In 2015, the Federal Drug Administration approved the proprotein convertase subtilisin-kexin type 9 inhibitors (PCSK9i) alirocumab and evolocumab for patients with clinical atherosclerotic cardiovascular (ASCVD) disease on maximally tolerated statin therapy who “require additional lowering of low density lipoprotein-cholesterol (LDL-C)”1 but did not specify an LDL-C threshold or statin intensity required before PCSK9i are considered for treatment. Subsequently, the FOURIER trial demonstrated efficacy of evolocumab, in ASCVD patients on a range of statin therapy (predominantly of moderate- or high-intensity), with LDL-C values ≥ 70 mg/dL.2 Given these variable criteria for PCSK9i use, we sought to assess the impact of LDL-C treatment thresholds and statin intensities on the proportion of patients potentially eligible for PCSK9i therapy seen in contemporary practice.
Using the prospective, ambulatory, national Cardiovascular Data Registry’s PINNACLE Registry, we assessed the proportion of patients aged 18 to 75 years with established ASCVD (prior acute coronary syndrome, coronary or other arterial revascularization, cerebrovascular accident, transient ischemic attack, or peripheral arterial disease) and available LDL-C data potentially eligible for PCSK9i therapy. Eligibility was variably defined using a range of LDL-C treatment thresholds (from ≥70 mg/dL to ≥160 mg/dL based on cardiovascular risk and previously-recommended LDL-C goals) and statin dosing (high-intensity, defined as atorvastatin ≥40 mg or rosuvastatin ≥20 mg; moderate-intensity, defined as atorvastatin 10 or 20 mg, rosuvastatin 5 or 10 mg, simvastatin 20–40 mg, pravastatin 40 mg, lovastatin 40 mg, fluvastatin 40 mg bid; or any intensity). Pearson χ2 tests were used to compare proportions of eligible adults by subgroups.
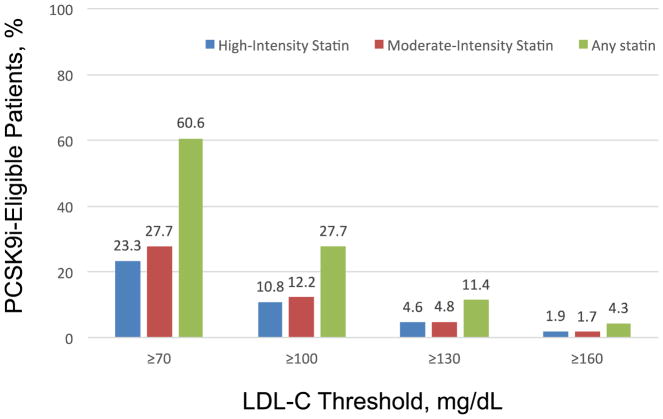
The study population consisted of 252,956 patients (mean age 63.6 years; 64.9% men) with ASCVD seen at 57 participating practices. ASCVD prevalence included 94.6% with prior coronary artery disease, 40.1% with a prior myocardial infarction, 11.0 % with prior cerebrovascular disease, 8.1% with a transient ischemic attack, and 18.0% with peripheral arterial disease. A total of 23.3% were on a high-intensity statin, 27.7% were on a moderate intensity statin, and 60.6% were on any statin. Among patients receiving high-intensity statins, the overall proportion of patients potentially eligible for PCSK9i therapy increased, from 1.9% with an LDL-C treatment threshold ≥ 160 mg/dL to 23.3% with an LDL-C treatment threshold ≥ 70 mg/dL (p<0.001). Among patients receiving moderate-intensity statins, PCSK9i eligibility increased from 1.7% with an LDL-C treatment threshold ≥ 160 mg/dL to 27.7% with an LDL-C treatment threshold ≥ 70 mg/dL (p<0.001). Among patients receiving any statin, PCSK9i eligibility increased from 4.3% with an LDL-C treatment threshold ≥ 160 mg/dL to 60.6% with an LDL-C treatment threshold ≥ 70 mg/dL (p<0.001) (Figure).
Figure.
Proportion of Patients With Atherosclerotic Cardiovascular Disease Potentially Eligible for PCSK9 Inhibitor Therapy According to LDL-C Treatment Thresholds and Statin Intensity.
Under the FDA criteria, the range of LDL-C thresholds and background statin use to determine eligibility is broad, as approval of alirocumab and evolucumab by the Federal Drug Administration was based on lowering of LDL-C, a surrogate marker of cardiovascular risk. Indeed, given an estimated 16.5 million American adults have cardiovascular disease,3 our analysis suggests that the number of patients potentially eligible for PCSK9i could range from approximately 700,000 to approximately 10 million American adults based on LDL-C threshold. If we use the FOURIER enrollment criteria to further guide these estimates, then approximately 8.4 million patients with ASCVD would be eligible for PCSK9i. This projection is in keeping with prior analyses4 and has substantial cost implications. Assuming a mean US price of $14 000 per patient per year, corresponding mean annual costs are approximately $118 billion. A cost-effectiveness study informed by FOURIER indicates price reductions of more than 70% are required to meet cost-effectiveness thresholds.4 Thus, reducing the price of PCSK9i therapy should be explored. To reduce the need for costly PCSK9i therapy, encouraging lifestyle modification, titrating statin therapy to maximally-tolerated doses, maximizing statin adherence, using lower-cost cholesterol-lowering medications such as ezetimibe,5 and targeting a subset of patients with ASCVD at higher risk of cardiovascular events based on clinical risk factors inclusive of higher LDL-C can be considered.5
Footnotes
Disclosures: This research was supported by the American College of Cardiology National Cardiovascular Data Registry. The PINNACLE Registry is an initiative of the American College of Cardiology. Bristol-Myers Squibb and Pfizer Inc are founding sponsors of the PINNACLE Registry. The PINNACLE Registry and the National Cardiovascular Data Registry had no role in the design or conduct of the study, the management or analysis performed in the study, or the interpretation of the data. Dr. Masoudi is the Chief Medical Officer for the NCDR. Other authors have no relevant disclosures.
References
- 1. [Accessed March 11, 2017]; https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm460082.htm.
- 2.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR FOURIER Steering Committee and Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:1851–1867. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazi DS, Penko J, Coxson PG, Moran AE, Ollendorf DA, Tice JA, Bibbins-Domingo K. Updated Cost-effectiveness Analysis of PCSK9 Inhibitors Based on the Results of the FOURIER Trial. JAMA. 2017;318:748–750. doi: 10.1001/jama.2017.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani SS, Akeroyd JM, Nambi V, Heidenreich PA, Morris PB, Nasir K, Michos ED, Bittner VA, Petersen LA, Ballantyne CM. Estimation of Eligibility for Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors and Associated Costs Based on the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk): Insights From the Department of Veterans Affairs. Circulation. 2017;135:2572–2574. doi: 10.1161/CIRCULATIONAHA.117.028503. [DOI] [PubMed] [Google Scholar]