Given the seemingly unstoppable diabetes crisis, new efficient therapies for the prevention and treatment of this devastating and costly disease are sorely needed, and bariatric surgeries intended for weight loss seem to fill this gap. In addition to weight loss, there is remarkable short- to medium-term diabetes remission, particularly after Roux-en-Y gastric bypass and sleeve gastrectomy (1). However, broad longer-term effectiveness and complete cure are more elusive (2,3). It is also clear that bariatric surgery is not the answer for the staggering numbers of people with obesity and type 2 diabetes on either a national or global scale. This, in turn, has stimulated intense research efforts to find out the molecular and behavioral mechanisms behind the success of bariatric surgeries so that they can eventually be exploited with improved pharmacological and lifestyle/behavioral strategies but without the surgery.
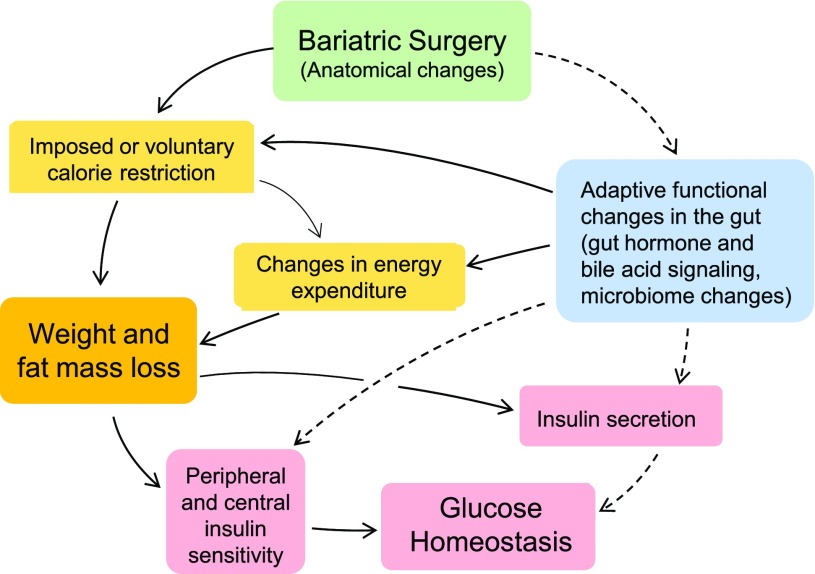
The question of whether diabetes remission is mainly due to weight loss or weight loss–independent effects of the surgery is fundamental, as it guides experimental approaches and may lead us to very different sets of key underlying pathways and molecular mechanisms (Fig. 1). If remission is mostly due to changes in food intake and energy expenditure resulting in weight loss, brain mechanisms involving the control of appetite and energy balance are key. If it involves mainly weigh loss–independent effects, mechanisms of insulin secretion and insulin action on various organs should attract greater attention. Given the severe dietary restrictions and the heavy dietary counseling before and after surgery, as well as difficulties in measuring food intake, clinical studies are not ideally suited to answer this question. A number of bariatric surgery models in both rats and mice have been established, allowing well-controlled and mechanistic studies.
Figure 1.
Schematic diagram showing the major factors and pathways involved in the beneficial effects of bariatric surgeries on body weight and glucose homeostasis, with emphasis on caloric restriction and weight loss–dependent (solid lines) and –independent (broken lines) factors.
In this issue of Diabetes, Abu-Gazala et al. (4) address this fundamental question. They used hyperglycemic leptin receptor–deficient db/db mice to demonstrate body weight–independent improvements of glycemia after sleeve gastrectomy. They first show that even though body weight quickly recovered after sleeve gastrectomy to above presurgical levels, the high blood glucose levels (>400 mg/dL) of sham-operated obese db/db mice decreased and remained at much lower levels (∼200 mg/dL) in db/db mice at 7 and 30 days after surgery. Furthermore, pair feeding mice with sham surgery to the reduced food intake after sleeve gastrectomy did not result in the same reduction of blood glucose, ruling out the early hypocaloric state as the main driver of glycemic improvement in this mouse model. These findings are in support of earlier reports of weight loss–independent effects of sleeve gastrectomy in obese diabetic rodent models (5,6) as well as conclusions reached from clinical studies with both sleeve gastrectomy and gastric bypass surgery.
Despite these clear and important findings, conclusions require careful consideration. First, these new findings may mostly apply to this special leptin-deficient diabetic mouse model but not to the more common prediabetic state that is seen in diet-induced obese rodents. Abu-Gazala et al. (Supplementary Data in ref. 4) addressed this important point by demonstrating a slightly higher glucose-lowering effect in high-fat diet–induced obese mice 7 days after sleeve gastrectomy compared with that obtained by calorie restriction in weight-matched mice with sham surgery. However, it should be noted that an impressive list of studies in humans (7–10) and rodents (11,12) find that calorie restriction–induced weight loss (to match body weight of surgical subjects) resulted in similar early improvements in glycemic control and insulin sensitivity. Also, longer-term studies with a mouse model of Roux-en-Y gastric bypass and utilizing weight-matched controls find no difference in the effectiveness to improve insulin levels and glycemic control (13). Semistarvation, as with very low-calorie diets, can significantly lower blood glucose and increase hepatic insulin sensitivity before any weight loss occurs (14,15), and as little as 5% weight loss sustained over 4 years was highly beneficial for diabetes remission in the Diabetes Prevention Program (DPP) (16). Thus, there is no doubt that dietary restriction and weight loss are major drivers of improvements in glucose homeostasis after bariatric surgeries.
Second and more importantly, although the study by Abu-Gazala et al. (4) points to changes in hepatic insulin sensitivity, we still do not know the mechanisms of weight loss–independent improvements in glycemic control after bariatric surgeries. Specifically, we do not understand the critical chain of events emanating from the anatomical changes made to the gastrointestinal tract that eventually lead to improved insulin sensitivity (Fig. 1). We may have many ideas regarding the involvement of gut hormones (17), bile acid signaling (18,19), the gut microbiome (20), and intestinal tissue reprogramming (21), but few of these potential mechanisms have been directly tested and confirmed so far.
Article Information
Funding. The authors’ research was partially supported by National Institutes of Health grants DK047348 (H.-R.B.), U01 DK114156 (J.P.K.), and DK092587 (H.M.)
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1079.
References
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminian A, Brethauer SA, Andalib A, et al. Can sleeve gastrectomy “cure” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg 2016;264:674–681 [DOI] [PubMed] [Google Scholar]
- 3.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219–234 [DOI] [PubMed] [Google Scholar]
- 4.Abu-Gazala S, Horwitz E, Schyr RB-H et al. Sleeve gastrectomy improves glycemia independent of weight loss by restoring hepatic insulin sensitivity. Diabetes 2018;67:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 2011;141:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lifante JC, Milone L, Korner J, Kopsombut G, Sebastian M, Inabnet WB 3rd. Sleeve gastrectomy improves glucose homeostasis in Zucker diabetic fatty rats. Obes Surg 2012;22:1110–1116 [DOI] [PubMed] [Google Scholar]
- 7.Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes 2013;62:3027–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care 2013;36:2741–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt JB, Pedersen SD, Gregersen NT, et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: a randomized controlled clinical trial. Int J Obes 2016;40:281–290 [DOI] [PubMed] [Google Scholar]
- 10.Steven S, Hollingsworth KG, Small PK, et al. Calorie restriction and not glucagon-like peptide-1 explains the acute improvement in glucose control after gastric bypass in type 2 diabetes. Diabet Med 2016;33:1723–1731 [DOI] [PubMed] [Google Scholar]
- 11.Hao Z, Mumphrey MB, Townsend RL, et al. Body composition, food intake, and energy expenditure in a murine model of Roux-en-Y gastric bypass surgery. Obes Surg 2016;26:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 2013;3:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Z, Münzberg H, Rezai-Zadeh K, et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes 2015;39:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steven S, Hollingsworth KG, Al-Mrabeh A, et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 2016;39:808–815 [DOI] [PubMed] [Google Scholar]
- 15.Perry RJ, Peng L, Cline GW, et al. Mechanisms by which a very-low-calorie diet reverses hyperglycemia in a rat model of type 2 diabetes. Cell Metab 2018;27:210–217.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007;246:780–785 [DOI] [PubMed] [Google Scholar]
- 18.Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun 2015;6:7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013;341:406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]