Abstract
Background
The expression of aldehyde dehydrogenase 1A1 (ALDH1A1) is increased in several human tumors, including colorectal carcinoma (CRC). The aim of this study was to compare the expression ALDH1A1 in CRC tumor tissue compared with non-tumor adjacent tissue (NAT), using immunohistochemistry (IHC), and to determine whether the expression of the ALDH1A1 protein was associated with prognostic factors in CRC.
Material/Methods
Formalin-fixed paraffin-embedded (FFPE) tissue from 424 patients diagnosed with CRC, and 196 matched NATs were used to prepare tissue microarrays (TMAs). IHC was performed using an immunoperoxidase method with a primary polyclonal rabbit anti-ALDH1A1 antibody. The IHC scores by light microscopy were the staining intensity (scored from 0–3) multiplied by the percentage area of positive immunostaining within the visual field (scored from 0–4). Associations between tumor expression levels of ALDH1A1 and patient clinicopathological characteristics, including tumor grade, size, and TNM stage at surgery were analyzed.
Results
ALDH1A1 protein expression was significantly increased in CRC tissues compared with matched NATs. In patients with CRC, increased expression of the ALDH1A1 protein was significantly associated with the presence of lymph node metastasis: 64.28% in N0 cases; 75.49% in N1 cases; and 82.14% in N2 cases, (P=0.002). Univariate and multivariate analysis showed that ALDH1A1 expression was an independent prognostic marker for CRC (P<0.001).
Conclusions
Using IHC, the expression of the ALDH1A1 protein in CRC tissues was significantly associated with the presence of lymph node metastases and might be a potential prognostic marker in patients with CRC.
MeSH Keywords: Aldehyde Dehydrogenase, Colorectal Neoplasms, Lymph Nodes, Tissue Array Analysis
Background
Colorectal cancer (CRC) is a common and malignant tumor of the gastrointestinal tract. In China, CRC is among the five most common cancers and is the fifth leading cause of cancer-related death [1]. The main treatment for CRC is surgery combined with systemic or local chemotherapy and radiotherapy. However, tumor recurrence in patients with CRC is common. The key to reducing mortality and improving the survival rate for patients with CRC is early detection, early diagnosis, and early treatment. Therefore, molecular studies of the expression of novel genes, and their protein products, which are associated with CRC may lead to improved diagnostic and prognostic biomarkers, or provide novel targets for treatment.
Previous studies have shown that aldehyde dehydrogenase (ALDH) can serve as a molecular marker for normal or cancer stem cell [2]. Further studies have shown that an isoform of ALDH, aldehyde dehydrogenase 1A1 (ALDH1A1), shows increased expression in bone marrow-derived hematopoietic progenitor cells and neural stem cells, and could be the key member of the ALDH family that can be used to identify stem cell populations [3]. ALDH1A1 is produced mainly by human liver tissue. Retinoic acid, a main product of ALDH1A1, can enter the nucleus to modulate gene transcription, thereby regulating cell proliferation and differentiation, and might also regulate cancer stem cell proliferation and differentiation [4].
Previously published studies have shown that detection of expression of the ALDH1A1 protein can be used as a cancer stem cell marker for CRC, breast cancer, lung cancer, and other malignant tumors [2,5–7]. However, the clinicopathological significance of expression of the ALDH1A1 protein in human cancer remains unclear.
Therefore, the aim of this study was to evaluate the expression of the ALDH1A1 protein in human CRC tissues, and compare the expression with non-tumor adjacent tissue (NAT), also known as normal tissue adjacent to the tumor, using immunohistochemistry (IHC), and to determine whether ALDH1A1 might be a potential prognostic biomarker in CRC. The study used tissue samples from a cohort of 424 patients with CRC and 196 matched NATs, prepared as tissue microarrays (TMAs). The study also aimed to investigate whether there were any associations between ALDH1A1 expression and patient clinicopathologic features and with any independent prognostic factors affecting long-term patient survival in CRC.
Material and Methods
Ethical approval and patient consents
This study was approved by the Ethics Committee of The First Affiliated Hospital of Jinzhou Medical University, China. Informed consent was obtained from all patients who participated in the study.
Patients and tissue samples studied
Colorectal tumor tissue samples were obtained from surgical resection specimens removed during radical surgery for the treatment of colorectal cancer (CRC), including regional lymph node dissection. All patients included in the study were general surgery patients who were treated at The First Affiliated Hospital of Jinzhou Medical University, China, from June 2008 to November 2011, with the approval of the Science and Education Department of the hospital and the consent of the Director of the Pathology Department.
Experimental tissue samples were selected from formalin-fixed paraffin-embedded (FFPE) specimens from 424 patients who were diagnosed with CRC, with 196 cases of non-tumor adjacent tissue (NAT), which were taken more than 2 cm beyond the tumor margin and were selected as study controls. All specimens and tissue sections were examined by an experienced histopathologist who confirmed the diagnosis of CRC in the samples and confirmed that the NAT tissue samples were tumor-free.
None of the patients included in the study had undergone chemotherapy or radiotherapy before surgical resection. Clinicopathological data collected for each patient in the study included age, gender, tumor location, tumor grade, tumor size, and tumor, node, metastasis (TNM) stage at surgery. The TNM staging was undertaken according to the American Joint Committee on Cancer (AJCC) staging manual (eighth edition). Patients who were included in the study had postoperative follow-up, and the details and dates of any postoperative recurrence of CRC, morbidity, or mortality were recorded.
Preparation of tissue microarrays (TMAs)
Tumor sections were screened by two pathologists and paired tissue blocks were selected, as previously described by Hedberg et al. [8]. A manual tissue array instrument was used to arrange tumor tissues and matched NAT control samples. The tissue microarrays consisted of 424 primary CRC tissue samples and 196 matched NAT samples.
Immunohistochemistry for aldehyde dehydrogenase 1A1 (ALDH1A1)
Tissue sections were obtained from the prepared TMAs. The expression of aldehyde dehydrogenase 1A1 (ALDH1A1) protein was detected using immunohistochemistry. Tissue sections were deparaffinized and rehydrated using xylene and alcohols, and incubated in 3% diluted hydrogen peroxide to block endogenous peroxidase. Antigen retrieval was performed using an autoclave method, followed by incubation with the primary antibody overnight at 4°C in a high humidity cabinet. The primary rabbit anti-ALDH1A1 polyclonal antibody (clone No. ab52492) (Abcam, Cambridge MA, USA) was used at a dilution of 1: 400 in phosphate-buffered saline (PBS). The secondary horseradish peroxidase (HRP)-conjugated antibody (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) was used according to the manufacturer’s instructions. Negative controls used included replacing the primary antibodies with PBS. After washing with PBS, tissues were incubated for 30 minutes at room temperature (37°C). The chromogen used was 3,3′-diaminobenzidine (DAB) (brown) (Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China). Tissue sections were lightly counterstained with hematoxylin, washed, and mounted with glass coverslips, ready for evaluation using light microscopy and photomicroscopy.
Evaluation of ALDH1A1 protein expression by IHC staining
The expression of the ALDH1A1 protein in colorectal tissue sections was scored using a visual coring system. The IHC scores were obtained by light microscopy as the staining intensity (scored from 0–3) multiplied by the percentage area of positive immunostaining within the visual field (scored from 0–4). Two experienced pathologists independently scored the immunostaining light microscopy. A third pathologist scored the slide if the initial two scores did not match. Immunostaining for the intensity of ALDH1A1 protein expression was scored as: 0 (no staining); 1 (weak staining); 2 (moderate staining); or 3 (strong staining). The percentage area of positive immunostaining, within the visual field, were scored as: 0 (<5%); 1 (5–25%); 2 (26–50%); 3 (51–75%); or 4 (>75%). The cut-off value for high versus low expression of the ALDH1A1 protein was determined using receiver-operating characteristic (ROC) curve analysis and SPSS statistical software, defining a final immunostaining score of >3.5 as ‘high’ ALDH1A1 protein expression.
Statistical analysis
All data were analyzed using SPSS version 20.0 software (Chicago, IL, USA). The Mann-Whitney U test was used for comparisons between the two groups, and the Kruskal-Wallis test was used to compare multiple groups. ROC curves were used to estimate the diagnostic accuracy of the tumor marker. Survival curves were assessed using the Kaplan-Meier method. By constructing a Cox regression model, survival analysis was performed to calculate hazard ratios (HRs) and the associated 95% confidence interval (CI). Multivariate survival analysis was performed on the variables shown to be significant in the univariate analysis to identify the most significant independent prognostic factors for predicting survival. A P-value of <0.05 was considered to be statistically significant.
Results
Expression levels of aldehyde dehydrogenase 1A1 (ALDH1A1) in colorectal cancer (CRC) tissues and non-tumor adjacent tissue (NAT)
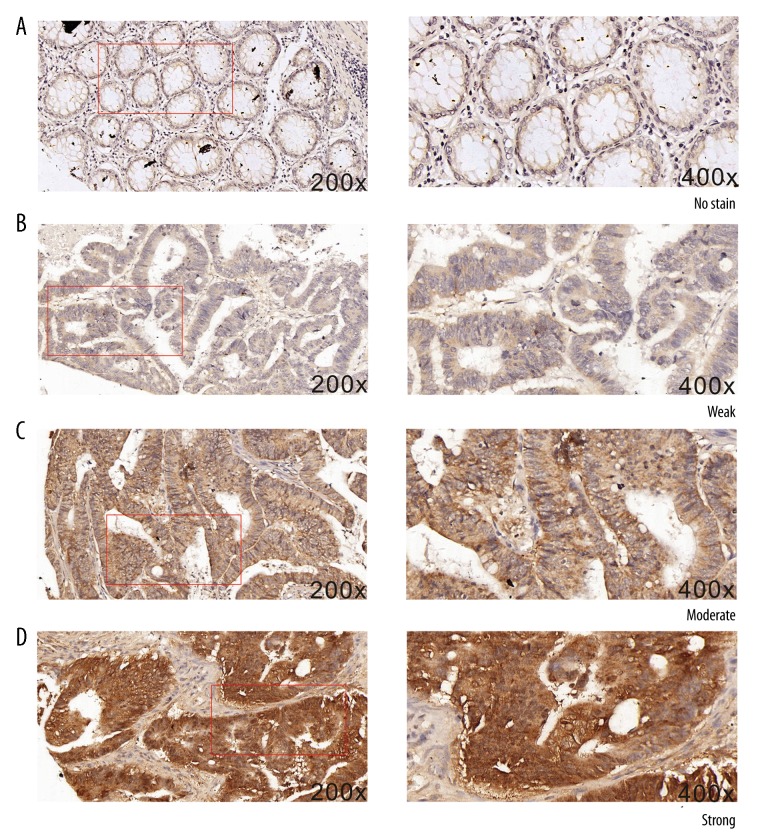
Immunohistochemistry (IHC) analysis of the tissue microarrays (TMAs) showed that the expression of the aldehyde dehydrogenase 1A1 (ALDH1A1) protein was significantly increased in tissues containing colorectal cancer (CRC) when compared with non-tumor adjacent tissue (NAT). As shown in Figure 1, cytoplasmic immunostaining of ALDH1A1 protein with various intensities was observed, from negative in normal tissues to strongly positive in CRC tumor tissues. Table 1 shows that the expression of the ALDH1A1 protein, using the light microscopic scoring system, was significantly increased in CRC tumor tissue samples compared with NAT (P <0.001).
Figure 1.
Photomicrographs showing the immunohistochemical expression of the aldehyde dehydrogenase 1A1 (ALDH1A1) protein in colorectal cancer (CRC) tissues and non-tumor adjacent tissue (NAT). (A) Negative immunostaining for aldehyde dehydrogenase 1A1 (ALDH1A1) protein expression is shown in normal colorectal tissue, or non-tumor adjacent tissue (NAT). (Magnification ×200). (B) Negative immunostaining for ALDH1A1 protein expression is shown in normal colorectal tissue or NAT. (Magnification ×400). (C) Weak immunostaining for ALDH1A1 protein expression is shown in colorectal carcinoma (CRC) tissue. (Magnification ×200). (D) Weak immunostaining for ALDH1A1 protein expression is shown in CRC tissue. (Magnification ×400). (E) Moderate immunostaining for ALDH1A1 protein expression is shown in CRC tissue. (Magnification ×200). (F) Moderate immunostaining for ALDH1A1 protein expression is shown in CRC tissue. (Magnification ×400). (G) Strong immunostaining for ALDH1A1 protein expression is shown in CRC tissue. (Magnification ×200). (H) Strong immunostaining for ALDH1A1 protein expression is shown in CRC tissue. (Magnification ×400).
Table 1.
The expression of of aldehyde dehydrogenase 1A1 (ALDH1A1) in colorectal carcinoma (CRC) tissue compared with non-tumor adjacent tissue (NAT), using immunohistochemistry (IHC).
| Variable | N | Mean | SD | P-value |
|---|---|---|---|---|
| CRC tissues | 424 | 4.87 | 2.555 | <0.001* |
| NATs | 196 | 2.40 | 1.855 |
Mean expression score (staining intensity multiplied by the percentage of tumor-positive area) for ALDH1A1. Standard deviation (SD).
P-value <0.05.
Correlation of between clinicopathological variables in patients with CRC and the expression of the ALDH1A1 protein in tumor tissues
Following IHC immunostaining and scoring of staining intensity, the association between ALDH1A1 protein expression and the clinicopathological features of patients with CRC who provided tumor tissues are presented in Table 2. In patients with CRC, increased expression of the ALDH1A1 protein was significantly associated with the presence of lymph node metastasis: 64.28% in N0 cases; 75.49% in N1 cases; and 82.14% in N2 cases, (P=0.002). No statistically significant correlation was found between increased ALDH1A1 protein expression and other clinicopathologic factors (P>0.05).
Table 2.
Correlation between clinicopathologic variables and expression of aldehyde dehydrogenase 1A1 (ALDH1A1) protein in colorectal carcinoma (CRC) tumor tissue using immunohistochemistry (IHC).
| Characteristic | Frequency | ALDH1A1 expression | Mean ±SD P-value | |
|---|---|---|---|---|
| Low | High | |||
| Total | 424 | 130 | 294 | 5.098±2.5122 |
| Location | 0.409 | |||
| Left colon | 91 | 24 | 67 | 5.033±2.1779 |
| Right colon | 110 | 39 | 71 | 4.591±2.3169 |
| Rectum | 223 | 67 | 156 | 5.374±2.6947 |
| Gender | 0.799 | |||
| Male | 242 | 73 | 169 | 5.205±2.5439 |
| Female | 182 | 57 | 125 | 4.956±2.4693 |
| Age | 0.818 | |||
| Min–Max: 26–92 | ||||
| Median: 62.5 | ||||
| Differentiation | 0.825 | |||
| Well | 11 | 3 | 8 | 5.000±3.0249 |
| Moderate | 359 | 110 | 249 | 5.085±2.4499 |
| Poor | 54 | 17 | 37 | 5.204±2.8443 |
| Tumor size | 0.075 | |||
| Min–Max: 1–15 | ||||
| Median: 4.5 | ||||
| T | 0.584 | |||
| 1 | 14 | 5 | 9 | 4.536±2.2657 |
| 2 | 43 | 16 | 27 | 5.116±2.6160 |
| 3 | 42 | 11 | 31 | 5.631±2.6871 |
| 4 | 325 | 98 | 227 | 5.051±2.4860 |
| N | 0.002* | |||
| 0 | 266 | 95 | 171 | 5.086±2.5657 |
| 1 | 102 | 25 | 77 | 5.069±2.4546 |
| 2 | 56 | 10 | 46 | 5.205±2.3965 |
| M | 0.387 | |||
| 0 | 383 | 115 | 268 | 5.094±2.4772 |
| 1 | 41 | 15 | 26 | 5.134±2.8527 |
| TNM stage | 0.080 | |||
| 1 | 48 | 18 | 30 | 5.063±2.6410 |
| 2 | 203 | 69 | 134 | 5.089±2.5206 |
| 3 | 132 | 28 | 104 | 5.080±2.3483 |
| 4 | 41 | 15 | 26 | 5.244±2.8942 |
Mean expression score (staining intensity multiplied by the percentage of tumor-positive area) for ALDH1A1. Standard deviation (SD).
P-value <0.05.
Univariate and multivariate survival analysis associated with expression levels of the ALDH1A1 protein in tumor tissue from patients with CRC
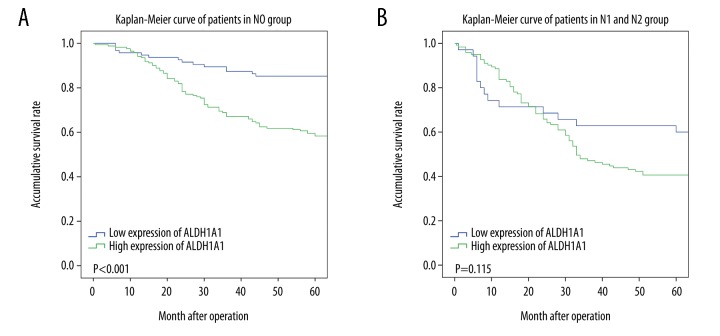
Patients with CRC with high expression of the ALDH1A1 protein in the CRC tumor tissue had a significantly reduced overall survival (OS) (P<0.001) (Figure 2). Univariate survival analysis assessed the prognostic value of clinicopathological features and ALDH1A1 protein expression (Table 3). Following univariate analysis, increased expression of the ALDH1A1 protein in CRC tumor tissue correlated with the prognostic factors of tumor differentiation (grade), T status, N status, and M status. Multivariate analysis further confirmed that expression of the ALDH1A1 protein by CRC tumor tissue was an independent prognostic marker for patients with CRC (P<0.001) (Table 3). ALDH1A1 expression levels were not found to differ significantly based on patient age, gender, tumor location, or tumor size. Further analysis between subgroups was undertaken to determine whether ALDH1A1 expression was a prognostic marker for subgroups of the patients with CRC (Figure 3). Patients stratified according to lymph node metastasis status (N status) at surgery showed that high ALDH1A1 expression was associated with poor prognosis in patients with no lymph node metastasis (N0). However, there was no significant correlation between ALDH1A1 protein expression and prognosis for patients presenting with lymph node metastasis (N1 and N2).
Figure 2.
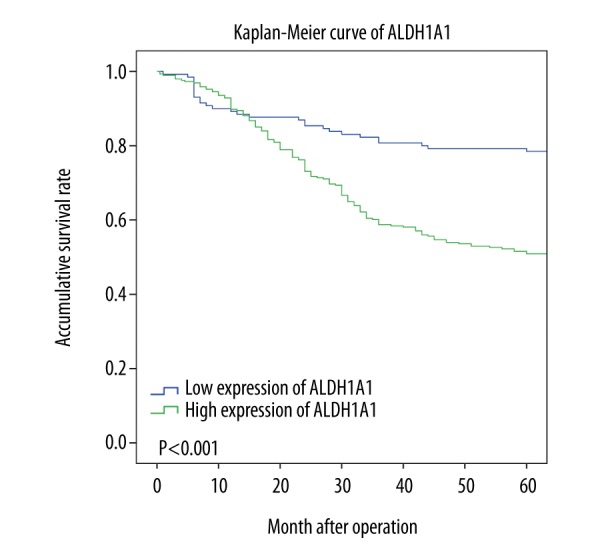
Kaplan-Meier overall survival (OS) curves, stratified according to the status of aldehyde dehydrogenase 1A1 (ALDH1A1) protein expression.
Table 3.
Univariate and multivariate analysis of prognostic variables in patients with colorectal carcinoma (CRC).
| Characteristic | Univariate Cox analysis | Multivariate Cox analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Location | 0.409 | |||||
| Right colon | 1 | 0.598 | ||||
| Left colon | 0.847 | 0.555–1.292 | 0.441 | |||
| Rectum | 0.844 | 0.599–1.190 | 0.333 | |||
| Gender | 1.274 | 0.945–1.717 | 0.112 | |||
| Age | 1.010 | 0.997–1.023 | 0.131 | |||
| Differentiation | 0.006* | |||||
| Well | 1 | 0.003 | 1 | 0.116 | ||
| Moderate | 0.951 | 0.390–2.321 | 0.913 | 0.838 | 0.339–2.073 | 0.702 |
| Poor | 1.853 | 0.723–4.750 | 0.199 | 1.276 | 0.485–3.356 | 0.622 |
| Tumor size | 0.986 | 0.914–1.063 | 0.713 | |||
| T | 0.003* | |||||
| 1 | 1 | 0.009 | 1 | 0.100 | ||
| 2 | 0.838 | 0.263–2.673 | 0.766 | 0.953 | 0.297–3.065 | 0.936 |
| 3 | 1.284 | 0.423–3.900 | 0.659 | 0.984 | 0.318–3.043 | 0.978 |
| 4 | 2.068 | 0.766–5.582 | 0.151 | 1.646 | 0.601–4.507 | 0.332 |
| N | <0.001* | |||||
| 0 | 1 | <0.001 | 1 | 0.078 | ||
| 1 | 1.791 | 1.279–2.509 | 0.001 | 1.481 | 1.042–2.104 | 0.028 |
| 2 | 2.395 | 1.622–3.534 | <0.001 | 1.320 | 0.857–2.031 | 0.207 |
| M (M1 vs. M0) | 5.117 | 3.519–7.440 | <0.001* | 4.378 | 2.958–6.478 | <0.001* |
| ALDH1A1 (high vs. low) | 2.696 | 1.820–3.993 | <0.001* | 2.577 | 1.727–3.846 | <0.001* |
HR – hazard ratio; CI – confidence interval; ALDH1A1 – aldehyde dehydrogenase 1A1; TNM – tumor, node, metastasis staging.
Indicates a P-value <0.05.
Age is a continuous variable. Tumor size is a continuous variable.
Figure 3.
Kaplan-Meier overall survival (OS) curves, stratified according to lymph node metastasis (N0 and N1–N2).
Discussion
Patient survival in colorectal cancer (CRC) requires early diagnosis, which depends on finding more sensitive and reliable diagnostic and prognostic biomarkers. Therefore, the aim of this study was to evaluate the expression of aldehyde dehydrogenase 1A1 (ALDH1A1), in CRC tumor tissue compared with non-tumor adjacent tissue (NAT), using immunohistochemistry (IHC). The findings of this study showed that the expression of the ALDH1A1 protein might have potential as a biomarker for predicting lymph node metastasis and prognosis in patients with a histological diagnosis of CRC.
The findings of the present study are supported by a study published in 2014, by Xu and colleagues, who studied the expression of ALDH1A1 in CRC using IHC, real-time polymerase chain reaction (PCR), and Western blot [9]. This previously published study showed that the RA/C, defined as a ratio of ALDH1A1 expression levels in adjacent normal tissue to ALDH1A1 expression levels in tumor tissue, reflected tumor invasion and metastasis, as patients with CRC tumors with RA/C <1 had significantly increased tumor invasion and metastasis compared with patients with RA/C ≥1 [9]. The findings of this study indicated that RA/C was a potential predictive biomarker for prognosis in patients with CRC [9].
However, other studies have found that the ALDH1A1 protein showed heterogeneous expression patterns using IHC staining of CRC tissues. Huang et al. found that the expression of ALDH1A1 was negative in some CRC tissues and positive in 29.3% of CRC tissues and that the cytoplasmic expression pattern of ALDH1A1 was highly variable, ranging from no staining to strong staining [10]. In this previously published study, approximately 20–50% of tumor cells were positive for any level of cytoplasmic ALDH1A1 expression [10]. In another large study, Deng et al. examined 1,287 CRC cases for ALDH1A1 expression by IHC, and showed that 987 (76.7%) of the tumor tissues were negative for cytoplasmic ALDH1A1 expression, and overexpression of ALDH1A1 (>25% positive tumor cells) was observed in the remaining 300 cases (23.2%) [11].
Some previously published studies have shown that a small subset of patients with CRC tissues that showed nuclear expression of ALDH1A1 was associated with a significantly reduced OS when compared with patients with CRC tissues with cytoplasmic staining [5,12]. In the present study, no nuclear ALDH1A1 protein expression was found, which is a finding supported by other studies, including that of Xu et al. [9]. This finding of the different intracellular localization of immunostaining may be due to differences in primary antibodies used, differences in tissue fixation and antigen retrieval methods or from the limited number of samples used in the present study. Therefore larger and more detailed further studies are required on the mechanism of expression of the ALDH1A1 protein in CRC.
Previously published studied have shown that ALDH1A1 overexpression was associated with poor clinical outcome in several types of tumor [2,5–7,11]. While the increased expression of the ALDH1A1 protein in CRC tissues has previously been shown to relate to clinicopathologic parameters such as tumor invasion depth, the prognostic value of ALDH1A1 expression remains controversial [11,13,14]. Lugli et al. studied tissues from 1,420 CRC tumors and showed that ALDH1A1 expression in CRC tumor tissues was not related to a significant difference in OS [15]. In the previously published study by Langan et al., increased expression of the ALDH1A1 protein showed a trend towards decreased patient survival, but this relationship did not show statistical significance (P=0.16) [13].
In this study, the expression level of the ALDH1A1 protein in tumor tissue was also significantly associated with the presence of lymph nodes metastasis in patients with CRC. Lymph node status is an important determinant of patient prognosis and survival. This relationship supports the view that high ALDH1A1 protein expression in CRC might be related to the development and progression of CRC. Further stratified analysis showed that there was a significant correlation between ALDH1A1 overexpression and shorter overall survival time for patients with CRC with no lymph node metastasis. AlDH1A1 was also found to be significantly correlated with tumor lymph node metastasis, indicating that this may be a critical predictor of metastasis in patients with CRC. Accurate tumor lymph node metastatic status is essential for successful surgical outcome, and so tumor tissue ALDH1A1 levels may have important clinical implications for selecting the optimal method of surgery for patients with CRC.
The Wnt/β-catenin signaling pathway plays a vital role in tumor invasion, metastasis and differentiation, most notably in CRC [16–18]. Following Wnt activation, β-catenin translocates to the nucleus and works with coactivators in the T-cell and lymphoid enhancer (TCF-LEF) family to transcriptionally target genes [16,18]. β-catenin is used as a supplemental biomarker in stratifying expression profiles of genes such as CD44, CD133, CD166, and ALDH1A1 in patients with CRC [19–22]. Xu et al. found that significant nuclear translocation of β-catenin was present in CRC tissues with high ALDH1A1 protein expression [9]. ALDH1A1 is known to synthesize retinoic acid and regulate its signaling [32]. Cross-talk between the Wnt/β-catenin signaling pathway and the retinoic acid signaling pathway has been previously reported [24–30]. Therefore, it may be assumed that the expression of the ALDH1A1 protein might affect tumor invasion and metastasis via the Wnt/β-catenin signaling pathway to influence the prognosis of CRC. However, this hypothesis requires evaluation by future studies.
This study had several limitations. The cut-off values used to define a high level of ALDH1A1 protein expression using IHC, when light microscopy rather than image analysis methods are used, vary between studies and may contribute to the observed differences in the findings of the present study and those of other studies. The evaluation of immunostaining and defining and detecting the cut-off for high and low expression using IHC are required. Future studies should be undertaken to confirm the preliminary findings of this study, using molecular techniques such as real-time PCR, and also with Western blots, for both CRC tissues and adjacent normal colorectal tissue, or using a combination of tissue microarray and automated quantitative assessment of immunofluorescence (TMA-AQUA), which measures the level of protein in the cells by immunofluorescence.
In the present study, the prognostic value of expression of the ALDH1A1 protein was investigated using a single polyclonal antibody in a single IHC method in CRC tissues. The ALDH1A1 protein was highly expressed in 294 of 424 (69.34%) of CRC tumor tissues, and the Cox regression model showed that increased ALDH1A1 expression was an independent factor in predicting overall survival (OS) time for patients with CRC. The findings of this preliminary study support the need for further studies to evaluate the role of ALDH1A1 as a prognostic or predictive biomarker of the clinical outcome for patients with CRC.
Conclusions
The findings of this study, using immunohistochemistry (IHC), showed that aldehyde dehydrogenase 1A1 (ALDH1A1) protein overexpression was significantly correlated with colorectal carcinoma (CRC) metastasis to regional lymph nodes and that ALDH1A1 overexpression was an independent prognostic factor for patients with CRC, especially in patients with no lymph node metastases.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by Clinical Research Special Fund of Wu Jieping Medical Foundation (No: 320.6750.1281)
References
- 1.Chen W, Zheng R, Zeng H, et al. Annual report on the status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–46. [PubMed] [Google Scholar]
- 4.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–25. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 5.Kahlert C, Gaitzsch E, Steinert G, et al. Expression analysis of aldehyde dehydrogenase 1A1 (ALDH1A1) in colon and rectal cancer in association with prognosis and response to chemotherapy. Ann Surg Oncol. 2012;19:4193–201. doi: 10.1245/s10434-012-2518-9. [DOI] [PubMed] [Google Scholar]
- 6.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–38. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang B, Liu G, Xue F, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22:817–23. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedberg Y, Ljungberg B, Roos G, Landberg G. Expression of cyclin D1, D3, E, and p27 in human renal cell carcinoma analysed by tissue microarray. Br J Cancer. 2003;88:1417–23. doi: 10.1038/sj.bjc.6600922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu SL, Zeng DZ, Dong WG, et al. Distinct patterns of ALDH1A1 expression predict metastasis and poor outcome of colorectal carcinoma. Int J Clin Exp Pathol. 2014;7:2976–86. [PMC free article] [PubMed] [Google Scholar]
- 10.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–89. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng S, Yang X, Lassus H, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Lin D, Fu Y, Lai M. Nuclear aldehyde dehydrogenase 1A1 (ALDH1A1) expression is a favorable prognostic indicator in colorectal carcinoma. Pathol Res Pract. 2016;212:791–99. doi: 10.1016/j.prp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Langan RC, Mullinax JE, Ray S, et al. A pilot study assessing the potential role of non-CD133 colorectal cancer stem cells as biomarkers. J Cancer. 2012;3:231–40. doi: 10.7150/jca.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessman CJ, Bubbers EJ, Billingsley KG, et al. Loss of expression of the cancer stem cell marker aldehyde dehydrogenase 1 correlates with advanced-stage colorectal cancer. Am J Surg. 2012;203:649–53. doi: 10.1016/j.amjsurg.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugli A, Iezzi G, Hostettler I, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–90. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19:634–64. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough NR. Focus issue: Wnt and beta-catenin signaling in development and disease. Sci Signal. 2012;5:eg2. doi: 10.1126/scisignal.2002806. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Tilló E, de Barrios O, Siles L, et al. Postigo. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA. 2011;108:19204–9. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogler T, Kriegl L, Horst D, et al. The expression pattern of aldehyde dehydrogenase 1 (ALDH1) is an independent prognostic marker for low survival in colorectal tumors. Exp Mol Pathol. 2012;92:111–17. doi: 10.1016/j.yexmp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. 2009;27:844–50. doi: 10.1080/07357900902744502. [DOI] [PubMed] [Google Scholar]
- 21.Horst D, Kriegl L, Engel J, et al. CD133 and nuclear beta-catenin: The marker combination to detect high-risk cases of low stage colorectal cancer. Eur J Cancer. 2009;45:2034–40. doi: 10.1016/j.ejca.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Horst D, Kriegl L, Engel J, et al. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–89. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Koppaka V, Thompson DC, Vasiliou V. Focus on molecules: ALDH1A1: From lens and corneal crystallin to stem cell marker. Exp Eye Res. 2012;102:105–6. doi: 10.1016/j.exer.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debeb BG, Lacerda L, Xu W, et al. Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/beta-catenin signaling. Stem Cells. 2012;30:2366–77. doi: 10.1002/stem.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froeling FE, Feig C, Chelala C, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–97. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 27.Lu D, Cottam HB, Corr M, Carson DA. Repression of beta-catenin function in malignant cells by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA. 2005;102:18567–71. doi: 10.1073/pnas.0509316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–45. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 29.Kielman MF, Rindapää M, Gaspar C, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 30.Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–18. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]