Abstract
Background
Canine mammary gland tumors (CMGTs) are the most common, spontaneous types of neoplasias in female dogs. Aberrant DAPK1 and MGMT methylation associated with tumor formation and development in various cancers. 5-Azacytidine is a known specific demethylation drug that covalently binds to DNA methyltransferase. However, the methylation of the DAPK1 and MGMT is unknown with respect to CMGTs. Therefore, we sought to demonstrate the effects of 5-azacytidine on the proliferation of CMGTs cell, and elucidate the potential molecular mechanisms of action in these cancerous cells.
Materials and methods
The effects of 5-azacytidine on CHMm and CHMp cell proliferation were evaluated by MTT assay. The DAPK1 and MGMT gene methylation patterns in CHMm and CHMp cells and CMGTs blood/tissue samples were analyzed by MSP assay. Effect of 5-azacytidine on the methylation of DAPK1 and MGMT gene, and DAPK1 and MGMT mRNA expression in CHMm and CHMp cells were analyzed by MSP assay and qRT-PCR assay, respectively.
Results
5-Azacytidine may suppress the proliferation of CHMm and CHMp cells. Furthermore, the DAPK1 and MGMT genes were hypermethylated in CHMm/CHMp cells and clinical malignant tumor samples, but not in normal female dogs’ blood and tissue. However, the DAPK1 and MGMT genes were re-inducible in CHMm and CHMp cells treated with 5 μM 5-azacytidine. Meanwhile, 5-azacytidine increased the expression of DAPK1 and MGMT mRNA.
Conclusion
These results suggest that DAPK1 and MGMT methylation can serve as sensitive diagnostic biomarkers and therapeutic targets for CMGTs. 5-Azacytidine also could be a potential therapeutic candidate for CMGTs.
Keywords: canine mammary gland tumor, CHMm, CHMp, 5-azacytidine, DAPK1, MGMT, DNA methylation
Introduction
Canine mammary gland tumors (CMGTs) are the most common, spontaneous types of neoplasias in female dogs.1,2 Approximately 50%–60% of CMGTs are considered malignant as determined by histologic examination.2 Human and CMGTs share similar molecular characteristics.3 Currently, surgical resection and chemotherapy are the standard treatment for these tumors.4 However, detection of metastasis after surgical resection results in a poor prognosis,5 and the subsequent chemotherapy currently in use has considerable side effects and the neoplasias may develop resistance to the drugs. Therefore, the development of novel and effective chemotherapeutic drugs against mammary gland tumor is of great importance.
CMGTs are a heterogeneous disease, involving multiple genes, cellular factors, and multistage complex processes.6 DNA methylation is one of the most widely studied epigenetic mechanisms that are believed to be involved in various cancers, and has been associated with tumor formation and development.7 Aberrant DNA methylation is a biochemical process, which is reversible through interventions with demethylating agents.8 5-Azacytidine is a known specific demethylation drug that covalently binds to DNA methyltransferase, thus reducing the biological activity of DNA methyltransferase.9 This results in gene demethylation and increases the expression of the inactivated gene through promoter CpG islands.10 The antineoplastic activity of 5-azacytidine has been demonstrated for non-Hodgkin lymphoma,10 breast cancer,11 hepatocellular cancer,9 melanoma,12 lung cancer,13 systemic masocytosis,14 and canine urothelial carcinoma15 based on in vivo and in vitro studies. However, little is known about the anticancer effects of 5-azacytidine on CMGTs.
The DAPK1 gene is located at the human chromosomal locus 9q34.1 and encodes a (CaM)-dependent serine–threonine kinase with a molecular mass of 160 kDa.16 This protein is known to be a positive regulator of apoptosis, inducing and accelerating programmed cell death, through the activation of various pathways, including p53, TNF-α, FAS, IFN, and TGF.16,17 As a tumor suppressor gene, DAPK1 expression is downregulated in a variety of tumors, which can be observed in 70% of B-cell lymphoma and leukemia cells18 and iñ30% of bladder cancers and breast cancers.19 The MGMT gene is a conserved DNA repair enzyme located on chromosome 10q21.20 Hypermethylation of the promoter CpG islands was observed to reduce the expression of MGMT genes. MGMT protects the cells from alkylating agents by irreversibly transferring alkylated groups from O6-methylguanine to the cysteine residues at position 145 of the MGMT protein.21 The expression levels and the gene structure of MGMT have been found to be affected by polymorphism in gene.22 Mutation in and hypermethylation of the promoter region have been shown to result in an increased incidence of tumor formation.22,23 Several previous studies have demonstrated that the MGMT gene promoter is almost universally methylated in neoplastic cells; however, it remains predominantly unmethylated in normal tissues.22,24 These studies clearly suggest that the methylated DAPK1 and MGMT promoters may be valuable clinical diagnostic biomarkers for most tumors, and that they appear to be promising molecular targets for many cancers. However, this potential is unknown with respect to CMGTs. Therefore, we sought to demonstrate the effects of 5-azacytidine on the proliferation of CMGT cells and elucidate the potential molecular mechanisms of action in these cancerous cells.
Materials and methods
Ethical statement
Written informed consent was obtained from all owners of pets that were involved in this study. These clinical trial dogs have received professional veterinary care and special treatment measures (thus reducing the chance of pain and infection, preventing wound complications, and accelerating the recovery time). Approval for this study (Clinical trial dogs and CHMm/CHMp cell line) was obtained from the Laboratory Animal Ethical Committee of Northeast Agricultural University, Harbin, China.
Clinical samples
With the pet owner’s explicit consent, tissue samples and 5 mL of blood from 24 female dogs with CMGTs were obtained at the College of Veterinary Medicine, Northeast Agricultural University, Harbin, China, between May 2011 and September 2015. The patients with CMGTs had not yet undergone chemotherapy or radiotherapy before or after surgery, at the time of sample collection. Some tumor tissues were snap frozen in liquid nitrogen and preserved at −80°C, while others were immediately fixed in 10% neutral buffered formalin and prepared for histopathologic analysis by conventional methods, and then the tissues were paraffin embedded. Sections (3 μm) were obtained from each sample and were subsequently stained with H&E. In addition, blood and tissues samples from six healthy female dogs were used as negative controls. The healthy dogs ranged in age from 3 to 8 years.
Cell culture
The canine CHMm and CHMp cell lines were kindly provided by the Department of Veterinary Medical Sciences, University of Tokyo, Japan. These cell lines were isolated from primary breast cancer and thoracic metastatic lesion of a 12-year-old female dog with metastasis of clinical stage IV.25 The cell lines were maintained in DMEM medium containing 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and 1% antibiotics (100 mg/mL streptomycin, 100 U/mL penicillin; Sigma-Aldrich), and were incubated at 37°C in a humidified 5% CO2 atmosphere. The culture medium was changed, and cells were passaged every 24–48 h depending on the cell density and separated by digesting with 0.25% trypsin. All experiments were performed during the logarithmic growth phase of the cells.
Cell proliferation assays (MTT assay)
The cell lines were seeded in 96-well plates at a concentration of ~3×104 cells/mL. When the cells formed a confluent monolayer, they were treated with 5-azacytidine (0, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, and 100 μM) for 24, 48, or 72 h, respectively. Next, 20 μL MTT agent (5 mg/mL; Sigma-Aldrich) was added to the cells and was incubated for 4 h at 37°C. The supernatants were then removed and 150 μL of dimethyl sulfoxide (Sigma-Aldrich) was added. The plates were shaken and incubated at 37°C for 10 min. Absorbency was measured using a microplate reader (BioTek, Vinooski, VT, USA) at 490 nm. Each experiment was repeated at least five times. Inhibition of cell growth was calculated using the following formula:
DNA methylation analysis
Genomic DNA was extracted using the High Pure PCR Template Preparation kit (Roche Applied Science, Pennsburg, Germany). DNA yields were measured using a Nanodrop® ND-1000 spectrophotometer (PEQLAB Biotechnologies, Erlangen, Germany). The DNA samples were treated with sodium bisulfite using the EpiTect Bisulfite kit (QiaGen, Hilden, Germany) according to the manufacturer’s protocol for the purification of modified DNA. Samples were stored at −80°C for subsequent analysis.
Methylation-specific polymerase chain reaction (MSP)
The modified DNA was used in the MSP assay with primers specific for either methylated or unmethylated DNA simultaneously. The sequence of promoter CpG islands of the relevant genes was analyzed using the Genome Browser Gateway (http://genome.ucsc.edu/cgi-bin/hgGateway). Methylation-specific primers were designed using Methyl primer Express v1.0 (Tables 1 and 2). Twenty-five microliter PCR reactions (16.2 μL ddH2O, 2.5 μL 10× PCR buffer, 2 μL dNTPs, 1 μL sense primers, 1 μL antisense primers, 2 μL DNA, and 0.3 μL ExTaq enzyme) were mixed, and amplification was performed under the following conditions: 94°C for 5 min, 30 cycles of amplification at 94°C for 30 s, 56°C–60°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 10 min. The PCR products were analyzed by 2% agarose gel electrophoresis. The gels were photographed using an automated gel imaging system (ChampGe 16000; Beijing Sage Creation Science Co. Ltd., Beijing, China).
Table 1.
Unmethylated specific primer sequences used for MSP
| Gene | Forward primer | Reverse primer | Annealing temperature/time | Amplicon |
|---|---|---|---|---|
| DAPK1 | GGAAGGAAGAGGGAGAGTTGTT | ACCAACAAAAAACTCAACAAAT | 58°C/30 s | 128 bp |
| MGMT | TTAAGGGAGAGAATGTTTTTATGT | AACAATAAAAACCTCAACTACAAAC | 60°C/30 s | 159 bp |
Abbreviation: MSP, methylation-specific polymerase chain reaction.
Table 2.
Methylated specific primer sequences used for MSP
| Gene | Forward primer | Reverse primer | Annealing temperature/time | Amplicon |
|---|---|---|---|---|
| DAPK1 | AAGGAAGAGGGAGAGTCGTC | CGACGAAAAACTCGACAAAT | 58°C/30 s | 128 bp |
| MGMT | AGGGAGAGAATGTTTTTACGC | AACGATAAAAACCTCGACTACG | 60°C/30 s | 159 bp |
Abbreviation: MSP, methylation-specific polymerase chain reaction.
Quantitative real-time polymerase chain reaction (qRT-PCR)
For the qRT-PCR assay, RNA was extracted from CHMm cells using the TRIzol reagent (Thermo Fisher Scientific). Total cellular RNA (1 μg) was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara, Kyoto, Japan) according to the manufacturer’s instructions. Gene expression analysis was performed on the Light Cycler 2.0 (Hoffman-La Roche Ltd., Basel, Switzerland) using the SYBR Premix Extaq™ II kits (Takara) in a 25 μL PCR reaction volume. The amplification protocol used is as follows: 95°C pre-denatured for 30 s, and 45 cycles of amplification at 95°C for 5 s, then 55°C for 30 s, 72°C for 45 s, followed by a final extension at 72°C for 5 min. Each sample was run in at least five replicates. β-actin was used as the internal control gene. The relative mRNA expression level was calculated using the 2−ΔΔCt method. Primers specific to the target genes were designed using the Primer 5 software (Table 3).
Table 3.
Sequences of qRT-PCR primers
| No | Gene | Forward primer | Reverse primer | Annealing temperature/time | Amplified fragments |
|---|---|---|---|---|---|
| NM001197157.1 | DAPK1 | GCTCATGTCTCTGCAGCAGTTTG | ACACATCCTGGACCGTTTCACTC | 60°C/30 s | 146 bp |
| NM001003376.1 | MGMT | CGCGATTCACCATCCCATT | AACAATAAAAACCTCAACTACAAAC | 60°C/30 s | 85 bp |
| Z_70044 | β-actin | GCTGTCCTGTCCCTGTATTCC | AGCCAAGTCCAGACGACGCAAG | 58°C/30 s | 132 bp |
Abbreviation: qRT-PCR, quantitative real-time polymerase chain reaction.
Statistical analyses
Statistical analyses were performed using the SPSS17.0 software package. The data were expressed as mean plus SD, unless otherwise specified. One-way analysis of variance was used to analyze multiple comparisons data. Results were considered to be statistically significant at P<0.05, and P<0.01 was considered to be highly significant.
Results
Epidemiology and histopathology of CMGTs
Twenty-four dogs with CMGTs were included in this study, none of which had been spayed prior to surgical resection of tumors. The average age of the dogs was 8.5 years (range 1–16 years). The age of the highest incidence of CMGTs was observed to be in 7- to 13-year-old dogs. The mean body weight of the enrolled dogs was 12.5 kg with a range of 3–30 kg. According to our analysis of the patient histories, the Pekingese breed was observed to be the most susceptible breed. Tumor-type determinations were made based on the criteria of the World Health Organization and were made separately by two pathologists.3 Thirteen of the 24 cases were diagnosed histologically as malignant CMGTs (54.17%). Eleven cases were determined to be benign CMGTs (45.83%). The predominant types of tumors observed included invasive ductal carcinoma, intraductal papillary adenoma, simple carcinoma, fibroadenoma, intraductal papillary adenoma, and those not specifically differentiated as a benign mixed tumor. Detailed information and pathologic characteristics of the samples are summarized in Table 4.
Table 4.
Epidemiology and histopathology of 24 dogs with CMGTs
| Type of clinicopathologic diagnoses | Number of samples |
|---|---|
| Age | |
| >8 years | 12 |
| ≤8 years | 12 |
| Tumor time course | |
| >6 months | 15 |
| ≤6 months | 9 |
| Weight | |
| Small (<15 kg) | 7 |
| Large (≥15 kg) | 17 |
| Spay status at the time of diagnosis | |
| Intact | 24 |
| Spayed before diagnosis | 0 |
| Tumor type | |
| Benign | 11 |
| Malignant | 13 |
| Type of growth | |
| Expansive | 14 |
| Invasive | 10 |
| Histologic grade | |
| I+II | 9 |
| III | 4 |
| TNM | |
| I+II+III | 23 |
| IV+V | 1 |
Abbreviation: CMGTs, canine mammary gland tumors.
Effect of 5-azacytidine on cell growth of CMGT cell lines in vitro
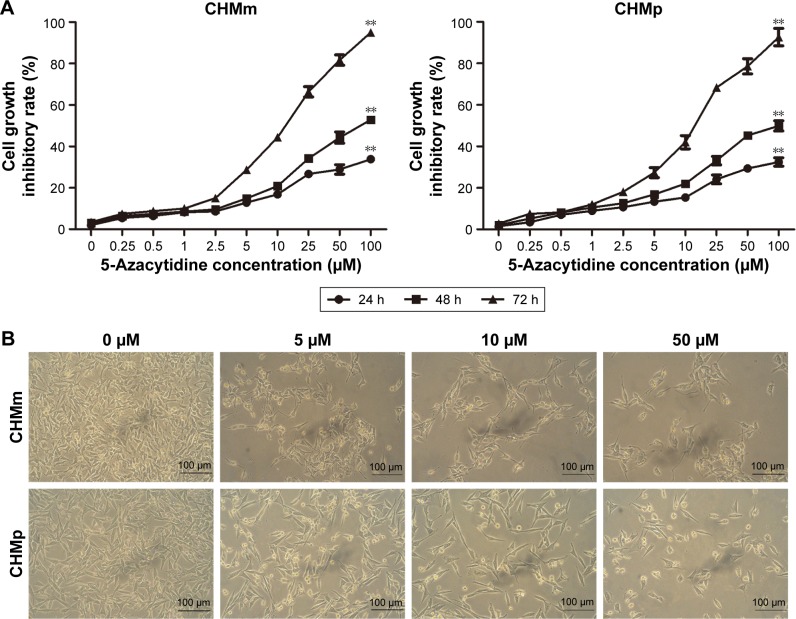
To determine whether 5-azacytidine inhibits the proliferation of CMGT cell lines in vitro, we evaluated the effect of the drug on CHMm and CHMp cell lines using an MTT assay at 24, 48, and 72 h, respectively. As shown in Figure 1A and B, 5-azacytidine inhibited the growth of CHMm cells in a treatment time- and dose-dependent manner; cells exhibited a poor condition and decrease with increasing concentration of 5-azacytidine. For example, for the CHMm cells treated with 0, 5, 10, and 50 μM 5-azacytidine for 72 h, the inhibition rates of growth were 3.49%±0.10%, 28.74%±1.49%, 44.39%±1.55%, and 81.67%±2.50%, respectively (P<0.01). Moreover, when CHMp cells were treated with 0, 5, 10, and 50 μM 5-azacytidine for 72 h, the inhibition rates of growth of CHMp cells were 2.76%±0.61%, 27.36%±2.43%, 42.01%±3.21%, and 78.62%±3.65%, respectively (P<0.01). Therefore, these results suggest that 5-azacytidine inhibited the proliferation of CMGT cells.
Figure 1.
Effect of 5-azacytidine on CMGT cell proliferation.
Notes: (A) CHMm and CHMp cell lines were treated with different concentrations of 5-azacytidine for 24, 48, and 72 h, respectively. Cell proliferation was determined using an MTT assay and analyzed as percentages relative to vehicle-treated control cells (100%). Bars represent the mean±SD of three independent experiments. Asterisks indicate significant differences compared to non-treated control cells (**P<0.01). (B) CHMm and CHMp cell morphologies are shown in the above light microscopic images (200×) at 72 h.
Abbreviation: CMGTs, canine mammary gland tumors.
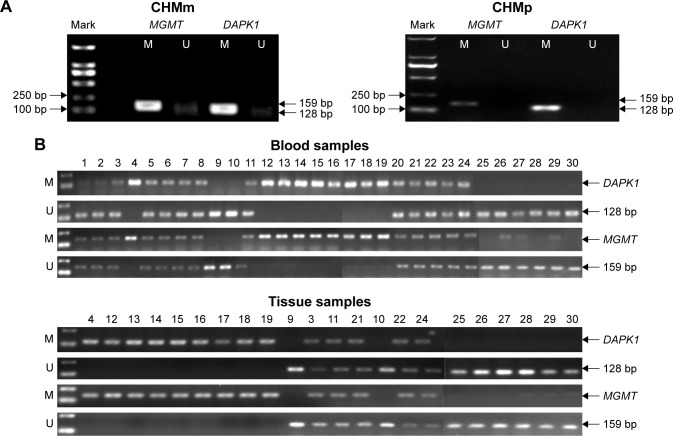
DAPK1 and MGMT gene methylation in CMGT cell lines and blood/tissue samples
The methylation patterns of the DAPK1 and MGMT genes in CMGT cell lines and blood/tissue samples from dogs with CMGTs were assessed with MSP assay. The results indicated that the promoters of DAPK1 and MGMT were completely methylated in CHMm and CHMp cells (Figure 2A). Among the 24 female dogs with CMGT blood samples tested, 37.50% (9/24) of the DAPK1 and MGMT gene promoters were completely methylated. Partial methylation was observed in 54.17% (13/24) of all cases, and unmethylated promoters were observed in 8.33% (2/24) of the samples (Figure 2B). In 69.23% (9/13) of the analyzed samples, malignant cases had completely methylated gene promoters, whereas the rest of the samples were only partially methylated. In contrast, in benign tumors, two of the samples were observed to be unmethylated, and the remaining nine benign tumors were partially methylated. On further investigation of the expression of DAPK1 and MGMT genes in 16 female dogs with CMGT tumor tissues, 9 of the malignant samples were observed to be methylated and 2 of the samples were observed to be unmethylated. The remaining five samples were partially methylated in seven benign tumors. Six of the normal samples were unmethylated. These results suggest that CMGTs may be at least partially induced by the methylation of DAPK1 and MGMT promoters, and the extent of methylation may be a prognostic indicator.
Figure 2.
Representative analyses of DAPK1 and MGMT methylation in CMGTs.
Notes: (A) The methylation status of DAPK1 and MGMT genes was measured in CHMm and CHMp cells via MSP assay (M, methylated primer; U, unmethylated primer). (B) The MSP results of DAPK1 and MGMT genes in part of blood and tissue samples (M, methylated primer; U, unmethylated primer). Methylated group (malignant tumor group): 4, 12, 13, 14, 15, 16, 17, 18, 19; unmethylated group (benign tumor group): 9, 10; partly methylated group: 1, 2, 3, 5, 6, 7, 8, 11, 20, 21, 22, 23, 24; normal group: 25, 26, 27, 28, 29, 30.
Abbreviations: CMGTs, canine mammary gland tumors; MGMT, O6-methylguanine-DNA methyltransferase; MSP, methylation-specific polymerase chain reaction.
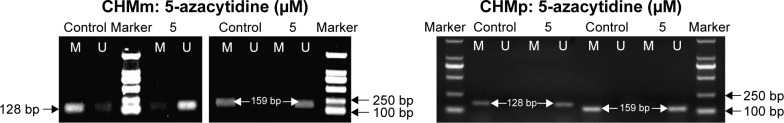
Effect of 5-azacytidine on the methylation of DAPK1 and MGMT genes in CMGT cell lines in vitro
It was evaluated whether 5-azacytidine-mediated decrease in viability of CMGT cells was caused by epigenetic regulation of DAPK1 and MGMT genes. MSP assay results showed that the DAPK1 and MGMT promoters were hypermethylated in CHMm and CHMp cells not treated with 5-azacytidine (Figure 3). However, partial demethylation of the DAPK1 gene and complete demethylation of the MGMT gene were detected in CHMm following a 72 h treatment with 5 μM 5-azacytidine. Moreover, treatment of CHMp cells with 5 μM 5-azacytidine led to demethylation of DAPK1 and MGMT at 72 h. These results suggest that 5-azacytidine induced further reduction of the methylation of DAPK1 and MGMT gene promoters in CMGT cells.
Figure 3.
Effect of 5 μM 5-azacytidine on the methylation of DAPK1 and MGMT genes in CMGT cell lines after 72 h of treatment.
Note: MSP assay was utilized to assess the methylation status of DAPK1 and MGMT genes (M, methylated PCR products; U, unmethylated PCR products).
Abbreviations: MSP, methylation-specific polymerase chain reaction; MGMT, O6-methylguanine-DNA methyltransferase.
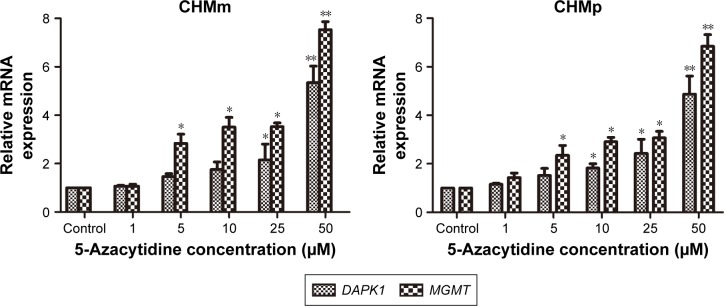
Effect of 5-azacytidine on DAPK1 and MGMT mRNA expression in CMGT cell lines in vitro
To better understand the effects of 5-azacytidine on DAPK1 and MGMT mRNA expression in CMGT cell lines in vitro, we evaluated the expression of DAPK1 and MGMT mRNA via qRT-PCR in CHMm and CHMp cells. Total RNA was extracted from both untreated cells and cells treated with 0, 1, 5, 10, 25, or 50 μM 5-azacytidine for 72 h. As shown in Figure 4, 5-azacytidine treatment increased both DAPK1 and MGMT mRNA expression in CHMm and CHMp cells in a dose-dependent manner, compared to untreated controls (P<0.01). These results demonstrate that the expression of DAPK1 and MGMT mRNA levels was increased in CMGT cells with increasing 5-azacytidine concentration.
Figure 4.
Effect of various concentrations of 5-azacytidine on the expression of DAPK1 and MGMT mRNA in CMGT cell lines after 72 h of treatment.
Notes: qRT-PCR was utilized to analyze the expression of DAPK1 and MGMT mRNA. Data are presented as the mean±SD of three independent experiments. Asterisks indicate significant differences compared to non-treated control cells (**P<0.01 and *P<0.05).
Abbreviations: CMGTs, canine mammary gland tumors; MGMT, O6-methylguanine-DNA methyltransferase; qRT-PCR, quantitative real-time polymerase chain reaction.
Discussion
5-Azacytidine is an agent of DNA demethylation, which has a potent inhibitory effect on DNA methyltransferase activity via the drug’s ability to incorporate into DNA during replication.13 This drug has been used to reactivate the expression of tumor suppressor genes, resulting in growth inhibition of various tumor cells, and to improve the chemosensitivity of tumor cells.9 However, it is not known whether 5-azacytidine exerts the same effects in CMGTs. Here, it was observed that 5-azacytidine may effectively suppress the proliferation of CMGT cells in a dose- and time-dependent manner. These observations can be corroborated by several previously published studies.9,13,15
Previous studies have demonstrated that the expression of DAPK1 is closely related to the development and progression of tumors in renal cell carcinomas,26 and the product of the DAPK1 gene can arrest cell motility and metastasis in gastric cancer cells.27,28 In addition, MGMT is a DNA methyltransferase, which is known to inhibit cell survival and proliferation. MGMT levels were found to be higher in tumors than in their normal tissue counterparts in several previous publications.29,30 It has also been reported that MGMT expression is significantly influenced by epigenetic regulation.24 Hypermethylation of the MGMT promoter was detected in several cancerous tissues, including cervical cancer and breast cancer.31 Tserga et al reported that in a cohort of 48 invasive breast cancer tissue samples, the levels of DAPK1 hypermethylation were as high as 37.5% (18/48). Of the 18 cancers with hypermethylated genes, 39.4% (13/33) were invasive ductal cancer and 50% (4/8) were invasive lobular carcinomas. In contrast, methylation of the MGMT promoter was observed to be ~16.6% and was primarily associated with age and tumor grade.11 The authors also reported similar methylation patterns in other genes, including DCR1, RASSF1A, and DCR1.11 Moreover, it has been reported that methylation of the DAPK1 gene is up to 32% in invasive ductal breast carcinomas.29 Yang et al showed that in 85 cases of cervical cancer specimens and 40 matching plasma samples, methylation of the DAPK1 gene was 60% and 28.2%, respectively.32 The same group also reported that normal cervical tissue is negative for MGMT methylation, whereas MGMT methylation is significantly increased in cervical cancer patients. Thus, MGMT methylation can be used as a diagnostic and prognostic marker in cervical cancer and glioblastoma patients.24,28,33 Based on the above study, we investigated the methylation patterns of DAPK1 and MGMT in blood/tissue samples from CMGT patients. We observed that the promoters of DAPK1 and MGMT genes in CHMm and CHMp cells and most malignant tumor samples were hypermethylated. The frequencies of methylated DAPK1 and MGMT genes in the clinical malignant tumor group were significantly higher compared to those in the benign tumor group and normal group, and the normal healthy dogs were unmethylated. Furthermore, hypermethylation of DAPK1 and MGMT could be correlated with the grade of malignancy of the tumor. These findings were expected, as the promoter regions of these tumor suppressor genes remain unmethylated in normal cells or benign tumor cells, and as a result, the cells can perform normal transcription. In contrast, in tumor cells, methylation of these gene promoters leads to inhibition of transcription, resulting in abnormal expression. Therefore, in the process of tumorigenesis, the oncogene is not fully methylated, causing abnormal expression. Additionally, tumor suppressor genes are predominantly hypermethylated, further reducing their expression. Alleviating the inhibition of the oncogene promotes the development of tumors. Taken together, these findings suggest that hypermethylation of the DAPK1 and MGMT promoters may be one of the potential mechanisms leading to the progression of CMGTs. Furthermore, this can be used as a diagnostic biomarker in CMGTs.
5-Azacytidine is part of a class of epigenetically active drugs, which function through the regulation of DNA methylation.34,35 However, it is unclear whether 5-azacytidine could inhibit the growth of CHMm cells by demethylation of DAPK1 and MGMT. It has been previously reported that 5-aza-2′-deoxycytidine, a DNA methyltransferase inhibitor, can inhibit the growth of cholangiocarcinoma cells through the regulation of DAPK1 promoter methylation and restoring DAPK1 expression. This suggests that DAPK1 may be an effective therapeutic target.36 Here, we demonstrated that CHMm and CHMp cells treated with 5 μM 5-azacytidine in vitro resulted in the demethylation of the DAPK1 and MGMT genes, compared to hypermethylation of DAPK1 and MGMT genes. In addition, qRT-PCR analysis showed that 5-azacytidine can increase DAPK1 and MGMT expression levels, which were positively correlated with the dose of the drug. Therefore, our results showed that 5-azacytidine could inhibit the growth of CMGTs cells via demethylation of DAPK1 and MGMT and enhancement of the expression of DAPK1 and MGMT genes. 5-Azacytidine can be used as a potential antitumor drug for the treatment of CMGTs; however, its antitumor mechanism requires further study.
Conclusion
Our study demonstrates that methylated DAPK1 and MGMT can serve as sensitive diagnostic biomarkers and therapeutic targets for CMGTs. We also conclude that 5-azacytidine could be a promising chemotherapeutic drug for CMGTs. Although we have presented some information indicating that the drug acts via demethylation of DAPK1 and MGMT genes, the specific antitumor mechanisms require further study.
Acknowledgments
The authors are grateful to N Sasaki, University of Tokyo, for kindly providing the CHMm and CHMp cell lines. They would like to thank all the veterinaries from the Northeast Agricultural University for their assistance with the animal sample collection work. This research was supported, in part, by the National Natural Science Foundation of China (grant no 31372492) and the National Key Research Projects, China (grant no 2016YFD0501008).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Moulton JE, Taylor DO, Dorn CR, Andersen AC. Canine mammary tumors. Pathol Vet. 1970;7(4):289–320. doi: 10.1177/030098587000700401. [DOI] [PubMed] [Google Scholar]
- 3.Shafiee R, Javanbakht J, Atyabi N, et al. Diagnosis, classification and grading of canine mammary tumours as a model to study human breast cancer: an Clinico-Cytohistopathological study with environmental factors influencing public health and medicine. Cancer Cell Int. 2013;13:79. doi: 10.1186/1475-2867-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Nieto A, Peña L, Pérezalenza MD, Sánchez MA, Flores JM, Castaño M. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: clinical and pathologic associations and prognostic significance. Vet Pathol. 2000;37(3):239. doi: 10.1354/vp.37-3-239. [DOI] [PubMed] [Google Scholar]
- 5.Egenvall A, Bonnett BN, Ohagen P, Olson P, Hedhammar A, von Euler H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev Vet Med. 2005;69(1–2):109–127. doi: 10.1016/j.prevetmed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Rijnkels M, Kabotyanski E, Montazer-Torbati MB, et al. The epigenetic landscape of mammary gland development and functional differentiation. J Mammary Gland Biol Neoplasia. 2010;15(1):85–100. doi: 10.1007/s10911-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koturbash I, Beland FA, Pogribny IP. Role of epigenetic events in chemical carcinogenesis – a justification for incorporating epigenetic evaluations in cancer risk assessment. Toxicol Mech Methods. 2011;21(4):289–297. doi: 10.3109/15376516.2011.557881. [DOI] [PubMed] [Google Scholar]
- 8.Saleem M, Abbas K, Manan M, et al. Review-Epigenetic therapy for cancer. Pak J Pharm Sci. 2015;28(3):1023–1032. [PubMed] [Google Scholar]
- 9.Ilyas A, Hashim Z, Zarina S. Effects of 5′-azacytidine and alendronate on a hepatocellular carcinoma cell line: a proteomics perspective. Mol Cell Biochem. 2015;405(1–2):53–61. doi: 10.1007/s11010-015-2395-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Wang J, Li Z, et al. Promoter hypermethylation of PTPL1, PTPN6, DAPK, p16 and 5-azacitidine inhibits growth in DLBCL. Oncol Rep. 2016;35(1):139–146. doi: 10.3892/or.2015.4347. [DOI] [PubMed] [Google Scholar]
- 11.Tserga A, Michalopoulos NV, Levidou G, et al. Association of aberrant DNA methylation with clinicopathological features in breast cancer. Oncol Rep. 2012;27(5):1630–1638. doi: 10.3892/or.2011.1576. [DOI] [PubMed] [Google Scholar]
- 12.Rajaii F, Asnaghi L, Enke R, Merbs SL, Handa JT, Eberhart CG. The demethylating agent 5-Aza reduces the growth, invasiveness, and clonogenicity of uveal and cutaneous melanoma. Invest Ophthalmol Vis Sci. 2014;55(10):6178–6186. doi: 10.1167/iovs.14-13933. [DOI] [PubMed] [Google Scholar]
- 13.Fuller M, Klein M, Schmidt E, et al. 5-Azacytidine enhances efficacy of multiple chemotherapy drugs in AML and lung cancer with modulation of CpG methylation. Int J Oncol. 2015;46(3):1192–1204. doi: 10.3892/ijo.2014.2792. [DOI] [PubMed] [Google Scholar]
- 14.Ghanim V, Herrmann H, Heller G, et al. 5-Azacytidine and decitabine exert proapoptotic effects on neoplastic mast cells: role of FAS-demethylation and FAS re-expression, and synergism with FAS-ligand. Blood. 2012;119(18):4242–4252. doi: 10.1182/blood-2011-09-382770. [DOI] [PubMed] [Google Scholar]
- 15.Hahn NM, Bonney PL, Dhawan D, et al. Subcutaneous 5-azacitidine treatment of naturally occurring canine urothelial carcinoma: a novel epigenetic approach to human urothelial carcinoma drug development. J Urol. 2012;187(1):302–309. doi: 10.1016/j.juro.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiloh R, Bialik S, Kimchi A. The DAPK family: a structure-function analysis. Apoptosis. 2014;19(2):286–297. doi: 10.1007/s10495-013-0924-5. [DOI] [PubMed] [Google Scholar]
- 17.Singh P, Ravanan P, Talwar P. Death associated protein kinase 1 (DAPK1): a regulator of apoptosis and autophagy. Front Mol Neurosci. 2016;9:46. doi: 10.3389/fnmol.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialik S, Kimchi A. The DAP-kinase interactome. Apoptosis. 2014;19(2):316–328. doi: 10.1007/s10495-013-0926-3. [DOI] [PubMed] [Google Scholar]
- 19.Celik S, Akcora D, Ozkan T, Varol N, Aydos S, Sunguroglu A. Methylation analysis of the DAPK1 gene in imatinib-resistant chronic myeloid leukemia patients. Oncol Lett. 2015;9(1):399–404. doi: 10.3892/ol.2014.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462(2–3):83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 21.Dolan ME, Schilsky RL. Silence is golden: gene hypermethylation and survival in large-cell lymphoma. J Natl Cancer Inst. 2002;94(1):6–7. doi: 10.1093/jnci/94.1.6. [DOI] [PubMed] [Google Scholar]
- 22.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4(4):296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 23.Kristensen LS, Treppendahl MB, Asmar F, et al. Investigation of MGMT and DAPK1 methylation patterns in diffuse large B-cell lymphoma using allelic MSP-pyrosequencing. Sci Rep. 2013;3:2789. doi: 10.1038/srep02789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Li S, Shen K, Ye S, Cao D, Yang J. DAPK1, MGMT and RARB promoter methylation as biomarkers for high-grade cervical lesions. Int J Clin Exp Pathol. 2015;8(11):14939–14945. [PMC free article] [PubMed] [Google Scholar]
- 25.Uyama R, Nakagawa T, Hong SH, Mochizuki M, Nishimura R, Sasaki N. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet Comp Oncol. 2006;4(2):104–113. doi: 10.1111/j.1476-5810.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang HL, Zhang J, Yan LI, Wang XQ. Effects of 5-Aza-CdR and low concentration CDDP on gastric cancer cell lines and expression of DAPK-1 gene. Pract Pharm Clin Remed. 2011 Epub. [Google Scholar]
- 27.Lin Y, Hupp TR, Stevens C. Death-associated protein kinase (DAPK) and signal transduction: additional roles beyond cell death. Febs J. 2010;277(1):48–57. doi: 10.1111/j.1742-4658.2009.07411.x. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Salehi F, Scheithauer BW, Rotondo F, Syro LV, Kovacs K. Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis. Anticancer Res. 2009;29(10):3759–3768. [PubMed] [Google Scholar]
- 29.Sharma G, Mirza S, Parshad R, et al. Clinical significance of promoter hypermethylation of DNA repair genes in tumor and serum DNA in invasive ductal breast carcinoma patients. Life Sci. 2010;87(3–4):83–91. doi: 10.1016/j.lfs.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Soejima H, Zhao W, Mukai T. Epigenetic silencing of the MGMT gene in cancer. Biochem Cell Biol. 2005;83(4):429–437. doi: 10.1139/o05-140. [DOI] [PubMed] [Google Scholar]
- 31.Spitzwieser M, Holzweber E, Pfeiler G, Hacker S, Cichna-Markl M. Applicability of HIN-1, MGMT and RASSF1A promoter methylation as biomarkers for detecting field cancerization in breast cancer. Breast Cancer Res. 2015;17:125. doi: 10.1186/s13058-015-0637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang HJ, Liu VW, Wang Y, et al. Detection of hypermethylated genes in tumor and plasma of cervical cancer patients. Gynecol Oncol. 2004;93(2):435–440. doi: 10.1016/j.ygyno.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Berghoff AS, Hainfellner JA, Marosi C, Preusser M. Assessing MGMT methylation status and its current impact on treatment in glioblastoma. CNS Oncol. 2015;4(1):47–52. doi: 10.2217/cns.14.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sood S, Srinivasan R. Alterations in gene promoter methylation and transcript expression induced by cisplatin in comparison to 5-Azacytidine in HeLa and SiHa cervical cancer cell lines. Mol Cell Biochem. 2015;404(1–2):181–191. doi: 10.1007/s11010-015-2377-3. [DOI] [PubMed] [Google Scholar]
- 35.Boumber Y, Issa JP. Epigenetics in cancer: what’s the future? Oncology (Williston Park) 2011;25(3):220–226. 228. [PubMed] [Google Scholar]
- 36.Liu XF, Jiang H, Zhang CS, Yu SP, Wang ZQ, Su HL. Targeted drug regulation on methylation of p53-BAX mitochondrial apoptosis pathway affects the growth of cholangiocarcinoma cells. J Int Med Res. 2012;40(1):67–75. doi: 10.1177/147323001204000107. [DOI] [PubMed] [Google Scholar]