Abstract
Bariatric surgery dramatically improves glycemic control, yet the underlying molecular mechanisms remain controversial because of confounding weight loss. We performed sleeve gastrectomy (SG) on obese and diabetic leptin receptor–deficient mice (db/db). One week postsurgery, mice weighed 5% less and displayed improved glycemia compared with sham-operated controls, and islets from SG mice displayed reduced expression of diabetes markers. One month postsurgery SG mice weighed more than preoperatively but remained near-euglycemic and displayed reduced hepatic lipid droplets. Pair feeding of SG and sham db/db mice showed that surgery rather than weight loss was responsible for reduced glycemia after SG. Although insulin secretion profiles from islets of sham and SG mice were indistinguishable, clamp studies revealed that SG causes a dramatic improvement in muscle and hepatic insulin sensitivity accompanied by hepatic regulation of hepatocyte nuclear factor-α and peroxisome proliferator–activated receptor-α targets. We conclude that long-term weight loss after SG requires leptin signaling. Nevertheless, SG elicits a remarkable improvement in glycemia through insulin sensitization independent of reduced feeding and weight loss.
Introduction
Bariatric surgery is currently the most effective measure to induce and sustain weight loss for obese individuals (1). Sleeve gastrectomy (SG) is a common bariatric surgery in which ∼80% of the stomach is removed, and the remaining stomach forms a sleeve that connects the esophagus to the small intestine. Both SG and very-low-calorie diets often result in glucose normalization even before dramatic weight loss (2), and the early effects of surgery have been attributed in part to reduced food consumption after surgery (3). SG was first considered to be a restrictive procedure but is now known to affect appetite and metabolic control through other mechanisms (3–10).
We sought to uncouple the effects of SG on food intake and weight loss from glucose homeostasis. The db/db mouse has a dysfunctional leptin receptor that leads to obesity and insulin resistance. SG does not lead to sustained weight loss in mice deficient in leptin signaling (11–15), but we observed improved glycemic control even after mice had surpassed their presurgical weight and when compared with pair-fed mice. The improvement was reflected mainly in increased peripheral hepatic insulin sensitivity and not in β-cell recovery and was characterized by hepatic regulation of peroxisome proliferator–activated receptor-α (PPARα) and hepatocyte nuclear factor-α (HNF4α) target genes.
Research Design and Methods
Eight- to 10-week-old db/db mice were fed normal chow and underwent SG or sham surgery (15) wherein the mouse stomach was exposed and a 12-mm clip placed by using a LIGACLIP Multiple Clip Applier horizontally across the greater curvature of the stomach. The excluded part of the stomach was excised. Sham surgeries included the abdominal incision and closure of body wall and skin. Mice were fasted the day before surgery and the day of surgery and then returned to normal chow. Lean controls were db/+ littermates of the db/db experimental group.
Clamp studies, triglycerides, cholesterol, and nonesterified fatty acid (NEFA) measurements were performed by the mouse phenotyping core of the Diabetes Research Center at the University of Pennsylvania. The joint ethics committee of the Hebrew University and Hadassah Medical Center and the institutional animal care and use committee of the University of Pennsylvania approved the animal experiments carried out in Jerusalem and Philadelphia, respectively. Values shown are mean ± SEM. See the Supplementary Data for additional information.
Results
SG Halts Weight Gain and Lowers Blood Glucose Levels in db/db Mice
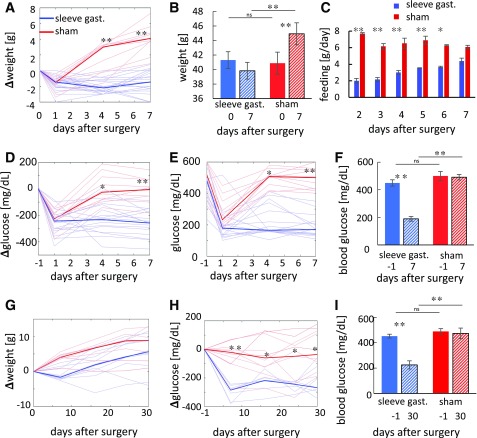
Obese diabetic db/db mice that underwent SG did not gain weight 1 week after surgery (Fig. 1A and B) but continued to gain weight afterword (Fig. 1G). Plasma glucose levels dropped in the week after surgery compared with sham-operated controls (Fig. 1B) and to preoperative levels (Fig. 1F and I). As expected, SG led to a dramatic early reduction in food consumption but reapproached the intake of sham-operated mice after 1 week (Fig. 1C), suggesting that hyperphagia caused by the absence of leptin signaling overrode the physical restriction on feeding imposed by the surgery. Both SG and sham-operated db/db mice gained weight compared with the presurgical starting point at a similar rate between days 10 and 30 after surgery (Fig. 1G). Nevertheless, blood glucose levels of SG-operated mice were improved significantly compared with sham-operated mice and to the presurgical levels, which agrees with previous results in leptin pathway–deficient rats (11) (Fig. 1E, F, H, and I).
Figure 1.
Weight and glucose levels in SG- and sham-operated mice. A–F: Weight and random glucose levels of SG- (n = 15–20) and sham-operated (n = 11) mice 1 week after surgery. Glucose and weight were measured on days 1, 4, and 7 after surgery. A: Change in weight in SG- and sham-operated mice relative to the weight at time of surgery. Median weight shown in dark colors and individual weight in light colors. B: Average weight of mice on the day of surgery and 7 days after surgery. C: Mean food consumption per mouse in the days after surgery. D: Change in nonfasting glucose levels compared with the day before surgery. Colors as in A. E: Glucose levels at the day before surgery and after surgery. Colors as in A. F: Mean glucose levels at the day before surgery and 7 days after surgery. G–I: Weight and nonfasting glucose levels of SG- and sham-operated mice 30 days after surgery. Glucose and weight were measured at days 1, 4, 7, 16, 24, and 30 (n = 8–11). G: Change in weight in SG- and sham-operated mice relative to weight at surgical time. Colors as in A. H: Change in nonfasting glucose levels compared with the day before surgery. Colors as in A. I: Mean nonfasting blood glucose levels at the day before surgery and 30 days after surgery. Error bars indicate SEM. *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni-corrected Tukey honest significant difference test. gast., gastrectomy; ns, not significant.
SG Improves Hepatic and Pancreatic Morphology One Week After Surgery
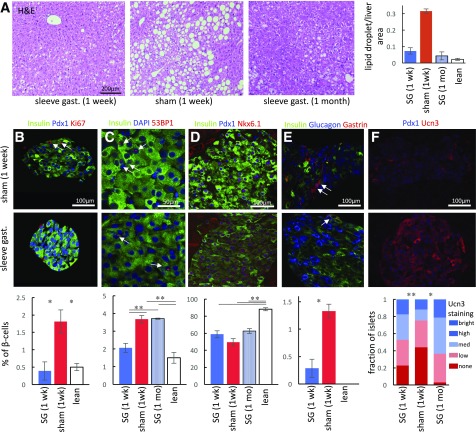
The liver of SG-operated mice displayed a dramatic decrease in the abundance of lipid droplets compared with sham-operated mice (Fig. 2A). This finding is especially striking considering that both sham-operated and SG-operated mice gained ∼5 g 7 and 30 days after surgery, respectively, indicating a weight-independent effect of SG on hepatic metabolism.
Figure 2.
Hepatic and pancreatic response to SG. A: Hematoxylin-eosin staining of liver section 1 week after SG (left) or sham surgery (middle) and 1 month after SG (right). Fractional area of lipid droplets in livers of operated mice shown on the right (n = 3–6). B–F: Immunohistochemical staining of pancreata from sham-operated (top) and SG-operated (bottom) mice. Quantification of staining shown below the images (n = 5–7 mice). B: SG reduces β-cell proliferation back to levels seen in lean mice. Nuclear Ki67 staining marking proliferating cells shown with arrows. C: SG transiently reduces DNA damage in β-cells to levels seen in lean mice. Red foci indicate nuclei experiencing DNA damage (arrows). D: SG increases nuclear colocalization of the β-cell transcription factors Pdx1 and Nkx6.1. E: SG reduces the number of gastrin-expressing cells (arrows), marking stressed endocrine cells. Gastrin is not expressed in pancreatic islets of lean mice. F: SG increases the fraction of islets expressing high levels of Ucn3, marking functional islets. All images taken under the same settings. Quantification shows pooled islets from all mice because of high intramouse variability among islets. Error bars indicate SEM. *P < 0.05, **P < 0.01 by unpaired Student t test. gast., gastrectomy.
Immunohistochemical staining of pancreatic marker proteins associated with type 2 diabetes and β-cell stress showed improvement in the β-cells of SG-operated mice primarily 1 week after surgery (Supplementary Fig. 1). Compared with db/+ lean control mice, β-cell proliferation was increased in db/db mice and reduced after SG to levels similar to those of controls (Fig. 2B). We have documented previously that diabetes leads to DNA damage in β-cells in mice and humans (16). SG reduced the frequency of 53BP1 foci that marked sites of DNA damage repair back to levels observed in lean mice. However, this improvement was transient, and 1 month after SG, the frequency of 53BP1 foci in β-cells had increased back to the levels present in sham-operated mice (Fig. 2C).
Diabetes leads to a partial loss of β-cell identity and a reduction in the expression of key β-cell transcription factors (17–19). Gastrin, expressed normally only in the fetal mouse pancreas and in diabetes (20), was detected in islets of sham-operated db/db mice but was virtually absent after SG (Fig. 2D). The frequency of Pdx1 and Nkx6.1 coexpression did not increase significantly after SG and remained much lower than in lean mice. However, a large fraction of islets (47%) restored high levels of the β-cell maturity marker Ucn3 in SG-operated mice 1 week and 1 month after surgery compared with 25% in sham-operated controls (Fig. 2F), supporting a partial return to the healthy state (21).
SG Reduces Glycemia Independent of Weight One Week After Surgery
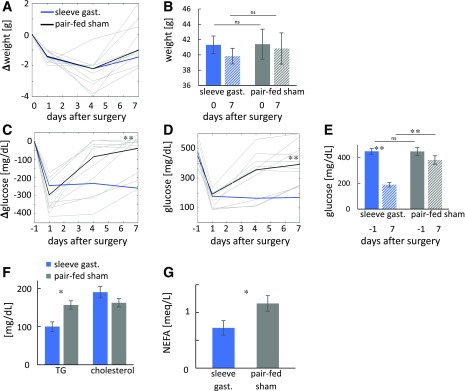
We pair fed sham-operated db/db mice to SG-operated db/db mice for the week after surgery. As intended, the two groups did not differ in weight or weight loss (Fig. 3A and B). Blood glucose levels of SG-operated mice were significantly lower compared with pair-fed mice and presurgical levels (Fig. 3C–E). Similar results were obtained in diet-induced obese SG-operated mice compared with sham-operated weight-matched controls (Supplementary Fig. 2). Serum triglyceride and NEFA levels also were improved 7 days after surgery, whereas no differences were observed in total cholesterol levels between the two groups (Fig. 3F and G).
Figure 3.
Glycemia is improved by SG- but not in pair-fed sham-operated db/db mice. A–E: Weight and nonfasting glucose levels of SG-fed (n = 15–20) and pair-fed sham-operated mice (n = 9–15) 1 week after surgery. Glucose and weight were measured at days 1, 4, and 7. Data for SG-operated mice as in Fig. 1. A: Change in weight in SG- and pair-fed sham-operated mice relative to weight at surgical time. Median weight shown in dark colors and individual weight in light colors. B: Mean weight at day of surgery and 7 days after surgery. C: Change in nonfasting glucose levels compared with the day before surgery. Colors as in A. D: Glucose levels at the day before surgery and after surgery. Colors as in A. E: Mean glucose levels at the day before surgery and 7 days after surgery. F: Mean triglycerides (TG) and total cholesterol. G: NEFAs in SG- and pair-fed sham-operated mice (n = 4–5). *P < 0.05, **P < 0.01 by two-way ANOVA with Bonferroni-corrected Tukey honest significant difference test (A–E) or Student t test (F and G). gast., gastrectomy; ns, not significant.
SG Does Not Affect Insulin Secretion Compared With Pair-Fed Mice
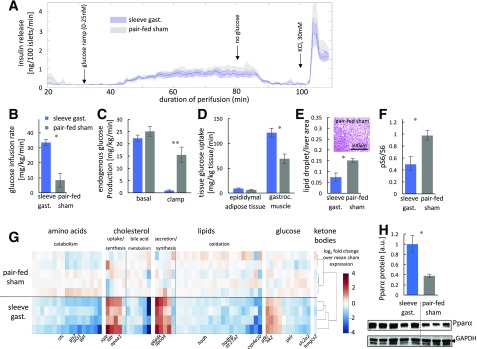
Islets of SG and pair-fed operated mice were perifused ex vivo with glucose to determine whether improved β-cell function underlies improved glycemia after SG. However, we found no difference between the two experimental groups. Islets from both groups did not display a first phase insulin secretion response but exhibited comparable second phase insulin release in response to a glucose ramp (Fig. 4A). This secretion pattern is characteristic of islets of patients and mice with type 2 diabetes, suggesting that neither SG nor pair feeding lead to islet recovery 1 week after surgery (22).
Figure 4.
Dramatic improvement in insulin sensitivity by SG- but not in pair-fed sham-operated db/db mice. A: Insulin secretion rate from isolated islets of SG- and pair-fed sham-operated mice. A glucose ramp from 0 to 25 nmol/L was applied from 30 to 80 min after perifusion. KCl was added at 100 min. There was no difference between the two groups. Shades indicate SEM (n = 4, 4). B–D: Mean glucose infusion rate (B), hepatic glucose production (C), and tissue glucose uptake (D) during a hyperinsulinemic-euglycemic clamp (n = 4, 5). E: Fractional area of lipid droplets in livers of SG- and pair-fed sham-operated mice (n = 4, 4). F: Quantification of the ratio of phosphorylated S6 (pS6) to total S6 by Western blot in SG- and pair-fed sham-operated mice (n = 4, 5). G: Heat map of log2 fold change in expression of differentially regulated genes (q < 0.05) from the livers of SG- or pair-fed sham-operated mice harvested after a hyperinsulinemic-euglycemic clamp. Expression was normalized to the mean of pair-fed sham-operated mice. Genes are grouped from amino acid metabolism (left) to cholesterol, lipid, and glucose metabolism (right). Selected individual key genes are shown at the bottom of the heat map. Unsupervised clustering accurately separated surgical groups (dendogram). H: Quantification of PPARα levels by Western blot in SG- and pair-fed sham-operated mice (n = 5, 3). Error bars indicate SEM. *P < 0.05, **P < 0.01 by unpaired Student t test. a.u., arbitrary unit; gast., gastrectomy; gastroc., gastrocnemius.
SG Increases Insulin Sensitivity Compared With Pair-Fed Mice
We applied the hyperglycemic-euglycemic clamp 5 days after surgery and found that SG-operated mice were more insulin sensitive than the pair-fed controls. Glucose infusion rate was significantly higher in SG-operated mice. Basal endogenous glucose production was equivalent in the two groups, but glucose production was inhibited by insulin infusion only in the SG-operated mice, revealing a striking difference in hepatic insulin sensitivity (Fig. 4B and C). In addition, 14C-2-deoxyglucose tracing demonstrated that skeletal muscle but not epididymal adipose tissue was more insulin sensitive in SG-operated mice (Fig. 4D). Histochemical staining of the liver showed that pair-fed mice retained a higher density of hepatic lipid droplets than SG-operated mice, suggesting a defect in hepatic lipid metabolism not normalized by weight loss alone (Fig. 4E). SG led to a reduction in the phosphorylation of S6 in the liver, indicating lower activation of mTORC1, which may contribute to the increased hepatic insulin sensitivity observed (23) (Fig. 4F and Supplementary Fig. 3).
RNA sequencing of liver tissue harvested immediately after the hyperinsulinemic clamp revealed a stronger transcriptional response to insulin signaling in SG-operated mice in agreement with the clamp results (Fig. 4F and Supplementary Table 1); genes involved in amino acid catabolism pathways were downregulated significantly in SG-operated mice compared with sham-operated mice (Supplementary Fig. 3 and Supplementary Table 2). Cholesterol production and uptake enzymes were upregulated after surgery, and genes involved in bile acid synthesis were expressed at lower levels in the SG group; however, farnesoid X receptor activation was not evident from the transcriptome data (4) (Supplementary Fig. 3 and Supplementary Table 3). Lipid synthesis and secretion pathway genes were activated after SG. Conversely, fatty acid oxidation enzymes were repressed (Supplementary Fig. 3 and Supplementary Table 4). By using ENCODE ChIP-seq data, we identified HNF4α as the transcription factor most enriched for binding differentially regulated genes in the current data (q < 10−3) (24) (Supplementary Table 5). HNF4α target genes identified from primary hepatocytes also were enriched in our data (P < 10−4) (25). HNF4α and PPARα-retinoid X receptor-α control hepatic metabolism (26,27), and both PPARα targets (P < 0.01) (28) and genes identified by retinoid X receptor-α ChIP-seq (q < 0.01) (24) were enriched in differentially regulated genes between SG and weight-matched sham control mice (Supplementary Table 6 and Supplementary Fig. 3). PPARα itself was upregulated at the protein and mRNA level (Fig. 4G, Supplementary Fig. 3, and Supplementary Table 7).
Discussion
SG reduces blood glucose levels in the short and medium term for most patients with diabetes (8,29,30). Decoupling weight loss and surgery-induced changes in diet from direct effects of the surgery on glycemic control is difficult because surgery leads to weight loss. Studies comparing nonobese patients and murine models who had lost weight after bariatric surgery or food restriction have identified weight-independent effects of surgery (10,31–33). Rodents deficient in leptin signaling are hyperphagic and have been shown by us and others to gain weight after surgery, highlighting the importance of leptin in weight loss after SG (11–15,34–36). Moreover, these models decouple surgical effects from weight loss while keeping experimental groups obese. In the current study, we show that SG lowers blood glucose levels up to 30 days after surgery, improves fatty liver and hepatic insulin sensitivity, and reduces expression of pancreatic markers associated with diabetes.
Leptin affects hepatic glucose production directly and indirectly through the central nervous system (37). The current results indicate that improvement in hepatic glucose metabolism is independent of direct leptin signaling. Nonetheless, the central nervous system likely contributes to postsurgically improved glycemia and hepatic metabolism through a neuronal gut-brain-liver axis (6), and this matter warrants further research.
Improved glycemic control can be a result of better β-cell function and increased peripheral insulin sensitivity. Although some improvement in β-cell histopathology after surgery was observed, β-cell function did not improve ex vivo. These results suggest that improvement in β-cell markers is an outcome rather than the main driver of improved glycemia. Of note, in vivo factors such as elevated incretin secretion contribute to improved glycemia (10,38).
Hepatic insulin resistance was markedly improved in clamp studies performed 5 days after surgery, and fatty liver was alleviated 7 and 30 days after SG, supporting the hypothesis that the liver drives improved glycemic control after SG. These results were obtained in pair-fed and weight-matched mice, highlighting the weight loss–independent effects of SG. Bioinformatic analysis suggests that HNF4α-PPARα signaling play a key role in the improved hepatic metabolism. Both factors affect lipid metabolism and fatty liver disease development (28,39), corresponding with the improvement in the accumulation of lipid droplets. Overall, the current results identify the liver as a driver of rapid and weight-independent improvement of glycemia after SG through the HNF4α-PPARα axis.
Supplementary Material
Article Information
Acknowledgments. The authors thank the University of Pennsylvania Diabetes Research Center for the use of the functional genomics, islet cell biology, and mouse phenotyping cores (P30-DK-19525). The authors thank Dr. Alon Chen (Weizman Institute of Science) for sharing the Ucn3 antibody, Dr. Gideon Zamir (Hadassah Medical Center), and members of our groups for critical discussions.
Funding. This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-102667 to K.H.K. D.B.-Z. is a fellow of the Zuckerman STEM Leadership Program.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A.-G., E.H, Y.D., K.H.K., and D.B.-Z. contributed to the conceptual design. S.A.-G., E.H., R.B.-H.S., A.B., H.I., A.H., J.S., S.S., Y.D., K.H.K., D.B.-Z. contributed to the methodology and investigation. Y.D., K.H.K., and D.B.-Z. contributed to the writing, supervision, and funding acquisition. D.B.-Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1028/-/DC1.
See accompanying article, p. 1043.
References
- 1.Rubino F, Nathan DM, Eckel RH, et al.; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–877 [DOI] [PubMed] [Google Scholar]
- 2.Perry RJ, Peng L, Cline GW, et al. Mechanisms by which a very-low-calorie diet reverses hyperglycemia in a rat model of type 2 diabetes. Cell Metab 2018;27:210–217.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes 2013;62:3027–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucharczyk J, Nestoridi E, Kvas S, Andrews R, Stylopoulos N. Probing the mechanisms of the metabolic effects of weight loss surgery in humans using a novel mouse model system. J Surg Res 2013;179:e91–e98 [DOI] [PubMed] [Google Scholar]
- 6.Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 2012;18:950–955 [DOI] [PubMed] [Google Scholar]
- 7.Stefater MA, Pérez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 2010;138:2426– 2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aminian A, Brethauer SA, Andalib A, et al. Can sleeve gastrectomy “cure” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg 2016;264:674–681 [DOI] [PubMed] [Google Scholar]
- 9.Cummings DE. Metabolic surgery for type 2 diabetes. Nat Med 2012;18:656–658 [DOI] [PubMed] [Google Scholar]
- 10.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 2011;141:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lifante JC, Milone L, Korner J, Kopsombut G, Sebastian M, Inabnet WB III. Sleeve gastrectomy improves glucose homeostasis in Zucker diabetic fatty rats. Obes Surg 2012;22:1110–1116 [DOI] [PubMed] [Google Scholar]
- 12.Hao Z, Münzberg H, Rezai-Zadeh K, et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes 2015;39:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokadem M, Zechner JF, Uchida A, Aguirre V. Leptin is required for glucose homeostasis after Roux-en-Y gastric bypass in mice. PLoS One 2015;10:e0139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K, Zhou X, Quach G, et al. Effect of sleeve gastrectomy plus side-to-side jejunoileal anastomosis for type 2 diabetes control in an obese rat model. Obes Surg 2016;26:797–804 [DOI] [PubMed] [Google Scholar]
- 15.Schlager A, Khalaileh A, Mintz Y, et al. A mouse model for sleeve gastrectomy: applications for diabetes research. Microsurgery 2011;31:66–71 [DOI] [PubMed] [Google Scholar]
- 16.Tornovsky-Babeay S, Dadon D, Ziv O, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in β cells. Cell Metab 2014;19:109–121 [DOI] [PubMed] [Google Scholar]
- 17.Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013;123:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonas JC, Sharma A, Hasenkamp W, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem 1999;274:14112–14121 [DOI] [PubMed] [Google Scholar]
- 19.Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun 2014;5:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahan T, Ziv O, Horwitz E, et al. Pancreatic β-cells express the fetal islet hormone gastrin in rodent and human diabetes. Diabetes 2017;66:426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blum B, Roose AN, Barrandon O, et al. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 2014;3:e02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest 2011;121:2118–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009;122:3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auerbach RK, Chen B, Butte AJ. Relating genes to function: identifying enriched transcription factors using the ENCODE ChIP-Seq significance tool. Bioinformatics 2013;29:1922–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odom DT, Zizlsperger N, Gordon DB, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science 2004;303:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalez FJ, Talianidis I. Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol Cell Biol 2010;30:565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras AV, Rangel-Escareño C, Torres N, et al. PPARα via HNF4α regulates the expression of genes encoding hepatic amino acid catabolizing enzymes to maintain metabolic homeostasis. Genes Nutr 2015;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakhshandehroo M, Knoch B, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res 2010;2010:612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011;254:410–420, discussion 420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219–234 [DOI] [PubMed] [Google Scholar]
- 31.Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev 2012;33:595–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 2010;61:393–411 [DOI] [PubMed] [Google Scholar]
- 33.Evers SS, Sandoval DA, Seeley RJ. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev Physiol 2017;79:313–334 [DOI] [PubMed] [Google Scholar]
- 34.Arble DM, Sandoval DA, Turek FW, Woods SC, Seeley RJ. Metabolic effects of bariatric surgery in mouse models of circadian disruption. Int J Obes 2015;39:1310–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson-Pérez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 2013;62:2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao Z, Mumphrey MB, Townsend RL, et al. Body composition, food intake, and energy expenditure in a murine model of Roux-en-Y gastric bypass surgery. Obes Surg 2016;26:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev 2011;91:389–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004;240:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuo S, Ogawa M, Muckenthaler MU, et al. Hepatocyte nuclear factor 4α controls iron metabolism and regulates transferrin receptor 2 in mouse liver. J Biol Chem 2015;290:30855–30865 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.