac surgery may experience enhanced atherothrombosis (22), increased perioperative autonomic instability (23), and upregulated pathways of inflammation and oxidative stress (24) relative to surgical patients without DM. These pathways may have influenced the unfavorable trends in perioperative outcomes for patients with DM that we observed. Future study is needed to determine whether these pathways can be modified to reduce the number of perioperative MACCEs among patients with DM.
Over recent decades, the incidence rates of ischemic stroke have declined for the overall U.S. population, including for patients with DM (10,25). Despite this decline, patients with DM remain at increased risk of ischemic stroke (26). In the NIS, patients with DM had greater adjusted odds in perioperative stroke than patients without DM. Because DM is a risk factor for perioperative stroke among patients undergoing cardiac and noncardiac surgery (27), it is possible that the incidence of perioperative stroke in the NIS increased in parallel with the increasing proportion of patients with DM. Alternatively, the increased odds of perioperative stroke among patients with DM in the NIS may be attributable to a greater burden of cardiovascular risk factors, atrial arrhythmias, or altered intraoperative hemodynamics (23,27). Perioperative medication use may also play a role in this finding. The Perioperative Ischemic Evaluation Study (POISE) (28) reported a small but statistically significant increased risk of stroke associated with perioperative use of long-acting metoprolol, but this observation is unlikely to fully explain the difference in the incidence of stroke between NIS patients with and without DM.
We observed a significantly greater association between DM and perioperative MACCEs in women compared with men, an observation that to our knowledge has not been described previously. Prior studies have shown an increased risk of fatal coronary heart disease (29), myocardial infarction (12), and stroke (30) among women with DM compared with men with DM. Further study is warranted to determine whether novel, sex-specific strategies can abrogate the increased risk of perioperative MACCEs that women with DM may experience.
Limitations
There are limitations to the current study. First, NIS analyses are based on administrative coding data, which may be subject to reporting bias or coding errors. In particular, we noted an increase in mortality in 2008–2009 for surgical patients with DM. The cause of this increase is unknown, but has been noted in other analyses of patients with DM from the NIS data set (31). The observed change in perioperative death after 2008 among patients with DM may be related to change in the use of secondary administrative codes for DM introduced as part of ICD-9-CM coding in 2008 (32). Second, the analysis was limited to patients ≥45 years of age, the population at risk for cardiovascular complications from noncardiac surgery. Third, there are limitations in the measures of DM control and complications used in NIS. There was no insulin use or levels of glucose control, a major predictor of CVD risk for patients with DM, that was reported (33,34). Definitions of DM control and complications were limited to administrative coding, which may not accurately reflect the severity and perioperative risk for some patients with DM. Administrative coding data have limited ability to distinguish type 1 DM from type 2 DM (35), and the prevalence estimates presented in Supplementary Table 1 are therefore only used for descriptive purposes. Similarly, the NIS administrative data set lacks granularity on clinical characteristics such as hypertensive control, appropriate use of anticoagulants for atrial fibrillation, or management of relevant comorbidities that may be associated with perioperative outcomes. Fourth, medical therapy was not available from this administrative data set. The use of cardiovascular therapies for the secondary prevention of CVD in patients with DM (36,37) in the perioperative period remains unknown. Fifth, data were analyzed as a simple random sample for some of the analyses (i.e., without using weights). This may introduce empirical weighting and thus may not be reflective of the overall population. Although observational analyses cannot establish causality, our findings of less favorable trends in perioperative MACCEs in patients with DM and increased risk among patients with uncontrolled DM may be useful for perioperative management. Finally, the results of perioperative laboratory testing, including cardiac biomarkers, were not available from this administrative data set.
Conclusion
Using the largest publicly available database for the accurate identification of trends in diseases and outcomes, we demonstrate that over a 10-year period and >10.5 million major noncardiac surgeries patients with DM experienced an increase in MACCEs and less favorable trends in AMI, stroke, and death than did patients without DM. This analysis highlights the need for additional strategies to reduce the risk of perioperative cardiovascular complications among patients with DM and the need for additional study to confirm the increases in perioperative stroke observed for patients with and without DM.
Supplementary Material
Article Information
Funding. J.D.N. was partially funded by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (grant K23-HL-125991) and an American Heart Association Mentored Clinical and Population Research Award (15MCPRP24480132). N.R.S. was supported by the NHLBI of the NIH under award 5T32-HL-098129. J.S.B. was partially funded by the NHLBI of the NIH (HL-114978).
The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the article.
Duality of Interest. No potential conflicts of interest relevant to this article were reported. Author Contributions. J.D.N. wrote the majority of the manuscript. T.W. made substantial critical revisions and aided with interpretation of the data. N.R.S. contributed significantly to study design and data analysis and made substantial critical revisions to the manuscript. J.S.B. made substantial critical revisions and aided with data analysis and interpretation. J.D.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Scientific Sessions of the American Heart Association, Anaheim, CA, 11–15 November 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2046/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Siddiqui NF, Coca SG, Devereaux PJ, et al. . Secular trends in acute dialysis after elective major surgery--1995 to 2009. CMAJ 2012;184:1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiser TG, Haynes AB, Molina G, et al. . Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385(Suppl. 2):S11. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Summary of revisions: Standards of Medical Care in Diabetes–2016. Diabetes Care 2016;39(Suppl. 1):S4–S5 [DOI] [PubMed] [Google Scholar]
- 4.Fleisher LA, Fleischmann KE, Auerbach AD, et al.; American College of Cardiology; American Heart Association . 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e77–e137 [DOI] [PubMed] [Google Scholar]
- 5.Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care 2014;37:611–616 [DOI] [PubMed] [Google Scholar]
- 6.Malone M, Lau NS, White J, et al. . The effect of diabetes mellitus on costs and length of stay in patients with peripheral arterial disease undergoing vascular surgery. Eur J Vasc Endovasc Surg 2014;48:447–451 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Diabetes report card [Internet], 2014. Atlanta, GA, Centers for Disease Control and Prevention. Available from https://www.cdc.gov/diabetes/library/reports/congress.html. Accessed 19 October, 2016
- 8.Centers for Disease Control and Prevention. CDC-number of discharges-hospitalizations for diabetes as any listed diagnosis [Internet], 2013. Atlanta, GA, Centers for Disease Control and Prevention. Available from https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 22 March 2017
- 9.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregg EW, Li Y, Wang J, et al. . Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 11.Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017;2:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarwar N, Gao P, Seshasai SR, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet 2010;376:958]. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. Healthcare cost and utilization project: trend weights for HCUP NIS data [article online], 2014. Available from https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed 1 May 2016
- 14.Lee TH, Marcantonio ER, Mangione CM, et al. . Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–1049 [DOI] [PubMed] [Google Scholar]
- 15.Noordzij PG, Boersma E, Schreiner F, et al. . Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol 2007;156:137–142 [DOI] [PubMed] [Google Scholar]
- 16.Schipper ON, Jiang JJ, Chen L, Koh J, Toolan BC. Effect of diabetes mellitus on perioperative complications and hospital outcomes after ankle arthrodesis and total ankle arthroplasty. Foot Ankle Int 2015;36:258–267 [DOI] [PubMed] [Google Scholar]
- 17.Dhatariya K, Levy N, Kilvert A, et al.; Joint British Diabetes Societies . NHS diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012;29:420–433 [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Liu Y, Paruch JL, et al. . Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013;217:833–842.e1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta PK, Gupta H, Sundaram A, et al. . Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011;124:381–387 [DOI] [PubMed] [Google Scholar]
- 20.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 21.Smilowitz NR, Berger JS. Perioperative management to reduce cardiovascular events. Circulation 2016;133:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 2006;55:202–208 [PubMed] [Google Scholar]
- 23.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–397 [DOI] [PubMed] [Google Scholar]
- 24.Wang CCL, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation 2016;133:2459–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koton S, Schneider AL, Rosamond WD, et al. . Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259–268 [DOI] [PubMed] [Google Scholar]
- 26.Kissela BM, Khoury J, Kleindorfer D, et al. . Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 2005;28:355–359 [DOI] [PubMed] [Google Scholar]
- 27.Selim M. Perioperative stroke. N Engl J Med 2007;356:706–713 [DOI] [PubMed] [Google Scholar]
- 28.Devereaux PJ, Yang H, Yusuf S, et al.; POISE Study Group . Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371:1839–1847 [DOI] [PubMed] [Google Scholar]
- 29.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–1980 [DOI] [PubMed] [Google Scholar]
- 31.Win TT, Davis HT, Laskey WK; American Heart Association . Mortality among patients hospitalized with heart failure and diabetes mellitus: results from the national inpatient sample 2000 to 2010. Circ Heart Fail 2016;9:e003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. ICD-9-CM TABULAR ADDENDA (FY09) [article online], 2008. Available from https://www.cdc.gov/nchs/data/icd/icdtab09add.pdf. Accessed 1 September 2017
- 33.Boyne MS, Saudek CD. Effect of insulin therapy on macrovascular risk factors in type 2 diabetes. Diabetes Care 1999;22(Suppl. 3):C45–C53 [PubMed] [Google Scholar]
- 34.Newman JD, Rockman CB, Kosiborod M, et al. . Diabetes mellitus is a coronary heart disease risk equivalent for peripheral vascular disease. Am Heart J 2017;184:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo-Ciganic W, Zgibor JC, Ruppert K, Arena VC, Stone RA. Identifying type 1 and type 2 diabetic cases using administrative data: a tree-structured model. J Diabetes Sci Technol 2011;5:486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 37.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.