Abstract
Nuclear translocation of stimulated Smad heterocomplexes is a critical step in the signal transduction of transforming growth factor β (TGF-β) from transmembrane receptors into the nucleus. Specifically, normal nuclear accumulation of Smad2/Smad4 heterocomplexes induced by TGF-β1 is involved in carcinogenesis. However, the relationship between nuclear accumulation and the nucleocytoplasmic transport kinetics of Smad proteins in the presence of TGF-β1 remains obscure. By combining a high-speed single-molecule tracking microscopy and Förster resonance energy transfer technique, we tracked the entire TGF-β1-induced process of Smad2/Smad4 heterocomplex formation, as well as their transport through nuclear pore complexes in live cells, with a high single-molecule localization precision of 2 ms and <20 nm. Our single-molecule Förster resonance energy transfer data have revealed that in TGF-β1-treated cells, Smad2/Smad4 heterocomplexes formed in the cytoplasm, imported through the nuclear pore complexes as entireties, and finally dissociated in the nucleus. Moreover, we found that basal-state Smad2 or Smad4 cannot accumulate in the nucleus without the presence of TGF-β1, mainly because both of them have an approximately twofold higher nuclear export efficiency compared to their nuclear import. Remarkably and reversely, heterocomplexes of Smad2/Smad4 induced by TGF-β1 can rapidly concentrate in the nucleus because of their almost fourfold higher nuclear import rate in comparison with their nuclear export rate. Thus, we believe that the determined TGF-β1-dependent transport configurations and efficiencies for the basal-state Smad or stimulated Smad heterocomplexes elucidate the basic molecular mechanism to understand their nuclear transport and accumulation.
Introduction
Transforming growth factor β (TGF-β) is a secreted cytokine that performs multiple important cellular functions, such as cell growth, proliferation, differentiation, and apoptosis (1, 2). Functioning as intracellular signals and transcription factor, Smad proteins play critical roles in transducing extracellular signals from TGF-β to the nucleus (3, 4, 5, 6, 7). In detail, the TGF-β/Smad signaling pathway is initiated with ligand-induced dimerization of receptor protein serine/threonine kinases on the plasma membrane and phosphorylation of the receptor-regulated Smad proteins (R-Smads) (8). R-Smads include Smad2 and Smad3, which are involved in the TGF-β/activin pathway (9). R-Smad proteins and the common signaling transducer Smad4 can form heterocomplexes and transport through nuclear pore complexes (NPCs) to accumulate in the nucleus under the stimulation of TGF-β (1, 10). At the basal state, however, without ligand stimulation, R-Smad or Smad4 proteins are present mostly in the cytoplasm or evenly distributed (11, 12). Moreover, the accumulation of ligand-induced Smad heterocomplexes within the nucleus plays multifaceted roles in carcinogenesis (13, 14, 15). Additionally, dysfunctional nucleocytoplasmic transport of Smad heterocomplexes in the TGF-β/Smad signaling pathway has been associated with many human diseases (16).
In eukaryotic cells, passive diffusion and facilitated translocation have been identified as two distinct nucleocytoplasmic transport modes through the NPCs (17, 18). The former is reserved for signal-independent diffusing molecules that are smaller than 60 kDa, and the latter ensures efficient and timely translocation of signal-dependent cargo molecules assisted by nuclear transport receptors through the NPCs (17, 18, 19). Facilitated translocation depends on interactions between nuclear transport receptors and intrinsically disordered nucleoporins (Nups) rich in phenylalanine-glycine (FG) repeats (19, 20, 21). These FG-Nups comprise one-third of all Nups of the NPC and together create a selectively permeable entropic barrier (22, 23, 24), allowing for facilitated transport of cargos that carry either nuclear location sequences (NLSs) or nuclear export signals (NES) recognized by transport receptors through the NPCs (25, 26). A concentration gradient of RanGTP (or RanGDP) across the nuclear envelope (NE) functions as the directionality regulator for nuclear import or export (27, 28).
Some insights into the nucleocytoplasmic transport mechanisms for Smad proteins in the absence or presence of TGF-β1 have been obtained in previous studies (12, 29). Here, we will briefly review the current knowledge of nuclear import and export for Smad4 and Smad2 proteins because these substrates will be our major targets in this study. Interestingly, either Smad4 or Smad2 can continuously shuttle between cytoplasm and nucleus through the NPC by facilitated translocation in basal states because they both possess NLSs for nuclear import and NES for nuclear export. Specifically, two transport receptors, importin7 and importin8, can recognize the NLSs within Smad4 and facilitate its nuclear import by directly interacting with the FG-Nups of NPCs (30). The CRM1 protein is responsible for mediating Smad4’s export through the NPCs by recognizing the NES within Smad4 and interacting with FG-Nups (31). Differently and surprisingly, a hydrophobic corridor in Smad2 is suggested to directly bind to the FG-Nups to promote its nuclear transport in the absence of TGF-β1 (32). The functionality of either the NLS or NES within Smad2 remains mysterious for the nucleocytoplasmic transport of Smad2 proteins. Moreover, previous studies have shown almost no nuclear accumulation of Smad4 or Smad2 in the absence of TGF-β1 stimulation. Although there is still a debate on the conformational status of Smad proteins in the basal state (8, 33), it is believed that the basal-state Smad2 or Smad4 could have the form of monomers or homodimers, or a mixture of monomers and homodimers. On the contrary, in the TGF-β1-stimulated state, Smad2 and Smad4 can form heterocomplexes and demonstrate rapid and significant nuclear accumulation (12, 29). Also, the stoichiometry of Smad heterocomplexes has been controversial, with evidence existing for both heterodimers and heterotrimers (34, 35). However, the molecular mechanism to explain the transition from the rare presence of Smads in the nucleus to the significant nuclear accumulation of Smad2/Smad4 heterocomplexes in the presence of TGF-β1 remains obscure. Schmierer et al. developed two models: the retention-only model and the alternative retention/enhanced complex import (RECI) model (36). The retention-only model hypothesizes that Smad heterocomplexes and basal-state Smads share an identical nuclear import rate, but only Smad heterocomplexes have nuclear retention, whereas the RECI model assumes that Smad heterocomplexes possess a faster import rate than basal-state Smad. Although fluorescence recovery after photobleaching has been applied to study nuclear accumulation of GFP-Smad2 (37), direct evidence to distinguish the above models is still unavailable.
In this study, we set out to directly measure the detailed nuclear transport kinetics of Smad heterocomplexes or basal-state Smads with or without the presence of TGF-β1 in live human cells. By combining a high-speed single-molecule tracking microscopy and Förster resonance energy transfer (FRET) technique, we have tracked the basal-state Smads or the Smad heterocomplexes as they transport through the NPCs and determined their transport time and efficiency at the single-molecule level. We found that the basal-state Smads exhibit a higher nuclear export efficiency than import efficiency in the absence of TGF-β1. Remarkably, the Smad heterocomplexes induced by TGF-β1 completely reverse this transport scenario by demonstrating an almost fourfold higher nuclear import efficiency than their nuclear export efficiency. Finally, single-molecule FRET data show that the Smad2/Smad4 heterocomplexes induced by TGF-β1 are formed in the cytoplasm, dissociated in the nucleus, and transported through the NPCs as entireties.
Materials and Methods
Plasmids, cell culture, and transfection
cDNAs of RFP-Smad2 and EGFP-Smad4 were gifts from Ali H. Brivanlou (Rockefeller University, New York, NY) and Xiaohong Fang (Chinese Academy of Sciences, Beijing, China), respectively (11, 38). HeLa wild-type cells were purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The cell line stably expressing POM121-GFP was previously described in our earlier publication (39). EGFP-Smad4 and RFP-Smad2 were transfected into wild-type HeLa or Pom121-florescence protein HeLa cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to standard protocols. For TGF-β1 treatment in the C2C12 cell line and the Hacat cell line, 1 h after 5 ng/mL TGF-β1 treatment shows a peak of nuclear accumulation for Smad2 and Smad4 (11)). Similarly, our cells were incubated with 5 ng/mL TGF-β1 (PeproTech, Rocky Hill, NJ) in Opti-MEM (Thermo Fisher Scientific) for 1 h before imaging. Trimerization of Smad proteins is dependent on the phosphorylation. SB-431542 could inhibit the phosphorylation of Smad and prevent Smad’s trimerization (40, 41). The cells without stimulation of TGF-β1 in our experiments were all treated for 4 h with 1 μM SB431542, so that they can serve as a negative control. For microscopy imaging in live-cell imaging, the medium was replaced with transport buffer (20 mM HEPES, 110 mM KOAc, 5 mM NaOAc, 2 mM MgOAc, and 1 mM EGTA (pH 7.3)) for 30 min before detection.
Instrumentation
Our high-speed single-molecule tracking microscope included an Olympus IX81 equipped with a 1.4-NA 100× oil-immersion apochromatic objective (UPLSAPO 100×; Olympus, Shinjuku, Tokyo, Japan), a 50-mW 488-nm semiconductor laser (Coherent OBIS, Santa Clara, CA), a 50-mW 561-nm laser (Coherent OBIS), an on-chip multiplication gain charge-coupled-device camera (Cascade 128+; Photometrics, Tucson, AZ), and a Slidebook software package (Intelligent Imaging Innovations, Denver, CO) for data acquisition and processing. EGFP and RFP were excited by 488- and 561-nm lasers, respectively. The 488-nm laser was combined with an excitation filter (FF01-469/35; Semrock, Rochester, NY). The 561-nm laser was also combined with an excitation filter (FF01-531/40; Semrock). The two lasers were collimated and focused into an overlapped illumination volume in the focal plane. The green fluorescence emission was filtered by a dichroic filter (FF497-Di01; Semrock) and an emission filter (FF01-525/39; Semrock). The red fluorescence emission was filtered by a dichroic filter (FF562-Di02; Semrock) and an emission filter (FF01-593/40; Semrock). The two fluorescence images were captured by the charge-coupled device (CCD) camera (Cascade 128+).
For FRET measurements, a 5-mW 488-nm laser was used for excitation. The donor and acceptor fluorescence were both collected by the same objective and split by a Dual View DV2 system (Photometrics) that includes a dichroic mirror (565dcxr; CHROMA, Bellows Falls, VT). The donor and acceptor channels were further filtered by 520 ± 15-nm band-pass (CHROMA) and 593-nm long-pass (FF01-593/LP-25; CHROMA) filters, respectively. The bleed-through signal of GFP and RFP signals with our band-pass filter is negligible because the cross talk index with the GFP-RFP pair in our setup is less than 10% (42). Single molecules were detected at 2 ms per frame. A 3 μm diameter circle area was illuminated at the focal plane during the experiment, and the illumination intensities at the focal plane were ∼70 kW/cm2 (488 and 561 nm).
Localization of the NE
The middle-plane position of the NE was determined by fitting the intensity-contrast image of the NE or the fluorescence of GFP-POM121 or mCherry-POM121, typically imaged with a CCD exposure time of 1–3 s, as follows. The pixel intensities within a row approximately perpendicular to the NE were fitted with a Gaussian function. The peak position of the Gaussian function for a particular set of pixel intensities was considered to be the NE center for that row. The peak positions of a series of such Gaussians were then fitted with a polynomial function, yielding the location of the NE within the entire image. The localization precision of the NE’s middle plane is ∼10 nm.
Single-molecule localization precision for isolated fluorescent spots
The localization precision for fluorescent Smad proteins was defined as how precisely the central point of each detected fluorescent diffraction-limited spot was determined by using two-dimensional Gaussian-fitting algorithms. For moving molecules, the influence of particle motion during image acquisition should be considered in the determination of localization precision. In detail, the localization precision for moving substrates (σ) was determined by the algorithm
where F is equal to 2, N is the number of collected photons, a is the effective pixel size of the detector, and b is the SD of the background in photons per pixel, and , where s0 is the SD of the point spread function in the focal plane, D is the diffusion coefficient of substrate, and Δt is the image acquisition time. Calculation of the diffusion coefficient was performed by plotting on a mean-square displacement versus time (Δt). The data was fitted with (43, 44, 45).
In our experiments, more than 1000 signal photons were collected from single-channel detection, and over 500 photons were collected from the donor or the FRET channel of the dual-channel detection. Thus, the localization precision was calculated to be <10 nm and <15 nm for the single-channel and dual-channel detection, respectively, on the basis of the above equations and the parameters determined experimentally (N > 1000 or N > 500, a = 240 nm, b ≈ 10, s0 = 150 ± 50 nm, and D = 1.7–2.6 μm2/s for the tested substrates). Given the localization precision for the NE’s middle plane of ∼10 nm, the systematical precision for tracking of molecules across the NE is approximately <14 nm and <18 nm for the single-channel or the dual-channel detections, respectively.
Statistics
Experimental measurements are reported as mean ± standard error of the mean unless otherwise noted. A binomial z-test for proportions was utilized for efficiency assays.
Results and Discussion
Subcellular distributions of the basal-state Smad proteins and the Smad heterocomplexes with or without the stimulation of TGF-β1
Enhanced green fluorescence protein (EGFP) and red fluorescence protein (RFP) are respectively or simultaneously used to label the N-termini of Smad4 and Smad2 proteins in live HeLa cells. These labeled fluorescence proteins not only indicate subcellular locations of Smad2 or Smad4, but also the FRET signals between EGFP-Smad4 and RFP-Smad2 (their approximate distance of ∼3–4 nm (Fig. 1 B) in heterocomplex is smaller than their Förster distance of 5 nm (34, 46, 47)) will highlight whether these Smad proteins would form heterocomplex before, during, or after moving through NPCs. With these fluorescent proteins, the FRET signals, and a setup of wide-field epifluorescence single-channel microscopy (Fig. 1, A and B), we first determined the subcellular spatial locations of Smads in live cells and confirmed that Smad4/Smad2 heterocomplex indeed accumulated in the nucleus in response to TGF-β1 stimulation and the basal-state Smad4 or Smad2 did not accumulate in the absence of TGF-β1 stimulation (Fig. 1, C–G). It is worth noting that SB431542, an inhibitor for TGF-β1 receptor on plasma membrane, was used to treat HeLa cells before epifluorescence microscopy imaging under the condition of TGF-β1 absence (36, 37).
Figure 1.
Simplified microscopy setup and subcellular distribution of Smad proteins. (A) A schematic optical setup of wide-field illumination with single-channel detection is shown. (B) Estimated distances between RFP-Smad2 and GFP-Smad4, based on the reported crystal structure of the Smad2/Smad4 heterotrimer or heterodimer (with or without a Smad2 within blue dashed circle) (34, 46), are shown. The subcellular distribution of EGFP-Smad4 (C), mCherry-Smad2 (D), and the FRET fluorescence (E) in the absence of TGF-β1 and the presence of SB-431542 is shown. The subcellular distribution of EGFP-Smad4 (F), mCherry-Smad2 (G), and the FRET fluorescence (H) under the stimulation of TGF-β1 is shown. The dashed line highlights the separation of cytoplasm and nucleus. C, cytoplasm; N, nucleus. Scale bars, 10 μm.
Narrow-field single-molecule single-channel microscopy revealed that the basal-state Smad proteins cannot accumulate within the nucleus because of higher nuclear export efficiencies
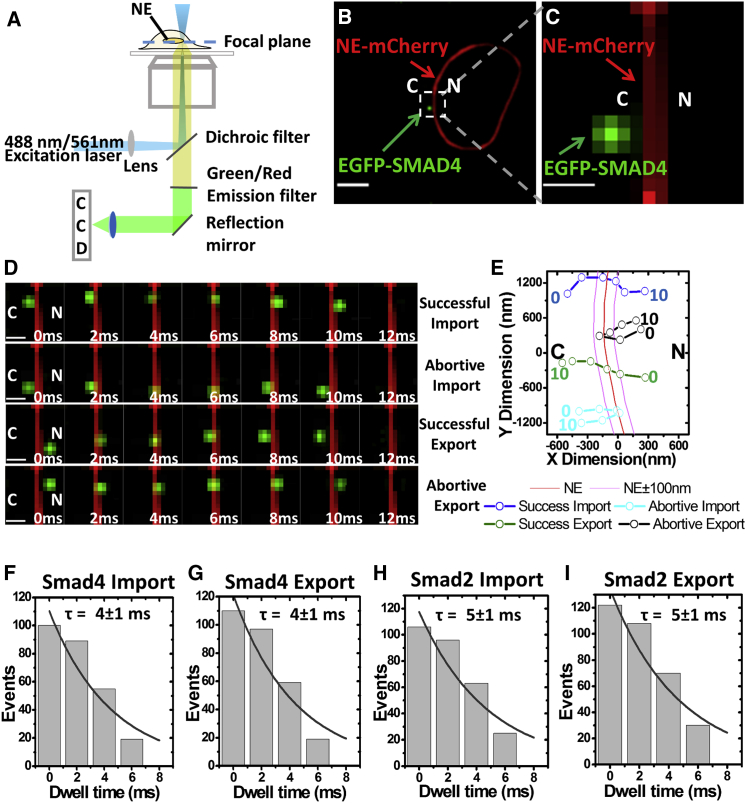
To further elucidate the nuclear transport details for Smads, we decided to track individual Smads as they moved through the native NPCs in live cells by employing a setup of narrow-field, single-molecule, single-channel microscopy (Fig. 2 A). We expected that the tracking of single Smad proteins through the NPCs under the absence of TGF-β1 could allow us to do the following: 1) determine the import or export time and efficiency for Smad proteins, and 2) compare the nuclear import and export kinetics to find clues for explaining the observed difference in their nuclear accumulations. Narrow-field single-molecule microscopy has been proven to be an effective approach in determining the nucleocytoplasmic transport time and efficiency of proteins, mRNAs, and viruses at the single-molecule level (39, 48). In our particular narrow-field microscopy setup, a 3 μm diameter illumination area in the focal plane was used to track labeled individual Smad4 or Smad2 proteins across the NE at the equator of cells with a single-molecule localization precision of <20 nm at a 2-ms detection time per frame (Materials and Methods). We hoped that the fast detection speed and the high localization precision would enable us to track the entire NE-crossing process of Smad proteins. Additionally, to separately label the NE and the transiting Smad proteins, we used a stable cell line expressing POM121-mCherry (a scaffold protein that locates at the center of the NPC and mCherry fusion protein is for the labeling of the NE (49, 50)) for single-molecule tracking of EGFP-Smad4 (Fig. 2, B and C; Videos S1, S2, S3, and S4), and a stable cell line of EGFP-POM121 for single-molecule tracking of RFP-Smad2 (Videos S5, S6, S7, and S8).
Figure 2.
Simplified narrow-field fluorescence microscopy setup and single-molecule imaging of nucleocytoplasmic transport of basal-state Smads. (A) A schematic optical setup of narrow-field illumination with single-channel detection is shown. (B) Individual EGFP-Smad4 proteins (green dot) were tracked around the NE labeled with mCherry-POM121 (red curved line). C, cytoplasm; N, nucleus. Scale bars, 10 μm. (C) An enlarged view of the boxed area in (B) is shown. Scale bars, 1 μm. (D) Snapshots from typical single-molecule videos of EGFP-Smad4 nuclear import and export events at 2 ms per frame are shown. Scale bars, 1 μm. (E) Single-particle trajectories (open dots) of the four single-molecule videos in (D) are shown. Numbers denote time in milliseconds. (F) An accumulative histogram for dwell-time distribution of EGFP-Smad4 nuclear import events fitted with exponential decay and import time, determined as τ here, is shown. (G) The export time for EGFP-Smad4 is shown. (H and I) Nuclear import and export times for mCherry-Smad2 are shown.
Both nuclear import (moving from the cytoplasm into the nucleus through the NPCs) and export (exiting the nucleus through the NPCs and entering the cytoplasm) processes of the basal-state Smads in the absence of TGF-β1 and presence of SB-431542 have been successfully tracked by our narrow-field single-molecule microscopy (Fig. 2 D). We typically collected 5000 frames in 10 s for each single-molecule video and determined the shift of the NE by comparing the NE images taken before and after the single-molecule video. We found the NE did not shift much within the 10-s detection time, and the middle plane of the NE was determined with a localization precision of ∼10 nm (Materials and Methods). Their single-molecule trajectories were determined after Gaussian fitting of each single-molecule fluorescent spots around the NE (Materials and Methods), and typical ones are shown in Fig. 2 E. Given the average length of NPCs (along the nucleocytoplasmic transport axis) in HeLa cells is ∼200 nm (51, 52, 53), the dwell time of a Smad protein within 100 nm on both sides of the middle of NE before it moves beyond this range is defined as transport time. Also, the Smad is considered to conduct a successful transport event if it starts from one compartment and reaches the other compartment after crossing the NE, or an abortive transport event if the protein comes back to the original compartment after interacting with the NE (Fig. 2 E). The percentage of the successful events over the summed successful and abortive events is referred to as transport efficiency. In the end, the total detected import and export events per second are defined as import and export frequency, respectively. From more than 100 single-molecule nuclear import or export events for basal state Smad4, we found that the protein spent ∼4 ms to cross the NE during either nuclear import or export (Fig. 2, F and G), which agrees with the nuclear transport times of transport receptors that could assist Smad4 through the NPCs such as ∼5 ms for importin β1 and ∼6 ms for CRM1 (54, 55).
Interestingly, the nuclear export efficiency of Smad4 is about twofold higher than its nuclear import efficiency (∼47 vs. ∼23%, as shown in Table 1). Moreover, the measurements of Smad2 almost repeated the above results of Smad4 in regard to transport time and efficiency (Fig. 2, H and I; Table 1). However, almost no difference was found between the import and export frequencies for either of these Smad proteins. The above studies suggest that either the basal-state Smad4 or Smad2 proteins prefer staying in the cytoplasm rather than the nucleus without the stimulation of TGF-β1 in live cells, which also provides direct evidence to explain why there is almost no nuclear accumulation for either of these proteins at bulk concentrations as observed in Fig. 1.
Table 1.
Nuclear Transport Kinetics for Smad Proteins
| Protein |
Import |
Export |
Net Import |
Import |
Export |
|---|---|---|---|---|---|
| Type | Efficiency |
Efficiency |
Efficiency |
Frequency |
Frequency |
| (%) | (%) | (%) | (events/(s · pore) | (events/(s · pore) | |
| Smad | 43.2 ± 2.9 | 12.2 ± 2.2 | 31 | 11 ± 2 | 10 ± 2 |
| Heterocomplex | |||||
| Basal state | 25.6 ± 4.0 | 42.0 ± 4.4 | −16.4 | 9 ± 2 | 10 ± 3 |
| Smad2 | |||||
| Basal state | 22.5 ± 4.6 | 47.2 ± 4.8 | −24.7 | 9 ± 2 | 10 ± 2 |
| Smad4 |
Net Import Efficiency = Import Efficiency − Export Efficiency. Please find definitions for the other terms in the main text.
Dual-channel single-molecule tracking revealed the mechanism of nuclear accumulation for Smad heterocomplexes in vivo
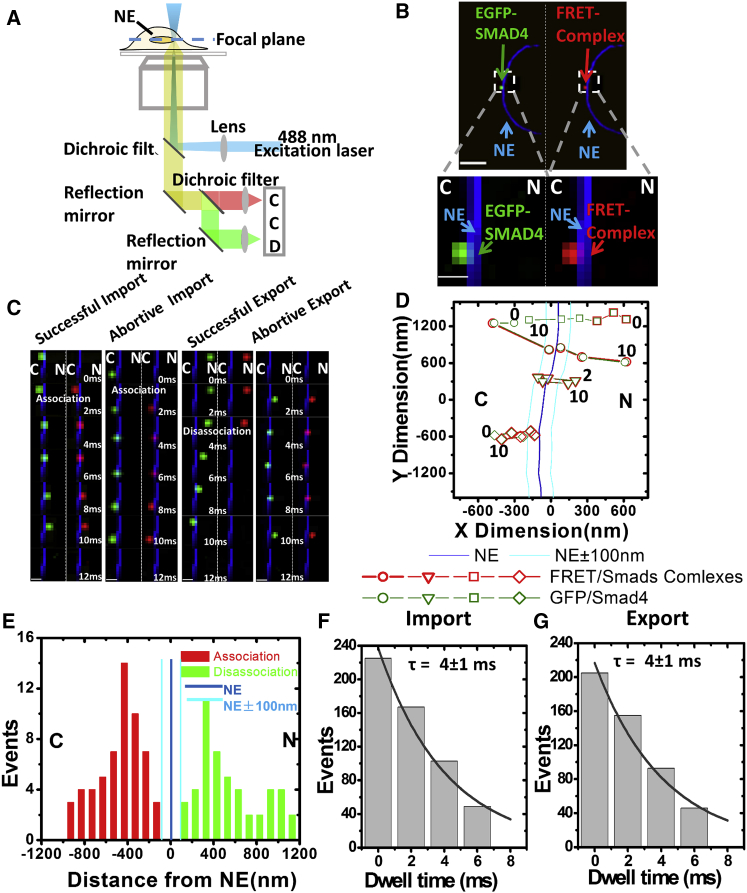
Next, we set out to further determine the nucleocytoplasmic transport details for Smad2/Smad4 protein heterocomplexes as they cross the NE under the condition of TGF-β1 stimulation in live cells by employing a narrow-field, dual-channel, single-molecule tracking microscopy setup (Fig. 3 A). In the dual-channel setup, we simultaneously monitored the fluorescent signal of the “donor” EGFP-Smad4 and the FRET signal between EGFP-Smad4 and the “acceptor” RFP-Smad2 around the NE (the NE’s middle plane was independently determined by a bright-field image, as stated in Materials and Methods) (Fig. 3 B). We expect that simultaneous single-molecule tracking of the “donor” and FRET signals can directly indicate where Smad2 and Smad4 begin to form heterocomplexes, whether they go through the NPCs separately or as a heterocomplex, and where the heterocomplex dissociates. As shown in Fig. 3, C–E, of 110 nuclear import events of Smad2/Smad4 heterocomplexes, we found that inevitably they began to form heterocomplexes in the cytoplasm before entering the NPCs, regardless of their later successful or abortive nuclear import. Based on all the observed successful import events, Smad2/Smad4 heterocomplexes transported through the NPCs always as entireties and never dissociated inside the NPC. After reaching the nucleus, the heterocomplexes need to first dissociate in the nucleus before entering the NPCs and completing their efficient export through the NPCs. Without these dissociations, we found that most of Smad2/Smad4 heterocomplexes failed their nuclear export even after already entering NPCs.
Figure 3.
Dual-channel single-molecule tracking of nucleocytoplasmic transport of Smad heterotrimer in live cells. (A) A schematic optical path of narrow-field illumination with dual-channel imaging is shown. (B) The donor (left half of the camera) and FRET signals (right half of the camera) were imaged simultaneously by narrow-field dual-channel fluorescence microscopy. An enlarged view of boxed areas to show simultaneous imaging of EGFP-Smad4 (green spot), the FRET signal (red spot), and the merged image (yellow spot) around the NE (blue ring) is shown. Scale bar at top, 10 μm; scale bar at bottom: 1 μm. (C) Snapshots from typical single-molecule videos of simultaneous tracking of the “donor” EGFP-Smad4 (green) and the FRET signal (red) as the complexes conduct nuclear import and export events at 2 ms per frame are shown. Scale bars, 1 μm. (D) Single-particle trajectories (open symbols: green for donor and red for FRET) of the four single-molecule videos in (C) are shown. Numbers denote time in milliseconds. (E) The association and disassociation sites of Smad heterocomplexes and their distances to NE were plotted. C, cytoplasm; N, nucleus. (F and G) Accumulative histograms for dwell-time distribution of Smad heterocomplexes during nuclear import and export, fitted with exponential decay to determine import and export times (τ), are shown.
Remarkably, through the above single-molecule trajectories, we also found that Smad2/Smad4 heterocomplexes possess a nuclear import efficiency of 43 ± 3%, which is approximately fourfold of its export efficiency (12 ± 2%) and about twofold of the nuclear import efficiencies (∼24%) for the basal-state Smads (Table 1). Apparently, the results support the aforementioned RECI model, in which Smad heterocomplexes possess a higher import rate than basal-state Smads (36, 56). Furthermore, the much higher nuclear import efficiency of the Smad2/Smad4 heterocomplexes over the basal-state Smads could result from a combination of multiple types of interactions with FG-Nups in the NPCs (57, 58), such as through the hydrophobic corridor from Smad2 and the transport receptors importin7/importin8 from Smad4. However, the much lower nuclear export efficiency of the Smad2/Smad4 heterocomplexes than the nuclear export of the basal-state Smads is also expected because the Smad2/Smad4 heterocomplexes have largely dissociated before entering the NPCs from the nucleoplasmic side (Fig. 3, C–E) (Videos S9, S10, S11, and S12). In addition, the low nuclear export efficiency of Smad heterocomplexes could also support a previous hypothesis that the Smad heterocomplexes would be dephosphorylated to dissociate after finishing their function as a transcription factor and disassociating from DNA (59, 60). Thus, we concluded that the fourfold higher nuclear import efficiency of Smad2/Smad4 heterocomplexes than their nuclear export efficiency is the fundamental cause for their significant nuclear accumulation under the stimulation of TGF-β1 (Fig. 1, F–H).
Finally, we observed the same dwell times for the nuclear import and the nuclear export of Smad heterocomplexes, which are also very similar to the nuclear transport times of the basal-state Smads (Figs. 2, F–I and 3, F and G). These similar nuclear transport times seem insensitive to their sizes and actually could be expected given the following reasons. First, the molecular weight of each of these substrates that were fused with GFP or RFP is far above the cutoff size limits (∼40–60 kDa) for passive diffusion through the NPCs. Thus, these Smad proteins are unlikely to passively diffuse through the NPCs (17, 18, 19). Instead, these substrates containing NLS and/or NES can be recognized by one or more types of transport receptors, and then their nuclear transport kinetics will heavily rely on the interactions between the transport receptors and FG-Nups in the NPCs (39). Previously, the nuclear transport times of different kinds of transport receptors have been determined to be 3–8 ms (39, 54), which largely agree with the determined transport times of Smad proteins in our study.
Conclusions
The Smad heterocomplexes regulate the expression of hundreds of TGF-β target genes and are involved in various functions, from cell proliferation to apoptosis to cancer growth (13, 14, 15). The Smad heterocomplex level in the nucleus is believed to be converted proportionately to transcriptional responses and expression of the target (61). Thus, nuclear translocation of phosphorylated Smad heterocomplexes plays critical roles in signal transduction of TGF-β1 from transmembrane receptors into the nucleus and regulates the transcriptional response and expression of target genes. On the other hand, dysfunctional nucleocytoplasmic transport of Smad heterocomplexes through the NPCs in the TGF-β/Smad signaling pathway has been associated with many human diseases (16, 62, 63). However, the detailed relationship between the nuclear accumulation of Smad heterocomplexes and their nucleocytoplasmic transport kinetics with the stimulation of TGF-β1 remains obscure. Moreover, the association and disassociation sites of Smad heterocomplexes in live cells were never systematically investigated (7). To answer these questions, we have combined a high-speed single-molecule tracking microscopy and FRET technique to track the TGF-β1-induced entire process of Smad2/Smad4 heterocomplex formation and their transport through the NPCs in live cells. With a high spatiotemporal single-molecule tracking resolution, we found that in TGF-β1-treated cells, the Smad2/Smad4-involved formation of Smad heterocomplexes was enhanced in the cytoplasm, and the heterocomplexes transported through the NPCs as entireties and finally dissociated in the nucleus before exiting. Moreover, our high-speed single-molecule tracking data revealed that the basal-state Smad2 or Smad4 cannot accumulate in the nucleus because of an approximately twofold higher nuclear export efficiency than its nuclear import in the absence of TGF-β1. On the contrary, the heterocomplexes of Smad2/Smad4 induced by TGF-β1 generated almost fourfold higher nuclear import efficiency than their nuclear export efficiency and thus can maintain their nuclear accumulation as needed. Overall, our study reveals the basic relationship between the nuclear accumulation and the nucleocytoplasmic transport kinetics for the basal-state Smads and the Smad heterocomplex in live cells.
Finally, our approach can be further applied to study the detailed roles of multiple transport receptors, such as importin7 and importin8 (30), played in mediating nucleocytoplasmic transport of Smad proteins. Also, we are interested in expanding the approach to study the nuclear transport details for various transcription factors, such as STAT1, ERK1, and JNK1 (64, 65), that were shown to accumulate in the nucleus under stimulated conditions.
Author Contributions
W.Y. designed experiments. Y.L. and W.L. performed experiments. Y.L., W.L., and W.Y. established cell lines, built microscopy equipment, conducted data analysis, and wrote the manuscript.
Acknowledgments
We greatly thank Ali H. Brivanlou (Rockefeller University, New York) for providing the plasmid of RFP-Smad2, Xiaohong Fang (Chinese Academy of Sciences, Beijing, China) for providing the plasmid of EGFP-Smad4, and Raymond Habas and Andrew Ruba (Temple University, Philadelphia, PA) for critical reading of this manuscript.
The project was supported by grants from the US National Institutes of Health (GM094041, GM097037, GM116204, and GM22552) to W.Y.
Editor: Elsa Yan.
Footnotes
Twelve videos are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30385-0.
Supporting Material
This video shows a typical successful nuclear import event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear export event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear import event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear export event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear import event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear export event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear import event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear export event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear import event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical nuclear export event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear import event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear export event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
References
- 1.Massagué J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakao A., Afrakhte M., ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-β signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 3.Heldin C.H., Miyazono K., ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 4.Attisano L., Wrana J.L. Mads and Smads in TGF β signalling. Curr. Opin. Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- 5.Massagué J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 6.Attisano L., Wrana J.L. Signal transduction by the TGF-β superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 7.Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y., Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 9.Derynck R., Zhang Y., Feng X.H. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmierer B., Hill C.S. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor β-dependent nuclear accumulation of Smads. Mol. Cell. Biol. 2005;25:9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warmflash A., Zhang Q., Brivanlou A.H. Dynamics of TGF-β signaling reveal adaptive and pulsatile behaviors reflected in the nuclear localization of transcription factor Smad4. Proc. Natl. Acad. Sci. USA. 2012;109:E1947–E1956. doi: 10.1073/pnas.1207607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill C.S. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19:36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- 13.de Caestecker M.P., Piek E., Roberts A.B. Role of transforming growth factor-β signaling in cancer. J. Natl. Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- 14.Muraoka-Cook R.S., Dumont N., Arteaga C.L. Dual role of transforming growth factor β in mammary tumorigenesis and metastatic progression. Clin. Cancer Res. 2005;11:937s–943s. [PubMed] [Google Scholar]
- 15.Moses H., Barcellos-Hoff M.H. TGF-β biology in mammary development and breast cancer. Cold Spring Harb. Perspect. Biol. 2011;3:a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blobe G.C., Schiemann W.P., Lodish H.F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 17.Yang W. Distinct, but not completely separate spatial transport routes in the nuclear pore complex. Nucleus. 2013;4:166–175. doi: 10.4161/nucl.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naim B., Brumfeld V., Reich Z. Passive and facilitated transport in nuclear pore complexes is largely uncoupled. J. Biol. Chem. 2007;282:3881–3888. doi: 10.1074/jbc.M608329200. [DOI] [PubMed] [Google Scholar]
- 19.Terry L.J., Shows E.B., Wente S.R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 20.Beck M., Förster F., Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. doi: 10.1126/science.1104808. [DOI] [PubMed] [Google Scholar]
- 21.Denning D.P., Patel S.S., Rexach M. Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc. Natl. Acad. Sci. USA. 2003;100:2450–2455. doi: 10.1073/pnas.0437902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Goryaynov A., Yang W. The selective permeability barrier in the nuclear pore complex. Nucleus. 2016;7:430–446. doi: 10.1080/19491034.2016.1238997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rout M.P., Aitchison J.D., Chait B.T. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cronshaw J.M., Krutchinsky A.N., Matunis M.J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macara I.G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001;65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wente S.R., Rout M.P. The nuclear pore complex and nuclear transport. Cold Spring Harb. Perspect. Biol. 2010;2:a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore M.S. Ran and nuclear transport. J. Biol. Chem. 1998;273:22857–22860. doi: 10.1074/jbc.273.36.22857. [DOI] [PubMed] [Google Scholar]
- 28.Schwoebel E.D., Ho T.H., Moore M.S. The mechanism of inhibition of Ran-dependent nuclear transport by cellular ATP depletion. J. Cell Biol. 2002;157:963–974. doi: 10.1083/jcb.200111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Xu L. Mechanism and regulation of nucleocytoplasmic trafficking of smad. Cell Biosci. 2011;1:40. doi: 10.1186/2045-3701-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X., Chen X., Xu L. Preferential utilization of Imp7/8 in nuclear import of Smads. J. Biol. Chem. 2008;283:22867–22874. doi: 10.1074/jbc.M801320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierreux C.E., Nicolás F.J., Hill C.S. Transforming growth factor β-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol. Cell. Biol. 2000;20:9041–9054. doi: 10.1128/mcb.20.23.9041-9054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L., Kang Y., Massagué J. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFbeta signaling complexes in the cytoplasm and nucleus. Mol. Cell. 2002;10:271–282. doi: 10.1016/s1097-2765(02)00586-5. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata M., Inoue H., Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J.W., Fairman R., Shi Y. Formation of a stable heterodimer between Smad2 and Smad4. J. Biol. Chem. 2001;276:20688–20694. doi: 10.1074/jbc.M100174200. [DOI] [PubMed] [Google Scholar]
- 35.Randall R.A., Howell M., Hill C.S. Recognition of phosphorylated-Smad2-containing complexes by a novel Smad interaction motif. Mol. Cell. Biol. 2004;24:1106–1121. doi: 10.1128/MCB.24.3.1106-1121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmierer B., Tournier A.L., Hill C.S. Mathematical modeling identifies Smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system. Proc. Natl. Acad. Sci. USA. 2008;105:6608–6613. doi: 10.1073/pnas.0710134105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolás F.J., De Bosscher K., Hill C.S. Analysis of Smad nucleocytoplasmic shuttling in living cells. J. Cell Sci. 2004;117:4113–4125. doi: 10.1242/jcs.01289. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Wolfram J., Ferrari M. Live-cell single-molecule imaging reveals clathrin and caveolin-1 dependent docking of SMAD4 at the cell membrane. FEBS Lett. 2013;587:3912–3920. doi: 10.1016/j.febslet.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J., Liu Z., Yang W. High-resolution three-dimensional mapping of mRNA export through the nuclear pore. Nat. Commun. 2013;4:2414. doi: 10.1038/ncomms3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hjelmeland M.D., Hjelmeland A.B., Rich J.N. SB-431542, a small molecule transforming growth factor-β-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol. Cancer Ther. 2004;3:737–745. [PubMed] [Google Scholar]
- 41.Inman G.J., Nicolás F.J., Hill C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 42.Albertazzi L., Arosio D., Beltram F. Quantitative FRET analysis with the EGFP-mCherry fluorescent protein pair. Photochem. Photobiol. 2009;85:287–297. doi: 10.1111/j.1751-1097.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 43.Ma J., Goryaynov A., Yang W. Super-resolution 3D tomography of interactions and competition in the nuclear pore complex. Nat. Struct. Mol. Biol. 2016;23:239–247. doi: 10.1038/nsmb.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortensen K.I., Churchman L.S., Flyvbjerg H. Optimized localization analysis for single-molecule tracking and super-resolution microscopy. Nat. Methods. 2010;7:377–381. doi: 10.1038/nmeth.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deschout H., Neyts K., Braeckmans K. The influence of movement on the localization precision of sub-resolution particles in fluorescence microscopy. J. Biophotonics. 2012;5:97–109. doi: 10.1002/jbio.201100078. [DOI] [PubMed] [Google Scholar]
- 46.Chacko B.M., Qin B.Y., Lin K. Structural basis of heteromeric smad protein assembly in TGF-β signaling. Mol. Cell. 2004;15:813–823. doi: 10.1016/j.molcel.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Más P., Devlin P.F., Kay S.A. Functional interaction of phytochrome B and cryptochrome 2. Nature. 2000;408:207–211. doi: 10.1038/35041583. [DOI] [PubMed] [Google Scholar]
- 48.Kelich J.M., Ma J., Yang W. Super-resolution imaging of nuclear import of adeno-associated virus in live cells. Mol. Ther. Methods Clin. Dev. 2015;2:15047. doi: 10.1038/mtm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallberg E., Wozniak R.W., Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J. Cell Biol. 1993;122:513–521. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Appen A., Beck M. Structure determination of the nuclear pore complex with three-dimensional cryo electron microscopy. J. Mol. Biol. 2016;428:2001–2010. doi: 10.1016/j.jmb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frenkiel-Krispin D., Maco B., Medalia O. Structural analysis of a metazoan nuclear pore complex reveals a fused concentric ring architecture. J. Mol. Biol. 2010;395:578–586. doi: 10.1016/j.jmb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Maimon T., Elad N., Medalia O. The human nuclear pore complex as revealed by cryo-electron tomography. Structure. 2012;20:998–1006. doi: 10.1016/j.str.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Akey C.W. Interactions and structure of the nuclear pore complex revealed by cryo-electron microscopy. J. Cell Biol. 1989;109:955–970. doi: 10.1083/jcb.109.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W., Musser S.M. Nuclear import time and transport efficiency depend on importin β concentration. J. Cell Biol. 2006;174:951–961. doi: 10.1083/jcb.200605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelich J., Goryaynov A., Yang W. vol. 25. American Society for Cell Biology; 2014. Super-resolution microscopy study of the pre-ribosomal subunit nuclear export mechanism. (Molecular Biology of the Cell). [Google Scholar]
- 56.Zi Z., Chapnick D.A., Liu X. Dynamics of TGF-β/Smad signaling. FEBS Lett. 2012;586:1921–1928. doi: 10.1016/j.febslet.2012.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribbeck K., Görlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu L.C., Fu G., Musser S.M. Large cargo transport by nuclear pores: implications for the spatial organization of FG-nucleoporins. EMBO J. 2013;32:3220–3230. doi: 10.1038/emboj.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dai F., Duan X., Feng X.H. Coupling of dephosphorylation and nuclear export of Smads in TGF-beta signaling. Methods Mol. Biol. 2010;647:125–137. doi: 10.1007/978-1-60761-738-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin X., Duan X., Feng X.H. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guzman-Ayala M., Lee K.L., Episkopou V. Graded Smad2/3 activation is converted directly into levels of target gene expression in embryonic stem cells. PLoS One. 2009;4:e4268. doi: 10.1371/journal.pone.0004268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura M., Ito H., Kusaka H. Phosphorylated Smad2/3 immunoreactivity in sporadic and familial amyotrophic lateral sclerosis and its mouse model. Acta Neuropathol. 2008;115:327–334. doi: 10.1007/s00401-007-0337-z. [DOI] [PubMed] [Google Scholar]
- 63.Ueberham U., Ueberham E., Arendt T. Altered subcellular location of phosphorylated Smads in Alzheimer’s disease. Eur. J. Neurosci. 2006;24:2327–2334. doi: 10.1111/j.1460-9568.2006.05109.x. [DOI] [PubMed] [Google Scholar]
- 64.McBride K.M., Banninger G., Reich N.C. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-α. EMBO J. 2002;21:1754–1763. doi: 10.1093/emboj/21.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plotnikov A., Zehorai E., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows a typical successful nuclear import event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear export event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear import event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear export event of single Smad4 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear import event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear export event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear import event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear export event of single Smad2 molecules (green spot) in live cells. Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. The compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical successful nuclear import event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical nuclear export event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear import event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).
This video shows a typical abortive nuclear export event of single Smad4/Smad2 protein complexes in live cells tracked by a dual-channel setup, in which the left channel is for the donor Smad4-GFP (green) and the right one for the FRET signal between Smad4-GFP and Smad2-mCherry (red). Pixels are 240-nm square, each frame was acquired in 2 ms, and the playback speed is 500× slower than real time. In each half of the CCD detection area, the compartment on the left side of the NE (the red fluorescent line) is the cytoplasm (C), and the right side is the nucleus (N).