Abstract
Actomyosin contractility is an essential element of many aspects of cellular biology and manifests as traction forces that cells exert on their surroundings. The central role of these forces makes them a novel principal therapeutic target in diverse diseases. This requires accurate and higher-capacity measurements of traction forces; however, existing methods are largely low throughput, limiting their utility in broader applications. To address this need, we employ Fourier-transform traction force microscopy in a parallelized 96-well format, which we refer to as contractile force screening. Critically, rather than the frequently employed hydrogel polyacrylamide, we fabricate these plates using polydimethylsiloxane rubber. Key to this approach is that the polydimethylsiloxane used is very compliant, with a lower-bound Young’s modulus of ∼0.4 kPa. We subdivide these monolithic substrates spatially into biochemically independent wells, creating a uniform multiwell platform for traction force screening. We demonstrate the utility and versatility of this platform by quantifying the compound and dose-dependent contractility responses of human airway smooth muscle cells and retinal pigment epithelial cells. By directly quantifying the endpoint of therapeutic intent, airway-smooth-muscle contractile force, this approach fills an important methodological void in current screening approaches for bronchodilator drug discovery, and, more generally, in measuring contractile response for a broad range of cell types and pathologies.
Introduction
Many adherent cells employ actomyosin contractility to exert traction forces on their surroundings. These forces are an essential part of cellular deformation (1, 2, 3), adhesion (4, 5, 6), spreading (7), and migration (8, 9, 10), as well as growth (11), homeostasis (12, 13), gene expression (14), and apoptosis (15). The significant role of traction force makes it a novel principal therapeutic target in diverse diseases; however, accurate measurements of traction forces are essential for this approach.
To quantify cell traction forces, researchers have employed a variety of techniques and tools. From the first wrinkling thin silicone sheets (16) to complex three-dimensional multicellular contractility (17), a multitude of biomechanical methods have been developed, collectively referred to as traction force microscopy (TFM), as reviewed here (18). Although these approaches have enabled the discovery of valuable mechanobiological connections, these methods are generally inherently slow and restricted to low-throughput implementation, limiting their utility as tools in broader pharmacological applications.
To address this need, we employ Fourier-transform TFM in a parallelized 96-well format, an approach we refer to as contractile force screening (CFS). Critically, rather than using the frequently employed hydrogel polyacrylamide (PAA), we fabricate these plates using polydimethylsiloxane (PDMS) rubber. Key to this approach is that the PDMS used is very compliant, with a lower-bound Young’s modulus of ∼0.4 kPa, unlike commonly used Sylgard 184 formulations (additional detailed mechanical characterization is presented in the Supporting Materials and Methods). Like PAA, soft PDMS elastomers possess several material-favorable properties: their stiffness is tunable over a large physiological range (Fig. 1), and they are nontoxic, nondegrading, and biologically inert. In addition to these aspects, this compliant PDMS has numerous advantages over PAA: 1) it is optically transparent, with a refractive index of ∼1.4, which is comparable to glass; 2) it is indefinitely stable after production without special storage considerations; 3) it is amenable to spin coating, providing a simple means of creating a uniform and flat surface; and 4) it is a uniformly nonporous surface, unlike PAA, whose porosity can vary strongly with cross-linking concentration (19). Critically, the monolithic, impermeable nature of these silicone substrates makes them easy and ideal to subdivide spatially into biochemically independent wells, creating a uniform multiwell platform for TFM. Taken together, our compliant PDMS presents numerous advantages in becoming a new standard in soft substrates for TFM; these advantages are particularly important for a standardized higher-throughput technology and will enable widespread adoption of CFS from our previous approach using PAA (20).
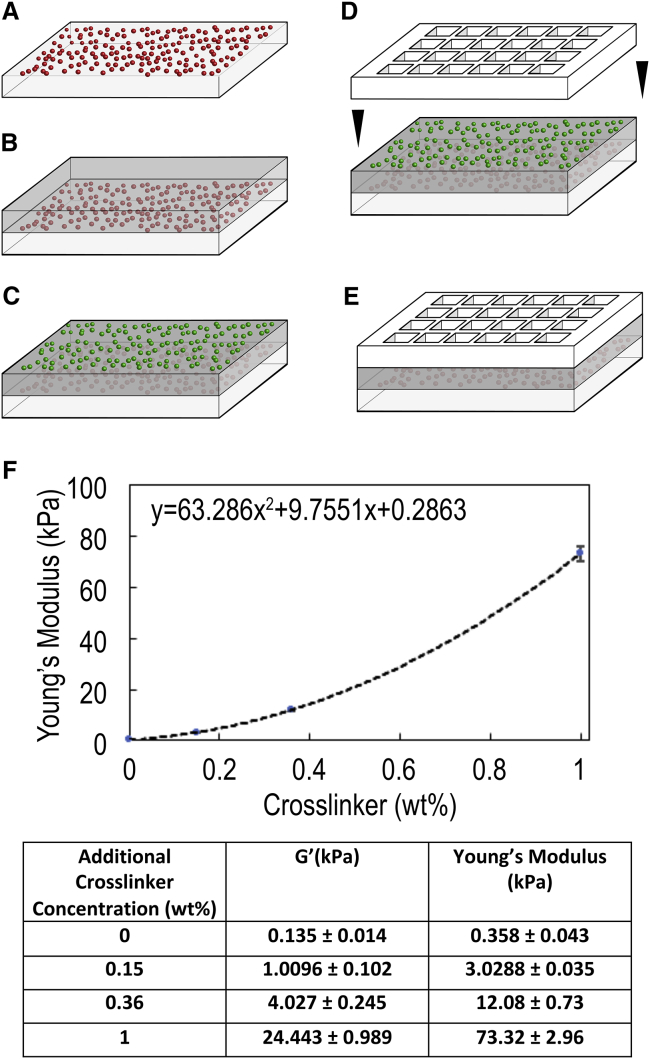
Figure 1.
Fabrication of multiwell substrates and mechanical characterization of tunable PDMS elastomer. Multiwell PDMS devices for TFM are fabricated by (A) optionally coating a layer of fluorescent microspheres (for de-drifting images) on the custom glass slide, (B) spin-coating a ∼100-μm-thick layer of compliant PDMS, (C) spin-coating a ∼1-μm-thick layer of compliant PDMS mixed with custom-synthesized beads with different fluorescence emission for measuring cell-induced deformation of the PDMS, (D) bonding a multiwell partitioning strip to the top, and (E) ligating and culturing cells. (F) A graph of PDMS moduli as determined by shear rheology (n = 4 independent preparations per data point) is shown. (G) A table of same-mean PDMS moduli ± SD is shown. To see this figure in color, go online.
Methods
Cell culture
Primary human airway smooth muscle (ASM) cells were obtained from the Gift of Hope Organ and Tissue Donor Network (Itasca, IL). These cells have been well-characterized previously, e.g., (21). All measurements were performed using cells at passage 5–8 from two nonasthmatic donors. ARPE-19 (retinal pigment epithelium) cells were obtained from American Type Culture Collection (Manassas, VA). All culture media formulations are provided in the Supporting Materials and Methods.
Preparation of silicone substrates in custom 96-well plates
We fabricate our multiwell TFM dishes by applying very compliant and tunable modulus PDMS onto custom-cut glass slides and then partitioning the wells with a plastic subdivider (Fig. 1 A–E). In brief, very compliant commercial PDMS (NuSil 8100; NuSil Silicone Technologies, Carpinteria, CA) is mixed with a small percentage by weight of Sylgard 184 cross-linking agent to make a tunable (E = 0.4–73 kPa) substrate, which is impregnated with an ∼1-μm-thick layer of fiduciary particles to reveal cell-induced deformations. This approach differs from existing PDMS TFM strategies, as this substrate is comparably compliant to PAA and linearly elastic, yet not a hydrogel. Full details of plate preparation, including detailed substrate functionalization and mechanical characterization, are provided in the Supporting Materials and Methods.
Mechanical characterization of PDMS substrates
We measured the frequency-dependent storage and loss-shear moduli for PDMS with different additional cross-linker formulations using shear rheology (MCR 302, 25-mm parallel plate tool; Anton Paar, Graz, Austria). Samples of ∼0.6 mL were loaded and cured at 100 C for 2–3 h, the normal force was reset, and the shear modulus was measured at 1 Hz and 0.5% strain. Young’s moduli, E, were calculated from shear moduli, G, as E = 2 × G(1 + v) by assuming the PDMS is incompressible with a Poisson ratio, v, of 0.5 (Fig. 1 F and G). Further mechanical characterization is described in the Supporting Materials and Methods.
Measurements of cell traction forces
The 96-well plate was mounted within a heated chamber (37°C) upon an automated computer-controlled motorized stage and imaged at 10× magnification using a monochrome camera (DFC365 FX; Leica, Wetzlar, Germany) affixed to an inverted microscope (DMI 6000B; Leica). We acquired fluorescent images of microspheres embedded in the elastic substrate immediately underneath the cells at 1) baseline with no treatment, 2) after treatment, and 3) after cell detachment with trypsin (reference null-force image). By comparing the fluorescent images at reference with the corresponding images at baseline and after treatment, we obtain a time series of bead displacement and hence substrate deformation fields (resolution = ∼15 μm). Using the measured substrate deformation, the predefined substrate modulus, and thickness, traction force maps and the root mean-squared value were calculated over a 732 μm × 732 μm area on a well-by-well basis, using the approach of Fourier-transform traction cytometry (22) modified to the case of cell monolayers (23).
Drugs
Histamine, isoproterenol, salbutamol, salmeterol, formoterol, thrombin, and H202 were purchased from Sigma-Aldrich (St. Louis, MO). Y27632 was purchased from EMD Millipore (Burlington, MA). Human VEGF-A165 and bevacizumab were purchased from R&D systems (Minneapolis, MN) and Genentech (South San Francisco, CA), respectively.
Statistics
Statistical comparisons for traction differences were performed using the nonparametric Wilcoxon matched-pairs signed rank test. Differences were considered significant when p < 0.05.
Results and Discussion
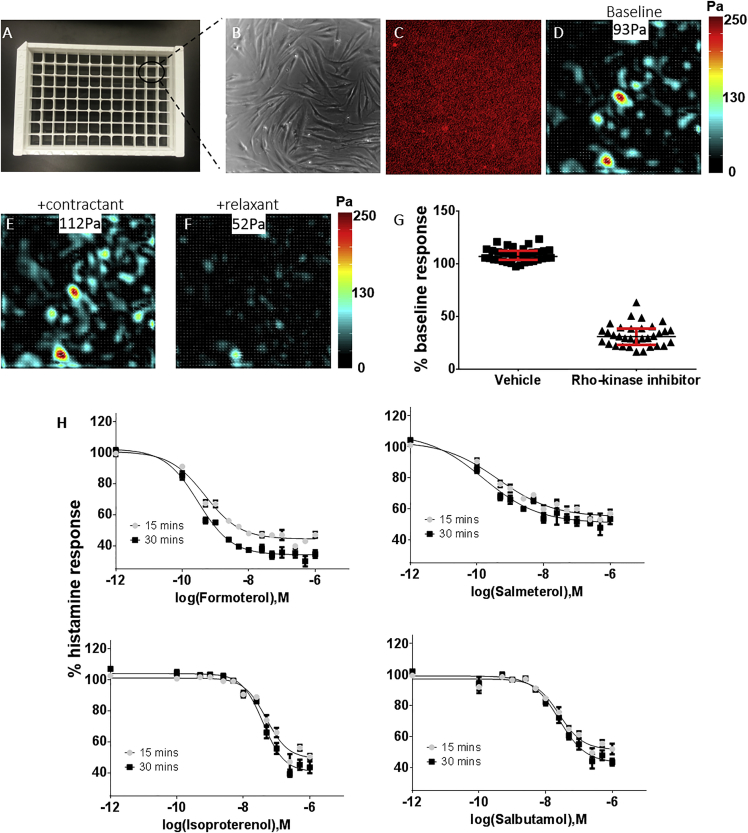
CFS entails quantifying the cell-generated forces by measuring fluorescent bead positions in each well of the 96-well plate: 1) without cells, 2) with cells adhered at baseline contractility, and 3) after treatment with the compound(s) of interest. For example, for a representative well of a 96-well plate (Fig. 2 A), shown are ASM traction force maps and the root mean-squared value (inset) at baseline (Fig. 2 D, 93 Pa), after treatment with the contractant, the H1 agonist histamine (Fig. 2 E, 112 Pa), and after additional treatment with the relaxant, the β2 adrenergic receptor agonist isoproterenol (Fig. 2 F, 52 Pa).
Figure 2.
CFS using soft elastomeric substrates recapitulates known ASM pharmacological responses. Human ASM cells were cultured to confluence upon Young’s modulus = 12 kPa (0.36% cross-linker) collagen-coated 96-well silicone substrates. (A–D) For a representative well of a 96-well plate, images of cells, fluorescent beads, traction force maps, and average magnitude (inset) at baseline are shown. (E and F) For the same well, shown are traction force maps and the average magnitude (inset) with the contractant compound histamine (10 μM, 30 min), and after additional treatment with the relaxant compound isoproterenol (0.5 μM, 30 min). (G) Over the 96-well plate, the force measurements are statistically different (p < 0.05) between positive and negative controls, as ascertained by an unpaired t-test. (H) Force measurements confirmed known differences in potency among a panel of functionally diverse ASM relaxation compounds (formoterol > salmeterol > salbutamol > isoproterenol). Plotted are the mean ± standard error calculated from three to eight wells per dose per ASM relaxation compound. Data were pooled from two to four 96-well plates tested on different days but under identical experimental conditions. To see this figure in color, go online.
First, we tested the suitability of our approach for higher-capacity measurements. We evaluated common factors associated with ASM contraction, including constituents of the culture medium and properties of the cellular substrate. Although serum deprivation only marginally affected the scope of ASM relaxation (contraction with 10 μM histamine–relaxation with additional 1 μM isoproterenol), substrate stiffness had a profound impact, with an optimal response on 12-kPa stiff substrates. Given these findings, we focused subsequent studies on 12-kPa stiff substrates prepared in 96-well plates. Individual wells of a representative plate were either assigned to a positive or negative control group. In the positive control group, cells were prestimulated with 10 μM histamine to induce maximal contractility, followed by poststimulation with the relaxant, 10 μM Y27632, for 30 min. In the negative control group, cells were prestimulated with vehicle (phosphate-buffered saline), followed by poststimulation with vehicle (phosphate-buffered saline) for 30 min. In both groups, traction-force-measured poststimulation was normalized to the corresponding prestimulation value on a well-by-well basis. From these measurements of normalized changes, we determined that the groups were statistically different (p < 0.05), as ascertained by an unpaired Student’s t-test.
Next, we verified the utility of our approach in pharmacology by examining traction force changes induced by a diverse set of well-known and clinically relevant ASM relaxation compounds (24). Each compound was evaluated in a 10-point dose-response manner across adjacent rows of the 96-well plate. Data were pooled from multiple plates and reported as a percentage of histamine response. The extent of ASM relaxation confirmed the known differences in potency of the β2 adrenergic receptor agonists (salmeterol > formoterol > salbutamol > isoproterenol) (24), and the full agonist, formoterol, provided a greater scope of relaxation than the partial agonist, salmeterol, as expected (25) (Fig. 2 H; Table S1). Notably, as supported by negligible standard errors and the small coefficients of variation, the data were highly reproducible.
Here we have focused on ASM response; yet this approach is applicable in pharmacology to any adherent contractile cell type and is therefore expected to be of broad utility. In ASM, this need is particularly exigent, as current efforts to screen new ASM relaxation drugs employ indirect assay methods that are poorly predictive of functional response. Commonplace examples include the dissociation of intracellular calcium regulation from the effects of bradykinin, bitter tastants (26), and proton-sensing receptor ligands (27) on ASM contraction, a similar dissociation of cAMP regulation from bronchorelaxant effect (procontractile receptor antagonists and again bitter tastants), and the limited predictive utility of membrane potential for almost all drugs whether they target receptors or other contractile effectors or signaling elements. A more relevant screen that directly quantifies the target output of ASM relaxation, as does CFS, is required to efficiently test the pending generations of ASM relaxation drugs. To this end, CFS fills an important methodological void in ASM biology, and, more generally, in measuring contractile response for a broad range of cell types and pathologies.
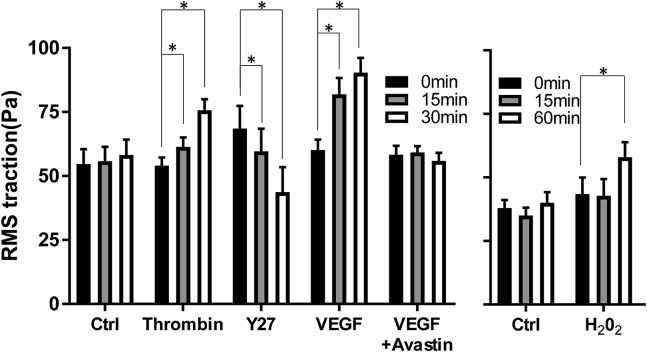
To demonstrate the versatility of CFS, we examined a key pathogenic mechanism common to many ocular pathologies—dysfunction of the retinal pigmented epithelium (RPE) (28). We discovered that the RPE barrier-disruptive agent thrombin (29), the proangiogenic cytokine VEGF-A (30), and the oxidative stressor H2O2 (31) each caused an increase in RPE traction forces (Fig. 3). Conversely, the rho kinase inhibitor Y27632 or the VEGF-A inhibitor bevacizumab ablated these forces. Taken together, these data reveal a novel role for traction force increase in RPE dysfunction and advocate for new discovery efforts targeted at reducing these forces. This might be especially pertinent to offset RPE dysfunction in the commonly occurring dry form of macular degeneration (32), wherein no therapeutic intervention currently exists.
Figure 3.
Mediators of retinal epithelial dysfunction increase cell traction forces. Human ARPE-19 cells were cultured to confluence upon Young’s modulus = 12 kPa (0.36% cross-linker) collagen-coated 96-well silicone substrates, and cell-contractile forces were measured at baseline (0 min) and after treatment (15, 30/60 min). Although thrombin (1 unit/mL), VEGF (100 ng/mL), and H202 (100 nM) increased baseline forces in a time-dependent manner, the rho kinase inhibitor Y27632 (10 μM) ablated them. Bevacizumab (0.05 mg/mL) prevented the VEGF-induced force increase. Plotted is the mean ± standard error pooled from 8–24 wells per time point per treatment. ∗ indicates significant difference compared to baseline.
Conclusions
We have demonstrated utility for a 96-well silicone-based substrate for CFS. This approach is advantageous over CFS using PAA (20), as the material itself is more robust and uniform, and the fabrication of multiwell silicone substrates eliminates time-consuming production steps, utilizes standard microfabrication procedures, and obviates the need for surface-bound fluorescent beads by embedding them as a monolayer by spin coating. Moreover, silicone elastomers possess many material-favorable properties over PAA. Specifically, they are predominantly elastic in the stiffness range that encompasses most physiological microenvironments, are nonporous and impermeable—thus obviating common concerns associated with PAA (19)—and possess superior optical properties.
Mechanical malfunction appears be an integral component of many diverse diseases, including asthma, ocular pathologies, acute lung injury, bladder dysfunction, vascular diseases, fibrosis, and cancer, wherein cell contractile forces play a pivotal role. CFS using elastic silicone substrates is expected to enable mechanistic studies for both quantitatively describing the aberrant contractile forces as well as mechanically rectifying the responses by directly evaluating potential therapeutic compounds.
Author Contributions
H.Y., N.K., R. Kaviani, and M.T. fabricated and measured PDMS TFM surfaces. K.R., S.Y., and Q.D. performed TFM in ASM cells. C.L. and J.K.S. optimized TFM in ASM cells. A.H. performed TFM in RPE cells. M.S.-G., R. Krishnan, and A.J.E. devised study, contributed reagents, and wrote the manuscript.
Acknowledgments
The authors thank Cynthia Crespo, Erin Hannen, and Luc Mongeau for technical assistance.
A.J.E. acknowledges Natural Sciences and Engineering Research Council grants RGPIN/05843-2014 and EQPEQ/472339-2015, Canadian Institutes of Health Research grant no. 143327, and Canadian Cancer Society grant no. 703930. R. Krishnan acknowledges National Institutes of Health grant no. R21HL123522 and Amgen Inc. H.Y. was supported by Fonds de recherche Santé Quebec. M.S.-G. acknowledges National Institutes of Health grant no. DP2-OD006649.
Editor: Philip LeDuc.
Footnotes
Ramaswamy Krishnan and Allen J. Ehrlicher contributed equally to this work.
Supporting Materials and Methods, nine figures, and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30439-9.
Supporting Citations
References (33, 34, 35, 36) appear in the Supporting Material.
Supporting Material
References
- 1.Pourati J., Maniotis A., Wang N. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am. J. Physiol. 1998;274:C1283–C1289. doi: 10.1152/ajpcell.1998.274.5.C1283. [DOI] [PubMed] [Google Scholar]
- 2.Wang N., Tolić-Nørrelykke I.M., Stamenović D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 3.Stamenovic D., Suki B., Fredberg J.J. Rheology of airway smooth muscle cells is associated with cytoskeletal contractile stress. J. Appl. Physiol. 2004;96:1600–1605. doi: 10.1152/japplphysiol.00595.2003. [DOI] [PubMed] [Google Scholar]
- 4.Balaban N.Q., Schwarz U.S., Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 5.Chen C.S., Alonso J.L., Ingber D.E. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 6.Lele T.P., Pendse J., Ingber D.E. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell. Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 7.Tan J.L., Tien J., Chen C.S. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tambe D.T., Hardin C.C., Trepat X. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.H., Serra-Picamal X., Fredberg J.J. Propulsion and navigation within the advancing monolayer sheet. Nat. Mater. 2013;12:856–863. doi: 10.1038/nmat3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrlicher A.J., Krishnan R., Pollak M.R. Alpha-actinin binding kinetics modulate cellular dynamics and force generation. Proc. Natl. Acad. Sci. USA. 2015;112:6619–6624. doi: 10.1073/pnas.1505652112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh K., Thodeti C.K., Ingber D.E. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc. Natl. Acad. Sci. USA. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan R., Park C.Y., Fredberg J.J. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS One. 2009;4:e5486. doi: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C., Krishnan R., Fredberg J.J. Fluidization and resolidification of the human bladder smooth muscle cell in response to transient stretch. PLoS One. 2010;5:e12035. doi: 10.1371/journal.pone.0012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prager-Khoutorsky M., Lichtenstein A., Bershadsky A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011;13:1457–1465. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.S., Mrksich M., Ingber D.E. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 16.Harris A.K., Wild P., Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 17.Steinwachs J., Metzner C., Fabry B. Three-dimensional force microscopy of cells in biopolymer networks. Nat. Methods. 2016;13:171–176. doi: 10.1038/nmeth.3685. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan R., Tambe D., Butler J. Traction Microscopy. In: Currier C.M., Pelling A.E., editors. Cells, Forces, and the Microenvironment. Pan Stanford; 2015. pp. 75–96. [Google Scholar]
- 19.Trappmann B., Gautrot J.E., Huck W.T. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 20.Park C.Y., Zhou E.H., Krishnan R. High-throughput screening for modulators of cellular contractile force. Integr. Biol. (Camb) 2015;7:1318–1324. doi: 10.1039/c5ib00054h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comer B.S., Camoretti-Mercado B., Gerthoffer W.T. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L727–L734. doi: 10.1152/ajplung.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler J.P., Tolić-Nørrelykke I.M., Fredberg J.J. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 2002;282:C595–C605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 23.Trepat X., Wasserman M.R., Fredberg J.J. Physical forces during collective cell migration. Nat. Phys. 2009;5:426–430. [Google Scholar]
- 24.Sturton R.G., Trifilieff A., Barnes P.J. Pharmacological characterization of indacaterol, a novel once daily inhaled 2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J. Pharmacol. Exp. Ther. 2008;324:270–275. doi: 10.1124/jpet.107.129296. [DOI] [PubMed] [Google Scholar]
- 25.Battram C., Charlton S.J., Trifilieff A. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J. Pharmacol. Exp. Ther. 2006;317:762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- 26.Deshpande D.A., Wang W.C., Liggett S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena H., Deshpande D.A., Penn R.B. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br. J. Pharmacol. 2012;166:981–990. doi: 10.1111/j.1476-5381.2011.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparrow J.R., Hicks D., Hamel C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klettner A., Kaya L., Roider J. Basal and apical regulation of VEGF-A and placenta growth factor in the RPE/choroid and primary RPE. Mol. Vis. 2015;21:736–748. [PMC free article] [PubMed] [Google Scholar]
- 30.Penn J.S., Madan A., Hartnett M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanus J., Zhang H., Wang S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis. 2013;4:e965. doi: 10.1038/cddis.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jager R.D., Mieler W.F., Miller J.W. Age-related macular degeneration. N. Engl. J. Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 33.Pham J.T., Schellenberger F., Butt H.J. From elasticity to capillarity in soft materials indentation. Phys. Rev. Materials. 2017;1:015602. [Google Scholar]
- 34.Notbohm J., Poon B., Ravichandran G. Analysis of nanoindentation of soft materials with an atomic force microscope. J. Mater. Res. 2011;27:229–237. [Google Scholar]
- 35.Klein S.M., Manoharan V.N., Lange F.F. Preparation of monodisperse PMMA microspheres in nonpolar solvents by dispersion polymerization with a macromonomeric stabilizer. Colloid Polym. Sci. 2003;282:7–13. [Google Scholar]
- 36.Zhang, W., X. Dong, …, X. Liu. 2015. A cost-effective microindentation system for soft material characterization. 2015 IEEE International Conference on Mechatronics and Automation (ICMA), pp. 825–830.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.