Abstract
Takotsubo cardiomyopathy is characterized by transient loss of systolic function in the absence of coronary artery disease. It is significantly more common in post-menopausal women and is typically brought on by intense emotional stress. Pathophysiology is not completely elucidated, but it appears to be related, in part, to excess catecholamine; this results in coronary artery vasospasm, ischemia and eventual ventricular dysfunction. Patient presentation can vary widely, but typically presents similar to acute coronary syndrome. Management involves acute stabilization and monitoring, as well as guideline-directed medical therapy for heart failure. We report a very unique case of a healthy male patient presenting with nonanginal symptoms of racing heart, who was found to have cardiomyopathy following a physical encounter. This case serves to bring into awareness that intense physical encounters may be sufficient to induce cardiomyopathy without presenting angina.
INTRODUCTION
First described in Japan in 1990, takotsubo cardiomyopathy (TTC) is more commonly known as ‘broken heart syndrome’. It has been described as a transient loss of systolic function precipitated by either physical or emotional stress that occurs in the absence of significant coronary artery disease. Epidemiological analysis shows that 90% of those affected are women between ages 62 and 76. Of patients presenting with acute coronary syndrome, 6–9% of women are diagnosed with TTC versus <0.5% in men.
Men are more likely to die suddenly in the early stages of TTC, so this could also contribute to decreased reporting. Moreover, the most common triggering event in men is physical stress, such as a major-medical illness or invasive surgical procedures. This contrasts with women, where emotional stress is the most common precipitating factor [1, 2].
A recent study evaluating 120 patient cases presenting with TTC did not reveal any such cases related to a physical fight as reported here [3]. Interestingly, a systematic review of TTC in young patients (under the age of 40) found that physical stress was more likely than mental stress [4]. Previously described precipitating factors that have been documented include: intracranial bleeds, head trauma, cerebrovascular accident, sepsis, invasive surgery, pheochromocytoma, anesthesia, allergic reactions, cocaine/methamphetamine use and opioid withdrawal. A vague, ‘Else’ category in the aforementioned systemic review accounted for 12.5% of young patients who suffered from TTC following a physical stressor.
It is possible some of these patients were involved in physical fights prior to presentation, but to date we could not find specific evidence of a physical fight leading to TTC. We report a unique case of a previously healthy 50-year-old male who developed acute heart failure following a physical bar fight. This case prompts the important question: should there be more screening for TTC in athletes, soldiers and first responders who present with a clinical picture similar to acute coronary syndrome?
CASE REPORT
A 50-year-old male with seasonal allergies and prior deep venous thrombosis presented to the emergency department (ED) at the insistence of his wife following an assault outside of a bar. The patient experienced feelings of his ‘heart racing’ during the altercation but attributed his symptoms to ‘adrenaline rush’. He reported several blows to the head but denied any loss of consciousness. He endorsed frequent palpitations but denied any chest discomfort, shortness of breath or pre-syncope. Patient also denied significant alcohol consumption on a regular basis and states that he only drinks socially on occasion. Ethanol levels on admission were elevated at 40 mg/dl. Patient demonstrated no signs of chronic alcoholism and did not have any tremors or withdrawal symptoms during his 4-day hospital admission. Aspartate aminotransferase levels were within normal limits 34 µ/l. Albumin levels were within normal limits (4.8 g/dl) and blood counts of red blood cells, and platelets were within normal limits.
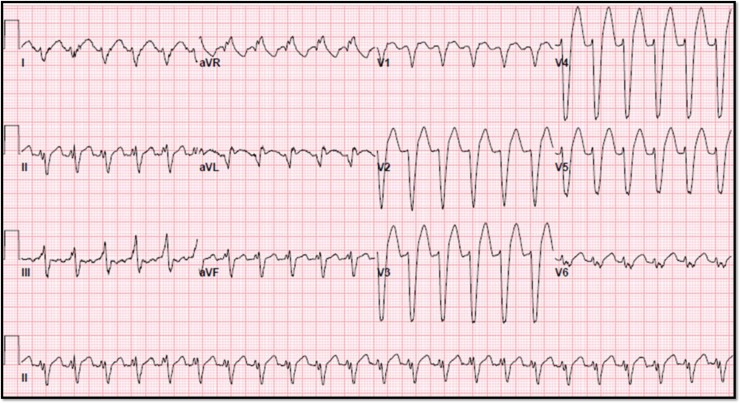
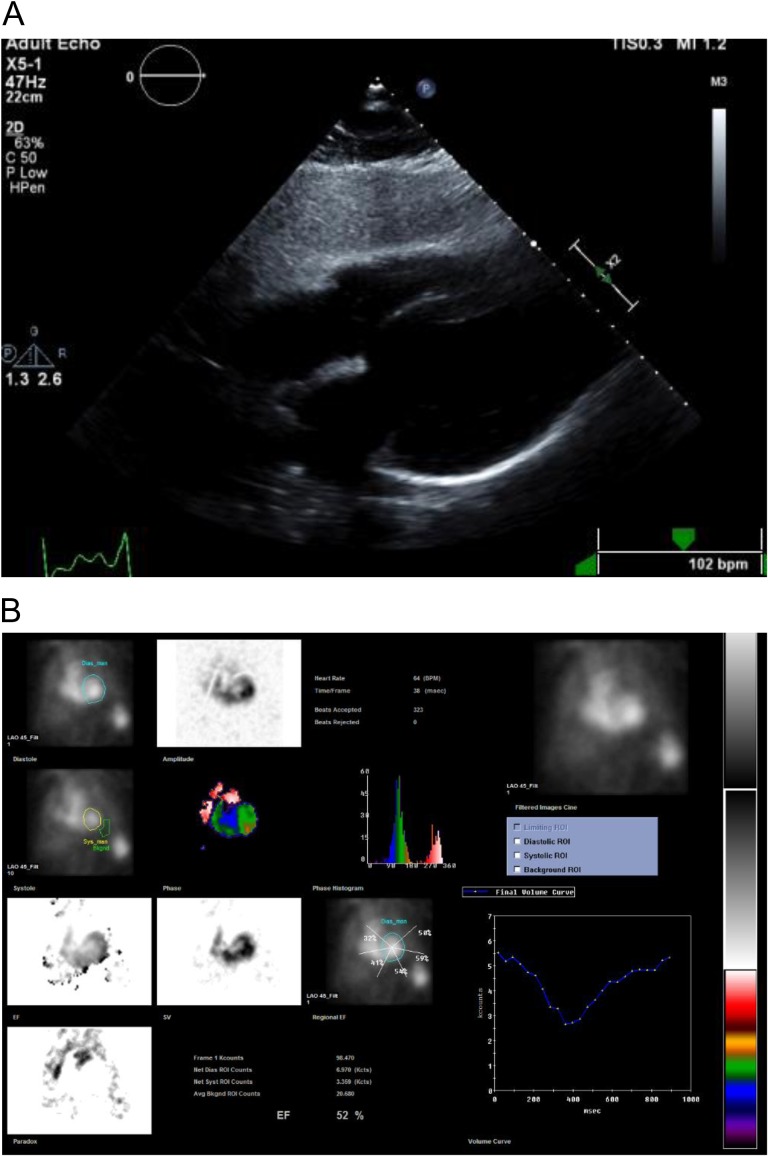
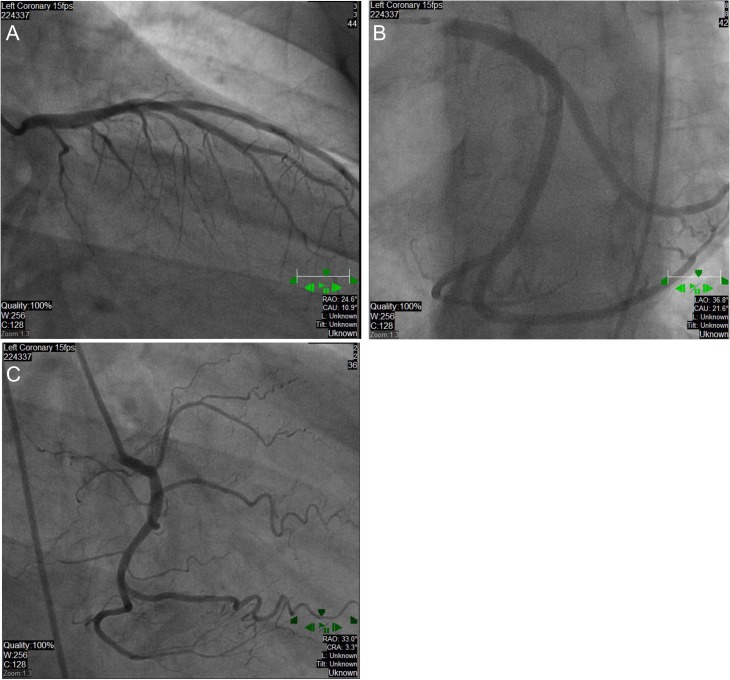
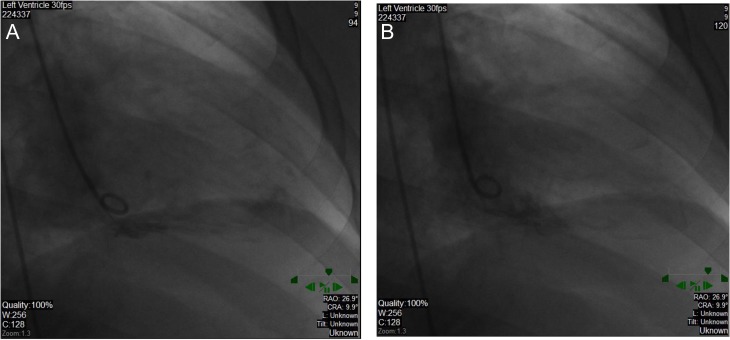
In the ED, his exam vitals were significant for tachycardia (150 bpm) and hypertension (191/95 mmHg). Physical exam was notable for lacerations and bruises of the face and cardiac exam revealed tachycardia, regular rhythm without murmurs, jugular venous distension or peripheral edema. Labs demonstrated a leukocytosis (18 300 cells/mm3) and mildly elevated troponin (0.261 ng/ml) (reference range normal <0.034 ng/ml). Chest X-ray and computed tomography of the head were unremarkable with no signs of interstitial edema. Electrocardiogram (EKG) showed sinus tachycardia with intraventricular conduction delay and left bundle branch block (LBBB) (see Fig. 1). He was given a full dose of aspirin and nitroglycerin due to concerns for acute coronary syndrome. The patient remained hemodynamically stable but became bradycardic. EKG showed a junctional escape rhythm, right bundle branch block and bi-fascicular block. Cardiology suspected Type II NSTEMI and repeat troponins were 2.66 ng/ml and 3.27 ng/ml. Transthoracic echocardiogram (TTE) revealed apical and mid-wall hypokinesis in a circumferential noncoronary distribution with left ventricular (LV) ejection fraction of 20% (see Fig. 2A). LV end diastolic dimension was measured at 5.9 cm and LV end systolic dimension was measured at 5.0 cm. Left heart catheterization revealed patent coronary arteries (see Fig. 3A–C) with apical ballooning consistent with stress cardiomyopathy on LV ventriculogram (see Fig. 4A and B). The patient was stabilized and discharged on guideline-directed medical therapy for cardiomyopathy, including carvedilol and lisinopril. He was also offered an external defibrillator and close cardiology follow-up. Repeat TTE 8 weeks later revealed a technically limited study but improving ejection fraction at 30–35%. A final repeat multigated acquisition scan (MUGA) revealed a normalization of the ejection fraction at 52% after 12 weeks from admission.
Figure 1:
Presenting EKG with sinus tachycardia, new LBBB.
Figure 2:
(A) Subcostal view four chamber with severe hypokinesis of apical and mid-walls with ejection fraction 20% and dilated LV; (B) MUGA scan with repeat EF of 52%.
Figure 3:
Patent coronary arteries. Left anterior descending with selective ostial engagement (A), Left circumflex artery with selective postal engagement (B). Right coronary artery (C).
Figure 4:
Dilated LV on ventriculogram in end diastole (A), normally contracting base with hypokinesis of anterior and inferior mid- and apical walls in end systole (B).
DISCUSSION
Although the underlying mechanism is not completely understood, the etiology of TTC is precipitated either by sudden, unexpected physical or emotional stress. It is hypothesized that abrupt release of catecholamine in excess due to stress can stun the myocardium [5]. Plasma levels of norepinephrine, epinephrine and dopamine have been shown to be elevated in patients with TTC [6]. This sympathetic stress results in high amounts of intracellular calcium and free radicals as evidenced by biopsy findings of contraction band necrosis. Other contributing factors include vasospasm of coronary arteries and coronary microvasculature impairment [7]. It is proposed that this vasospasm causes ischemia to the myocardium resulting in LV dysfunction which then leads to the hypokinesis and apical ballooning classically seen. This idea of regionally injured myocardium contradicts the minimal rise in troponins seen in some patients. Coronary microvascular impairment is another proposed mechanism as evidenced by positron emission tomography. It has been shown that changes in glucose metabolism are more severe than changes in perfusion in the affected areas of the heart [8]. This phenomenon could also be explained by excess sympathetic stimulation, which would decrease glucose uptake into the cells.
Kounis syndrome, or allergic acute coronary syndrome, was briefly endorsed in light of seasonal allergies and elevated troponin. Patient did have a history of seasonal allergies, though his March admission was in a low allergy period for the location. Moreover, the patient did not demonstrate any signs of sinus congestion. He had neither other inciting events nor exposures at the time other than the physical altercation at the bar.
Patients present with signs and symptoms closely resembling acute coronary syndrome, including chest pain, dyspnea as well as elevated troponins or evidence of ST-segment elevation on EKG. Rarely, patients present with cardiac arrhythmia, syncope, cardiac arrest, seizures and gastrointestinal symptoms [4]. The patient we describe presented with nonanginal symptoms of palpitations; intriguingly, he denied chest discomfort, shortness of breath or pre-syncope. The diagnosis is confirmed when angiography reveals patent coronary arteries or evidence of wall motion abnormalities that do not correspond to coronary disease. Although reversible, these patients are at high-risk for life-threatening pulmonary edema, shock, sudden cardiac death and arrhythmias; this can warrant offering an external defibrillator.
At this time, there are few evidence-based guidelines on the treatment of TCC, partly because the majority of cases have normal cardiac function within three weeks [9]. In the acute setting, monitoring with telemetry and assessment either with echocardiography or magnetic resonance imaging is reasonable to assess for mitral regurgitation, left or right ventricular dysfunction, mural thrombus, or outflow tract obstruction. Arrhythmias from QT prolongation have been observed but presently prophylactic anti-arrhythmic agents are not standard practice. Inotropic agents tend to be avoided secondary to the proposed underlying catecholamine excess mechanism [9]. Anticoagulation with heparin is common practice to prevent thrombus formation secondary to wall hypokinesis. However, anticoagulation is not without controversy because of the risk of cardiac rupture due to the apical ballooning. Nevertheless, anticoagulation is commonly continued until regional wall motion abnormalities resolve [10]. Chronic treatment almost always includes beta blockers, which theoretically is reasonable because of the sympathetic overload discussed earlier. Angiotensin converting enzyme inhibitors can be used for reduced LV function. A repeat ejection fraction measurement after 3 months of guideline-directed medical therapy is necessary to assess return of ventricular function.
Prognosis is generally considered fair as majority of patients have complete return of LV function [11]. Rare cases of persistent regional wall motion abnormalities have been reported. In-hospital mortality rate is estimated at 8% secondary to arrhythmias, ventricular rupture, apical thrombosis and cardiogenic shock [10]. Recurrence has been reported and is estimated to occur in about 2–10% of cases and is highest within first four years of initial presentation. It is thought that recurrence rates are higher in patients not taking long-term beta-blocker therapy, but another study showed no difference in medical treatment of those with and without recurrence at 4 years [7, 12].
In conclusion, TTC presents very similarly to acute myocardial infarction but intervention and prognosis is quite different. Although classically described in post-menopausal females responding to an emotional stressor, this case illustrates a middle aged-man following a physical fight. Therefore, it is imperative for physicians to be aware of unique patient populations and risk factors to prevent unnecessary intervention such as thrombolytic therapy, stent placement or bypass operations. Moreover, it can often times be difficult to distinguish whether the symptoms relate to Takosubo or undiagnosed dilated cardiomyopathy. Part of the trend with TTC is a return of function at 12 weeks. If this does not occur as in this case, it may otherwise have been a pre-existing dilated cardiomyopathy; magnetic resonance imaging may also be useful to determine an etiology in this alternative scenario. This case highlights that more research into sudden cardiac deaths related to athletics or the military could reveal more cases TTC. More research is necessary to elucidate pathophysiology and offer evidence-based diagnostic criteria and treatment regiments.
CONFLICT OF INTEREST STATEMENT
None declared.
CONSENT
Written consent was obtained from the patient for publication of this article.
REFERENCES
- 1. Schneider B, Athanasiadis A, Sechtem U. Gender-related differences in takotsubo cardiomyopathy. Heart Fail Clin 2013;9:137–46. [DOI] [PubMed] [Google Scholar]
- 2. Patel SM, Chokka RG, Prasad K, Prasad A. Distinctive clinical characteristics according to age and gender in apical ballooning syndrome (takotsubo/stress cardiomyopathy): an analysis focusing on men and young women. J Card Fail 2013;19:306–10. [DOI] [PubMed] [Google Scholar]
- 3. Sharkey S, Windenburg D, Lesser JR, Maron MS, Hauser RG, Lesser JN, et al. . Natural history and expansive clinical profile of stress (takotsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–41. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Xia L, Shen X, Han G, Feng D, Xiao H, et al. . A new insight into sudden cardiac death in young people: a systematic review of cases of takotsubo cardiomyopathy. Medicine (Baltimore) 2015;94:e1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneider B, Sechtem U. Influence of age and gender in takotsubo syndrome. Heart Fail Clin 2016;12:521–30. [DOI] [PubMed] [Google Scholar]
- 6. Wittstein IS, Theimann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. . Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. [DOI] [PubMed] [Google Scholar]
- 7. Veillet-Chowdhury M, Hassan S, Stergiopoulos K. Takotsubo cardiomyopathy: a review. Acute Cardiac Care 2014;16:15–22. [DOI] [PubMed] [Google Scholar]
- 8. Kurowski V, Kaiser A, vonHof K, Killermann DP, Mayer B, Hartmann F, et al. . Apical and midventricular transient left ventricular dysfunction syndrome (takotsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest 2007;132:809–16. [DOI] [PubMed] [Google Scholar]
- 9. Akashi Y, Goldstein D, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008;16:2754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurisu S, Kihara Y. Clinical management of takotsubo cardiomyopathy. Circ J 2014;78:1559–66. [PubMed] [Google Scholar]
- 11. Kubena P, Bohata S, Manousek J, Baughman KL, Schulman SP, Gerstenblith G. Takotsubo cardiomyopathy, clinical experience with the disease and one-year prognosis of patients. Vnitr Lek 2015;61:619–25. [PubMed] [Google Scholar]
- 12. Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 2007;50:448–52. [DOI] [PubMed] [Google Scholar]