Abstract
We examined phenotypes of autism spectrum disorder (ASD) based on trajectories of intellectual development from early (ages 2–3 ½) to middle (ages 5–8) childhood in a recent clinically ascertained cohort. Participants included 102 children (82 males) initially diagnosed with ASD from the Autism Phenome Project longitudinal sample. Latent class growth analysis was used to identify distinct IQ trajectories. Baseline and developmental course differences among groups were assessed using univariate techniques and repeated measures regression models, respectively. A four class model best represented the data. Using the highest posterior probability, participants were assigned to High Challenges (25.5%), Stable Low (17.6%), Changers (35.3%), and Lesser Challenges (21.6%) groups. The High Challenges and Stable Low groups exhibited persistently low IQ, although, the High Challenges group experienced declines while the Stable Low group’s scores remained more constant. Changers showed IQ improvement of > 2 standard deviations. The Lesser Challenges group had IQs in the average range at both times that were about 1 standard deviation higher at T2. In summation, 75% of the participants experienced some relative improvements in intellectual and/or other areas of functioning between ages 2 and 8 years. The Changers group demonstrated the most significant IQ change that was accompanied by adaptive communication improvement and declining externalizing symptoms. Only the Lesser Challenges group showed a significant reduction in ASD symptom severity, such that by age 8, 14% of them no longer met ADOS-2 criteria for ASD. All groups showed reductions in internalizing symptoms. Intervention history was not associated with group status.
Keywords: intellectual development, phenotypes, longitudinal, ASD, IQ, early childhood, autism spectrum disorder
INTRODUCTION
For the parents of the 1 in 68 toddlers (Christensen et al., 2016) who have been diagnosed with autism spectrum disorder (ASD), there is no more salient concern than what the future will hold for their children. Given the heterogeneity characteristic of ASD (Geschwind & Levitt, 2007), it remains difficult to answer this question. While some with ASD will never acquire functional language, sustain interpersonal relationships or live without significant support, others will develop meaningful friendships, obtain post-secondary education, and work and live independently in the community. Some may cease to meet the diagnostic criteria for ASD (Fein et al., 2013). Longitudinal studies offer the best hope of unravelling the diverse and complex pathways between early development and later outcomes that would permit better prediction (Karmiloff-Smith, 1998). Multivariate cluster analytic techniques that isolate subgroups of distinct developmental trajectories/phenotypes of such groups also offer a potentially informative way of identifying more etiologically and developmentally homogeneous phenotypes that may share similar courses, prognoses, and responses to targeted treatments (Lord, Bishop, & Anderson, 2015; Szatmari, 2017).
We focus on intellectual ability level, which is most commonly assessed using a developmental quotient (DQ) or intelligence quotient (IQ), (henceforth both referred to as IQ). We do so because IQ is the strongest predictor of outcomes in individuals with ASD and typical development (Gillespie-Lynch et al., 2012; Howlin, Goode, Hutton, & Rutter, 2004; Piven, Harper, Palmer, & Arndt, 1996), and also constitutes the most significant source of heterogeneity within the ASD phenotype (Miller & Ozonoff, 2000; Munson et al., 2008). Thus, identifying developmental courses defined by IQ, may constitute an effective way of helping families to answer the critical question raised above.
Given its importance, there now have been several investigations of IQ development in ASD from early to middle childhood. These studies have shown that IQ typically increases through early childhood in those with ASD (Eaves & Ho, 2004; Flanagan et al., 2015; Turner, Stone, Pozdol, & Coonrod, 2006); that IQ growth is most rapid during the first 6–7 years (Baghdadli et al., 2012; Fountain, Winter, & Bearman, 2012; Pickles, Anderson, & Lord, 2014); and that nonverbal IQ (NVIQ) and verbal IQ (VIQ) show comparable development (Anderson, Liang, & Lord, 2013). However, none of these studies has isolated individual phenotypes of IQ developmental trajectories or has specified the correlates of trajectory membership that are suggested by the extant literature, which suggests that symptom severity, patterns of relative verbal and non-verbal strength, problem behaviors, and intervention are the variables most strongly associated with intellectual functioning in persons with ASD. These areas are reviewed below.
IQ and ASD symptom severity
ASD diagnoses show considerable stability in young children (Jonsdottir et al., 2007; Lord et al., 2006). However, most of the 3%–25% losing their diagnoses (Helt et al., 2008) have average or better IQs (Kim, Macari, Koller, & Chawarska, 2015). Receiving the milder Pervasive Developmental Disorder--Not Otherwise Specified (PDD-NOS) diagnosis also has been associated with having a higher IQ (Jonsdottir et al., 2007). Across the broader range of functioning, those with intellectual disability (ID; IQ<70 and significant adaptive behavior deficits) versus those with average IQs, differ in symptom severity. Within these broad groupings, however, IQ and symptom severity associations are less consistent (Munson et al., 2008). In fact, calibrated severity scores (CSS) used with the gold standard Autism Diagnostic Observation Schedule-2 (ADOS-2; (Lord et al., 2012)), recently have been developed to explicitly quantify autism severity apart from the influence of overall cognitive and language abilities (Gotham, Pickles, & Lord, 2009; Gotham, Risi, Pickles, & Lord, 2007) NVIQ is not predictive of CSS (Gotham, Pickles, & Lord, 2012), and symptom severity and VIQ can be assessed relatively independently (Gotham et al., 2012; Venker, Ray-Subramanian, Bolt, & Weismer, 2014). Thus, while it is true that across the entire autism spectrum, the majority of individuals who lose their diagnoses have higher IQs, for the remainder of affected individuals, there is little relationship between IQ and ASD symptom severity.
Patterns of relative verbal and non-verbal strength and IQ development
Language-related abilities may be a critical catalyst for general cognitive development. For example, Munson et al. (Munson et al., 2008) conducted a latent class analysis (LCA) of patterns of relative VIQ and NVIQ scores at a single time point in a large sample (N = 456) of children ages 2–5 ½ years, and derived four classes based on IQ. One class was defined by a markedly lower VIQ than NVIQ that was comparable to the NVIQ found in the higher IQ classes. They proposed that this group might later develop more language skills and merge with the two more able classes. Subsequent longitudinal studies have provided support for this contention. Using growth curve analyses, Anderson et al. found that NVIQ predicted language growth between ages 2 to 9 years in a sample of 206 children (Anderson et al., 2007). Pickles et al. (Pickles et al., 2014) implemented latent class growth analysis in a sample of 192 participants aged 2–19 years and observed that the less impaired of two low language groups had NVIQs one standard deviation higher than those with persistent low language abilities, and that those experiencing “catch up” exhibited a NVIQ>VIQ discrepancy until developing language. Thurm et al. (Thurm, Manwaring, Swineford, & Farmer, 2015) found that higher NVIQ was predictive of spoken language development in minimally verbal children (N = 70), also suggesting that strong non-verbal abilities can scaffold communication development. In sum, it would appear that developing language abilities can be an engine for intellectual development, especially if underlying non-verbal intellectual abilities are strong.
Problem behaviors and IQ
Internalizing and externalizing-related problem behaviors can negatively influence functioning (Anderson, Maye, & Lord, 2011). Historically, internalizing problems have been associated with higher IQ in children and adolescents with ASD (Mazurek & Kanne, 2010), while externalizing problems have been associated with lower IQ (Gray et al., 2012). However, using trajectory-based analysis, a more recent longitudinal study of children with ASD from ages 3 to 6 found that both internalizing and externalizing behaviors occurred at high rates, were co-morbid, and declined slightly over time. Membership in subgroups with higher and lower levels of these problem behaviors was not associated with IQ (Vaillancourt et al., 2016). Despite these findings in younger children, findings above for children and adolescents suggests that relationships may emerge with development.
Intervention and IQ
Increasing intellectual development is an important goal of early intervention in ASD. Some studies find positive associations between outcomes related to intellectual function and participation in intervention, (Anderson et al., 2013) intensity of intervention, (Mazurek, Kanne, & Miles, 2012) and early age of treatment (Anderson, Oti, Lord, & Welch, 2009; Pellicano, 2012). However, others have argued that innate cognitive capacity is more predictive of later IQ than intervention effects (Fernell et al., 2011), and that studies of intervention are difficult to interpret because those doing poorly receive more services, while those doing well receive less.
We used group-based trajectory modeling to isolate significant developmentally and potentially more etiologically homogeneous intellectual ability phenotypes of ASD in a recent Northern California sample of children. We then investigated potential correlates of these intellectual developmental trajectories. We hypothesized that: (1) There would be four IQ trajectory groups between ages 2 and 8 years, as suggested by the Munson et al. (2008) study, which identified four groups using LCA at one time point, including one group showing relative IQ improvement over time (Fein et al., 2013; Georgiades et al., 2014; Stevens et al., 2000; Szatmari, Bryson, Boyle, Streiner, & Duku, 2003), (2) The approximately 10% (Anderson et al., 2013) of children losing their diagnoses by age 8 would be more likely to have higher IQs at age 2; but that apart from this group, ASD symptom severity would be relatively independent of IQ trajectory membership (Gotham et al., 2012; Venker et al., 2014), (3) Those experiencing the largest positive changes in IQ would have relatively higher non-verbal abilities at age 2, and demonstrate strong adaptive communication development from ages 2 to 8, and (4) Groups with positive IQ change would show lower levels of internalizing problem behaviors (Gray et al., 2012; Mazurek & Kanne, 2010). We also sought to replicate findings from the literature that there would be no differences in the amount or intensity of intervention received.
METHOD
A sample of both children with suspected ASD and typically developing (TYP) children was recruited through the MIND (Medical Investigation of Neurodevelopmental Disorders) Institute of the University of California, Davis (UCD) for the Autism Phenome Project (APP) beginning in 2006. The original APP sample consists of 279 participants -- 189 participants with ASD and 90 typically developing controls that consented to be part of the longitudinal study. We currently are in the middle of the fourth assessment of these individuals We have maintained close contact with these families, and during the period of the current study, attrition rates never exceeded 25%. This manuscript reports results from data collected at the first (T1) and third (referred to herein as T2) assessments. Inclusion criteria for ASD were based on the NIH Collaborative Programs of Excellence in Autism standards. Participants had received a best estimate diagnosis of autism, PDD-NOS, or Asperger syndrome from a licensed site clinician; and having met the ADOS-2 cut-off score for either autism or ASD, or the Autism Diagnostic Interview-revised (ADI-R;(Lord, Rutter, & Le Couteur, 1994)) cut-off for autism on either the Social or Communication subscale while being within two points of this criterion on the other subscale. Participants needed to live with at least one biological parent, be English speaking and ambulatory, and have no severe motor, vision hearing or chronic health problems that would preclude them from being assessed. They had to have completed the baseline behavioral assessment at the time of enrollment when they were 23–44 months of age (referred to as age 2–3 1/2 or T1) and the follow-up behavioral assessment at 51–91 months of age (referred to as age 5–8 or T2) to be included in the current study. This age range was selected because it represents the toddlerhood to middle childhood period and enabled us to maximize sample size. The T1 and T2 age spans differed due to resource limitations early in T2. The mean age difference between T1 and T2 was 33 months (range = 24–59 months). Final participants were 102 individuals with ASD (82 males; 20 females). This study was approved by the UCD Institutional ReviewBoard, and informed consent was obtained from the parent or guardian of each participant.
Measures
Common ASD diagnostic, cognitive, and adaptive communication, and problem behavior measurements were used including: Mullen Scales of Early Learning (MSEL) (Mullen, 1995), the Differential Abilities Scales-II (DAS-II) (Elliot, 2007), the ADOS-2 (Gotham et al., 2007; Lord et al., 2000), the ADI-R (Lord et al., 1994), Vineland Adaptive Behavior Scales, Second Edition: Parent/Caregiver Rating Form)(VABS-2) (Sparrow, Balla, & Cicchetti, 2005), Child Behavior Checklist (CBCL)-Preschool (Achenbach & Rescorla, 2000) and School-Age (Achenbach & Rescorla, 2001) versions. All diagnostic assessments were conducted or directly observed by trained, licensed clinical psychologists who specialize in ASD and were trained according to research standards for these tools. A form detailing participants’ intervention was also completed by parents. Descriptions of these instruments follow.
Mullen Scales of Early Learning (MSEL) (Mullen, 1995)
Cognitive and developmental functioning was measured using the MSEL at study entry. The MSEL is a standardized measure of cognitive and developmental functioning for children 0–68 months of age. MSEL yields subscale standard scores, age-equivalents, and composite standard scores. Four subscales were administered to provide measures of nonverbal (Visual Reception, Fine Motor) and verbal abilities (Expressive and Receptive Language). Since a significant proportion of the ASD group achieved the lowest possible MSEL standard score, ratio developmental quotients (mental age/chronological age *100) were calculated to provide nonverbal, verbal and combined IQ estimates.
The Differential Abilities Scales-II (DAS-II) (Elliot, 2007)
The DAS-II is a standardized measure of cognitive abilities for children ages 2.5–17 years. Participants completed the core battery of the DAS-II Upper Early Years or School Age form, which consists of verbal, nonverbal, and spatial reasoning clusters. This produced standardized cluster scores, a cognitive composite (General Conceptual Ability, GCA) and a combined nonverbal and spatial ability composite (Special Nonverbal Composite; SNC). DAS-II verbal cluster, SNC and GCA scores provided estimates of verbal, nonverbal and combined IQ. Children who were unable to achieve basal scores on the DAS-II (n=18) were administered the MSEL at Time 2, and development quotients were used to provide nonverbal, verbal, and combined IQ estimates.
The Autism Diagnostic Observation Schedule-2 (ADOS-2)
ADOS-2 is a semi-structured standardized observation. Diagnostic classification is based upon exceeding a threshold in a combined Social Affect and Restricted and Restricted and Repetitive Behavior score. Calibrated severity scores (CSS) provide a common metric for comparison of scores across ADOS modules, yielding estimates of overall ASD symptom severity (Gotham et al., 2009) as well as separate social affect and restricted and repetitive behavior scores (Hus, Gotham, & Lord, 2014). CSS range from 1–10 (1–3 Non-ASD; 4–5 Autism Spectrum; 6–10 Autism)
Autism Diagnostic Interview-Revised (ADI-R; (Lord et al., 1994))
This comprehensive parent interview probes for symptoms of ASD. It is administered by a trained clinician using a semi-structured interview format. ADI-R elicits information on over 100 questions about the child’s current behavior and developmental history. The significant developmental time point in the ADI-R is age 4 to 5 years; research indicates behaviors are at their peak by this age, making it a most sensitive time for identification. The items that empirically distinguish individuals with autism from those with other developmental delays are summed into three algorithm scores -- social difficulties, communication deficits, and repetitive behaviors.
Vineland Adaptive Behavior Scales, Second Edition: Parent/Caregiver Rating Form) (VABS-2) (Sparrow et al., 2005)
The Parent/Caregiver Rating Form was completed by caregivers to assess adaptive behavior in Communication, Daily Living Skills and Socialization domains. The Communication domain score was used in our analysis.
Child Behavior Checklist (CBCL)-Preschool (Achenbach & Rescorla, 2000) and School-Age (Achenbach & Rescorla, 2001) versions
The CBCL, which is a part of the Achenbach System of Empirical Behavioral Assessment (ASEBA) is a standardized caregiver rating scale, assessing a broad range of behavioral, social and emotional problems, yielding standard symptom, syndrome, and composite t-scores (≥ 64 clinical range). Standardized scores for the Internalizing and Externalizing scales were used.
Services, Treatment and Intervention Data
At each visit, caregivers completed a form inquiring about current and previous intervention involvement, providing information about the type and duration of multiple types of intervention the child had received. An intensity score for each type of intervention was calculated using the following formula (weeks of intervention * hours per week) * (number of adults/number of children). This form was adapted from the Collaborative Programs of Excellence in Autism.
Data Analysis
At Time 1, verbal, nonverbal, and combined IQ were estimated by calculating ratio developmental quotient scores, dividing average verbal, nonverbal and combined MSEL subscale age equivalents by chronological age. At Time 2, DAS-II Verbal cluster standard score, Special Nonverbal Composite and General Conceptual Ability Scores were used within analyses as verbal, nonverbal, and combined IQ estimates. For the 18 children unable to achieve basal scores on the DAS-II at Time 2 were administered the MSEL, and DQ scores were used. To identify distinct intellectual developmental trajectories based on these IQ scores obtained at ages 23–44 and 51–95 months, group-based trajectory analysis was performed in Mplus version 8 (https://www.statmodel.com/). The optimal number of trajectories was based on both statistical goodness-of-fit criteria and interpretability, taking into account whether the classes captured clinically meaningful features. The Bayesian information criterion (BIC) and Akaike information criterion (AIC), which penalize more complex models, as well as the bootstrap likelihood ratio test (BLRT) were used to assess the model fit (Nylund, Asparoutiov, & Muthen, 2007). The local maximum problem was addressed by using a large number of starting points (up to 300) to replicate each model. The optimal model was used to obtain for each child estimates of the posterior probabilities of belonging to each subgroup and to assign each child to the subgroup with the highest posterior probability. Using this classification, we examined differences across the trajectory groups using demographic information and measurements presented above. Chi-squared tests (or McNemar or Fisher’s exact tests when appropriate) were used to examine differences across groups in categorical variables. Repeated measures linear models (Laird & Ware, 1982) were used to investigate baseline and developmental course differences for trajectory groups. Following significant overall tests, Tukey-Kramer adjustment for multiple comparisons was used to estimate pairwise group differences. These analyses were implemented in SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Hypothesis #1: Analysis of Trajectories
Latent class models with 2–5 classes were fit using linear curves over time. Model fit statistics are presented in Table 2. A model with 4 distinct trajectories of intellectual development between ages 23–44 months and 51–95 months was chosen as the best fit for the data. This model had the lowest AIC and sample size adjusted BIC and BLRT p-value < 0.0001 indicating that it fit the data better than a three trajectory model. Table 3 presents the details of the final model. In latent class analysis, each participant’s trajectory is modeled as a mixture of all trajectories and posterior group probabilities are calculated for each participant and each class. For the selected four group model, 79.4% of the participants had over 90% of their probability focused on a single trajectory and only 8 participants did not have at least 70% probability concentrated on just one trajectory class. No participants had a highest single posterior probability below 55%. The average posterior probabilities for those assigned to the groups were 98%, 85%, 92%, and 92%, respectively.
Table 2.
Model fit statistics and the number of children assigned to each group for latent class growth models with two to five classes for IQ
| Number of classes |
BIC1 | AIC1 | aBIC1 | BLRT | Number of individuals assigned to each class | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 2 | 3 | 4 | 5 | |||||
| Two | 1845 | 1827 | 1823 | <.0001 | 64 | 38 | – | – | – |
| Three | 1811 | 1785 | 1780 | <.0001 | 46 | 34 | 22 | – | – |
| Four | 1812 | 1777 | 1770 | <.0001 | 26 | 18 | 36 | 22 | – |
| Five | 1822 | 1780 | 1771 | 0.67 | 22 | 13 | 32 | 13 | 22 |
Lower numbers indicate more optimal model fit.
BIC = Bayesian Information Criterion; AIC = Akaike Information Criterion; aBIC = Sample Adjusted Bayesian Information Criterion; BLRT = Parametric bootstrapped likelihood ratio test. Small p-values of the BLRT test support the model with k vs. the model with k-1 classes.
Table 3.
Final latent class growth model for IQ
| Parameter | Estimate | SE | p-value | |
|---|---|---|---|---|
| Group (%) | ||||
|
| ||||
| High Challenges (26.6%) | Intercept | 44.01 | 2.24 | <.001 |
| Linear | −8.21 | 2.36 | <.001 | |
| Stable Low (17.5%) | Intercept | 61.28 | 3.58 | <.001 |
| Linear | 4.76 | 4.47 | .29 | |
| Changers (33.7%) | Intercept | 64.51 | 1.79 | <.001 |
| Linear | 34.07 | 3.20 | <.001 | |
| Lesser Challenges (22.1%) | Intercept | 97.46 | 2.16 | <.001 |
| Linear | 13.64 | 3.15 | <.001 | |
Note: Due to rounding percentages do not sum to 100. SE = standard error.
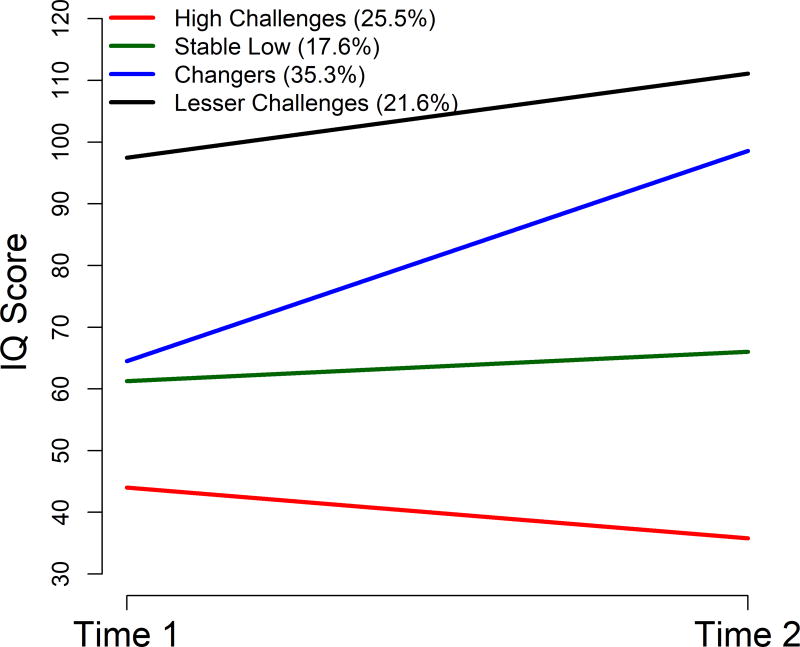
This resulted in the following groups: High Challenges (n = 26), Stable Low (n = 18), Changers (n = 36), and Lesser Challenges (n = 22). Figure 1 provides a visual depiction of the fitted mean trajectories. As shown in Table 1, the four trajectory groups did not differ in age at either time point. At T2, 16 participants from the High Challenges and 2 participants from the Stable Low groups completed the MSEL because they could not achieve basal scores on the DAS-II. The High Challenges group (25.5%) had the lowest mean IQ at T1 (estimate 43.4, 95% CI 39.8 – 47.1) and demonstrated an 8.6 point decline at T2 relative to their peers. The Stable Low group (17.6%) had significantly higher IQ scores at T1 (estimate 60.6, 95% CI 56.2 – 65.0) than the High Challenges group and had a nonsignificant change from T1 to T2 (estimate = 3.5, p = .26). The Changers group (35.3%) had comparable scores at T1 with the Stable Low group (estimate 64.7, 95% CI 61.6 – 67.8), but showed a 33.8-point increase in IQ from T1 to T2. The final class, the Lesser Challenges group (21.6%), displayed significantly higher IQ scores than all other groups at T1 (estimate 98.2, 95% CI 94.2 – 102.2) and had a 13-point increase from T1 to T2. Contrary to expectations, no trajectory was defined based on differential patterns of VIQ and NVIQ change, so we report only composite IQ trajectories since trajectory membership was similar for VIQ and NVIQ (85% percent of the participants were assigned to the same trajectory for VIQ and composite IQ, and 89% percent of the participants were assigned to the same trajectory for NVIQ and composite IQ).
Figure 1.
Trajectories of intellectual development in the Autism Phenome Project (APP) cohort. Results of trajectory analysis for the 102 members of the APP cohort for whom IQ scores were available at Time 1 (23–44 months) and Time 2 (51–95 months). Four classes were produced using DQ and/or IQ scores. Lines represent fitted mean trajectories.
Table 1.
Summary of characteristics for the four trajectory groups at early childhood behavioral assessments
| Variable | High Challenges (n = 26) |
Stable Low (n = 18) |
Changers (n = 36) |
Lesser Challenges (n = 22) |
|---|---|---|---|---|
| Male | 22 (85%) | 17 (94%) | 25 (75%) | 16 (73%) |
| Age at IQ testing (months) | ||||
| Age 2–3.5 years | 35.6 (5.6) | 32.9 (4.5) | 34.1 (4.8) | 34.4 (6.3) |
| Age 6–7.5 years | 68.8 (9.8) | 66.6 (10.4) | 67.1 (9.9) | 67.1 (11.6) |
| Time between IQ tests (months) | ||||
| 33.2 (7.5) | 33.7 (8.8) | 32.9 (8.3) | 32.7 (6.8) | |
| ADOS CSS ≤3 at T2 | 0 (0%) | 0 (0%) | 2 (6%) | 3 (14%) |
| PPD-NOS | 0 (0%) | 1 (6%) | 3 (8%) | 4 (18%) |
| ADOS Severity | ||||
| Age 2–3.5 years | 8.2 (1.6)a | 7.2 (1.6) | 7.0 (1.8) | 6.8 (1.7)b |
| Age 6–7.5 years | 8.3 (1.2)a | 8.3 (1.5)a | 6.7 (2.0)b | 5.4 (2.0)c |
| Verbal IQ Score < 70 | ||||
| Age 2–3.5 years | 25 (96%)* | 15 (83%)* | 27 (75%)* | 0 (0%)* |
| Age 6–7.5 years | 26 (100%)* | 11 (61%)* | 0 (0%)* | 0 (0%)* |
| Non-Verbal IQ Score < 70 | ||||
| Age 2–3.5 years | 24 (92%)* | 7 (39%)* | 21 (58%)* | 0 (0%)* |
| Age 6–7.5 years | 26 (100%)* | 9 (50%)* | 0 (0%)* | 0 (0%)* |
| VABS communication domain | ||||
| Age 2–3.5 years1 | 63.5 (12.1)a | 73.7 (15.2)b | 77.7 (11.4)b | 87.9 (11.1)c |
| Age 6–7.5 years2 | 56.7 (12.7)a | 81.4 (11.4)b | 90.8 (13.6)b,c | 95.0 (11.4)c |
| CBCL Externalizing | ||||
| Age 2–3.5 years3 | 55.6 (8.4) | 62.1 (10.2) | 61.5 (11.2) | 56.8 (12.6) |
| Age 6–7.5 years4 | 56.2 (10.3) | 58.2 (7.7) | 53.8 (9.3) | 54.6 (10.2) |
| CBCL Internalizing | ||||
| Age 2–3.5 years3 | 60.7 (7.0) | 63.2 (8.2) | 62.5 (9.5) | 63.7 (10.0) |
| Age 6–7.5 years4 | 58.5 (9.2) | 56.6 (8.5) | 58.4 (12.8) | 60.7 (9.4) |
ADOS = Autism Diagnostic Observation Schedule; CBCL Child Behavior Checklist; VABS = Vineland Adaptive Behavior Scales.
Fisher’s exact test p-value < 0.05;
Groups with different superscripts differ significantly (p < .05) after Tukey-Kramer multiple comparison adjustment
Missing data = 3 in High Challenges, 1 in Stable Low, and 1 in Changers;
Missing data = 1 in High Challenges, 3 in Stable Low, 5 in Changers and 1 in Lesser Challenges;
Missing data = 1 in High Challenges, 1 in Stable Low, and 2 in Changers;
Missing data = 5 in High Challenges, 3 in Stable Low, 5 in Changers and 3 in Lesser Challenges.
Hypothesis #2: IQ and ASD symptom severity
By T2, lose to 5% of the sample no longer met ADOS-2 criteria for ASD (CSS≤3. See Table 1). These children were either in the Lesser Challenges group (3, 14%) or the Changers (2, 6%) group. A similar proportion had PDD-NOS diagnoses at T1.
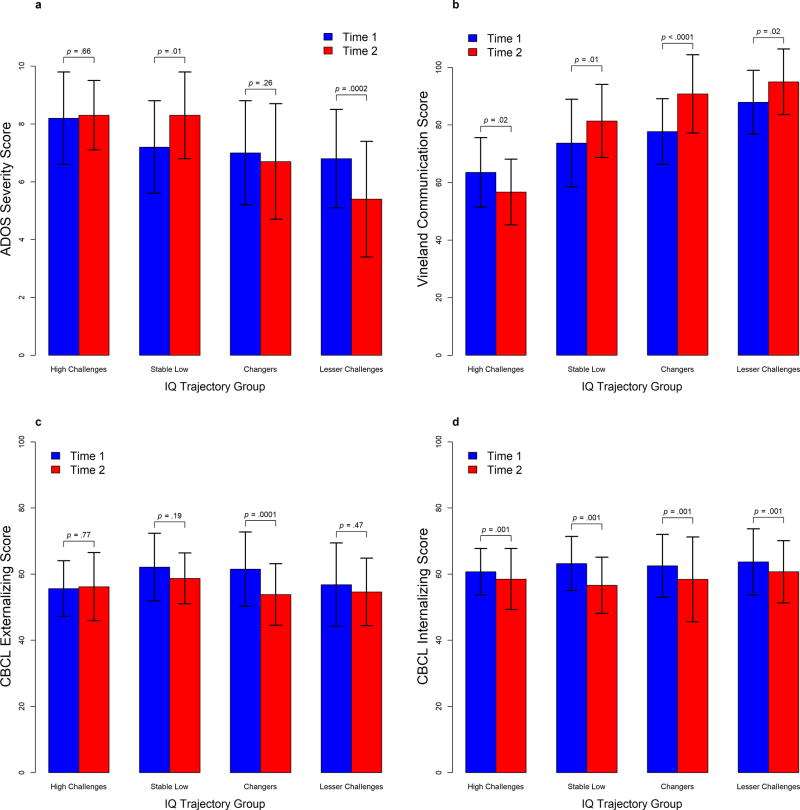
Table 4 and Figure 2 summarize the results of the repeated measures analyses of ADOS-2 CSS scores. After controlling for multiple comparisons, at T1 the High Challenges group showed a significantly greater CSS than the Lesser Challenges (by 1.3 points, p = .04) group and the difference between the High Challenges and the Changers groups approached statistical significance (1.1 points, p = .06). The pattern of changes from T1 to T2 differed significantly across trajectory groups (F(3, 98) = 7.05, p <.001), driven by a 1.5 point decrease in CSS for the Lesser Challenges group (p <.001) and a 1.1 point increase in CSS for the Stable Low group (p = .01). This resulted in the Lesser Challenges group having significantly lower scores at T2 than the Changers (by 1.3 points, p = .03), the Stable Low (by 2.9 points, p < .0001), and the High Challenges (by 2.9 points, p < .0001) groups. At T2, the difference between the High Challenges group and the Changers group increased in magnitude (1.6 points, p = .002). See Figure 2a.
Table 4.
Parameter estimates (standard errors) for the mixed-effects regression models predicting T1 and T2 scores
| ADOS Severity Score |
Vineland Communication Score |
CBCL Externalizing |
CBCL Internalizing |
|
|---|---|---|---|---|
|
| ||||
| Model term | ||||
| Estimated trajectory for High Challenges group | ||||
|
| ||||
| Baseline (T1) | 8.15 (0.34)*** | 63.36 (2.56)*** | 55.60 (2.04)*** | 61.38 (1.72)*** |
| Time effect (T2-T1) difference) | −0.15 (0.35) | −6.74 (2.87)* | 0.70 (2.42) | −3.84 (1.15)** |
|
| ||||
| Estimated difference between Stable Low and High Challenges groups | ||||
|
| ||||
| Baseline (T1) | −0.93 (0.53) | 10.36 (3.94)** | 6.59 (3.20)* | 0.60 (2.55) |
| Time effect (T2-T1) difference) | 0.90 (0.55) | 15.60 (4.56)*** | −4.58 (3.79) | – |
|
| ||||
| Estimated difference between Changers and High Challenges groups | ||||
|
| ||||
| Baseline (T1) | −1.13 (0.44)* | 14.46 (3.30)*** | 5.96 (2.68)* | 0.87 (2.15) |
| Time effect (T2-T1) difference) | −0.49 (0.46) | 19.53 (3.77)*** | −8.92 (3.16)** | – |
|
| ||||
| Estimated difference between Lesser Challenges and High Challenges groups | ||||
|
| ||||
| Baseline (T1) | −1.34 (0.50)** | 24.50 (3.68)*** | 1.22 (2.98) | 2.67 (2.39) |
| Time effect (T2-T1) difference) | −1.61 (0.52)** | 14.09 (4.14)*** | −2.57 (3.52) | – |
Note:
p < .05,
p < .01,
p < .001.
ADOS = Autism Diagnostic Observation Schedule; CBCL = Child Behavior Checklist
Figure 2.
Autism severity, Vineland, Internalizing, and Externalizing behavior scores over time by trajectory group. Panel a: Combined severity scores (CSS) based on the autism diagnostic observation schedule-2 (ADOS-2). Panel b: Vineland adaptive behavior scales (VABS-2) scores. Panel c: Children’s behavior checklist scores for Internalizing behaviors. Panel d: Children’s behavior checklist scores for Externalizing behavior
Hypothesis #3: Improvement over time driven by increases in VIQ and adaptive communication related abilities
As shown in Table 1, at T1, 75% of those who would be classified as Changers had VIQs < 70, while 58% had NVIQs <70. By T2, there were no Changers with VIQ or NVIQ <70, suggesting that, contrary to hypotheses, there had been significant changes in both verbal and non-verbal domains of cognitive ability level for the Changers (Mc Nemar’s test p < .001 for both domains). Neither the High or Lesser Challenges groups experienced growth in the percentage of individuals with VIQ>70 between T1 and T2. In the Stable Low group, more modest numbers of individuals showed VIQ change than Changers (Mc Nemar’s test p = .045), while the percentage of individuals with NVIQ>70 declined.
After controlling for multiple comparisons, at T1 the High Challenges group showed a significantly lower mean score on the VABS-2 communication domain than the Changers (by 14.5 points, p = .0002), the Lesser Challenges (by 24.5 points, p <.0001), and the Stable Low (by 10.4 points, p = .048) groups. The Lesser Challenges group also had significantly higher scores at T1 than the Stable Low (by 14.1 points, p = .003) and the Changers (by 10.0 points, p = .02). The pattern of changes from T1 to T2 differed significantly across the trajectory groups (F(3, 91.4) = 9.42, p < .0001), driven by a 12.8 point increase in the Changers (p <.0001). There were also increases in the Stable Low (by 8.9 points, p = .01) and Lesser Challenges (by 7.4 points, p = .02) groups, and the High Challenges had a significant decrease (by 6.7 points, p = .02). This resulted in the Lesser Challenges group having significantly higher scores at T2 than the Stable Low (by 12.6 points, p = .02) and the High Challenges (by 38.6 points, p < .0001) groups. There was no longer a significant difference between the Changers and the Lesser Challenges groups (p = .55). The High Challenges group continued to show significantly lower mean score on the VABS-2 communication domain than the Changers (by 34.0 points, p < .0001) and Stable Low (by 26.0 points, p <.0001) groups. See Figure 2b.
Hypothesis #4: Problem behavior and IQ change
The group differences in Externalizing at T1 were modest, with Changers and Stable Low displaying scores about 5 points higher than the other 2 groups, but none of the differences remained significant after controlling for multiple comparisons. There was a significant interaction with time (F(3, 88.1) = 2.93, p = .04), driven by an 8.2 point decline in the Changers (p = .0001) and a non-significant 3.9 point decline in the Stable Low group. See Figure 2c.
For the Internalizing scale, there was no group difference in the patterns of change from T1 to T2. The four trajectories had similar scores at baseline (all pair-wise differences less than 3 points) and their scores decreased by an average of 4 points (p =.001). See Figure 2d.
There were no significant differences in the percentage of children in each of the groups receiving applied behavior analysis (ABA) or language therapy or in the mean hours of treatment (See Table 5).
Table 5.
Summary of intervention received by the four groups during early childhood
| Variable | High Challenges (n = 26) |
Stable Low (n = 18) |
Changers (n = 36) |
Lesser Challenges (n = 22) |
|---|---|---|---|---|
| Before T1 | ||||
|
| ||||
| Received ABA1, n (%) | 18 (72%) | 13 (76%) | 15 (45%) | 12 (57%) |
| Weekly ABA hours, mean (SD) | 18.2 (9.0) | 19.7 (10.9) | 12.7 (5.5) | 19.1 (9.2) |
| Received ST1, n (%) | 15 (60%) | 13 (76%) | 27 (82%) | 18 (86%) |
| Weekly ST hours, mean (SD) | 1.3 (0.5) | 1.1 (0.3) | 1.6 (1.4) | 1.6 (1.1) |
|
| ||||
| Between T1 and T2 | ||||
|
| ||||
| Received ABA2, n (%) | 20 (83%) | 13 (76%) | 25 (76%) | 15 (68%) |
| Weekly ABA hours, mean (SD) | 18.1 (8.5) | 15.1 (10.0) | 16.3 (9.5) | 17.7 (11.0) |
| Received ST2, n (%) | 22 (92%) | 15 (88%) | 28 (85%) | 18 (82%) |
| Weekly ST hours, mean (SD) | 1.2 (0.8) | 1.2 (0.7) | 1.3 (0.7) | 0.8 (0.3) |
ABA = Applied Behavioral Analysis; ST = Speech-Language Therapy;
Missing data = 1 in High Challenges, 1 in Stable Low, 3 in Changers and 1 in Lesser Challenges;
Missing data = 2 in High Challenges, 1 in Stable Low, and 3 in Changers.
DISCUSSION
We investigated early to middle childhood IQ-based developmental trajectories and their correlates in a recently ascertained sample of children recruited at the MIND Institute. A four-class solution best fit the data. The High Challenges group had IQs at the most severe level of ID, and their IQs and their adaptive communication abilities declined by T2, relative to their peers. The Stable Low group began with IQs in the milder range of ID than the High Challenges group. While their relative IQ scores were stable, and their adaptive communication scores increased, the severity of their ASD symptoms increased by T2. Changers exhibited early IQ scores in the ID range with progression to the average range by age 8. Both NVIQ and VIQ increases contributed to these >2 standard deviation increases. Changers also exhibited significant improvements in adaptive communication abilities that rendered them indistinguishable from the members of the Lesser Challenges group, and significant reductions in externalizing behavior symptoms. Finally, the Lesser Challenges group had IQs in the average range throughout the ages 2 to 8 year old period, with a significant decline in ASD symptom severity such that 14%% no longer met ADOS-2 CSS criteria for ASD. Internalizing symptoms declined for all groups. The groups also did not differ in the amount or intensity of intervention received.
We return to the question of how this manuscript advances understanding of prognosis and treatment planning. As shown in Table 6, findings provide some guidance about how parents can begin to think about their children’s future functioning by middle childhood. Regardless of ABA or speech and language intervention intensity, between the ages of 2 and 8, about one quarter of the sample experienced a slowing in relative intellectual development and adaptive communication abilities alongside an increase in ASD symptom severity. The remaining three quarters fared at least somewhat better in that their relative intellectual ability levels were stable or improving, their adaptive communication abilities increased, and their externalizing behaviors declined, albeit only significantly so for the Changers. Despite the current literature, which suggests there is little relationship between IQ and ASD symptom severity, we found that the Lesser Challenges group demonstrated significant severity score reductions over time, while the Stable Low group saw increases, suggesting a more nuanced relationship.
Table 6.
Clinical Implications of findings based on the examination of 2–8 year olds with ASD
| Group | High Challenges | Stable Low | Changers | Lesser Challenges |
|---|---|---|---|---|
| Percentage in the Group | *About 25% | *About 20% | *About 33% | *About 20% |
| Cognitive Functioning (T1) | *Moderate ID | *Mild ID | *Mild ID | *Average |
| Cognitive Change | *Relative Declines in IQ | *Stable IQ | *Improves 2 SD | *Improves about 1 SD |
| Autism Symptom Severity | *Stable severe ASD sx | *Worsening ASD sx | *Moderate ASD sx | *ASD sx decline |
| Loss of Diagnosis | *Don’t lose dx | *Don’t lose dx | *May lose dx | *May lose dx |
| Adaptive Communication | *Low and declining | *Improves with time | *Strong Increase | * High and improving |
| Externalilzing Symptoms | *Similar to others & stable | *Similar to others & stable | *Declining | *Similar to others & stable |
| Internalizing Symptoms | *Declining | *Declining | *Declining | *Declining |
In summation, we thus believe that these results offer a hopeful message to many. One third of children with ASD –including those who have ID at age 2 – even can experience dramatic intellectual development by middle childhood, reaffirming that it is important to be conservative when diagnosing ID in young children with ASD (Fountain et al., 2012). Finally, another positive finding of our study was that there was a decline in internalizing symptoms across all groups, replicating the work of (Vaillancourt et al., 2016).
A very similar recent study offers support for many of our findings (Visser et al., 2017). These authors examined trajectories of development of ASD symptom severity scores at 3 time points in a larger sample of slightly younger children with ASD ages 1–4 years. They too found that attention symptom reduction and increased verbal skills were present in individuals who improved; that about 20% of their sample experienced decrements in functioning relative to their peers over the study period; and that autism severity scores were relatively stable. While we found a small insignificant increase in externalizing symptoms in our lowest functioning group, our findings diverge from those of Visser et al. who found large increases. These inconsistencies may be due to the fact that our study included older children.
In our study, there were no group differences in retrospective parent reports of ABA or speech therapy received. This underscores the great need to better understand the mechanisms of intellectual development in members of the High Challenges and the Stable Low groups to improve the psychosocial and pharmacological treatment armamentarium, and to illuminate what leads one to be a member of the Changers versus the Stable Low group. Two findings in the Changers – that they achieved adaptive communication abilities comparable to the Lesser Challenges group, and that they experienced the greatest reduction in externalizing behaviors, also offer clues that language and attention interventions may be particularly useful.
The current study had several limitations. First, although ours is a relatively large longitudinal dataset, sub-group cells became small with a consequent reduction in statistical power. Second, the T1 and T2 age spans differed. However, there was a minimum of two years between observations, no trajectory group differences in the length of this period, and no difference in findings about trajectory membership when the 7 individuals with greater than 4-year spans between assessments were excluded from the analysis. Third, recent work raises questions about the comparability of the MSEL and DAS-II. Farmer and colleagues (Farmer, Golden, & Thurm, 2016) examined the concurrent validity of DAS-II and MSEL DQ scores in children with ASD and TYP, and reported a 10–13 point mean difference (DAS-II scores were higher). Additionally, analyses revealed curvilinear relationships between DAS-II standard IQ and MSEL ratio IQ scores, such that the greatest degree of discrepancy was observed within the middle of the IQ distribution, which may include our Changers. We would point out, however, that Changers’ IQ increases of 34 points are still approximately 1 SD above what could be attributed to Thurm et al. measurement differences. Finally, ours was not a population-based sample. Children from poorer or more rural areas were not well-represented in our largely northern California-based sample recruited at the MIND Institute, and our findings might not generalize to the larger population.
In conclusion, findings raise important questions for further investigations during the lifespan. These include whether IQ development levels off over time; whether the High Challenges and Stable Low groups progress and why; whether Changers continue their robust development; and whether more children in the Lesser Challenges group lose their diagnoses? When combined with other behavioral and biological markers of group inclusion, such information will help provide a clearer answer to the central question posed in this paper.
LAY SUMMARY.
We examined how the IQs of children with autism spectrum disorder change between ages 2 and 8, and identified four patterns. Two groups exhibited persistently lower IQs. One group showed IQ increases of greater than 30 points with improved communicate abilities and declining disruptive behaviors. The final group had IQs in the average or better range at both time points, and 14% of them lost their diagnoses. Over half of the children experienced improved intellectual functioning between ages 2 and 8, whereas about 25% showed declines. Findings were not associated with intervention history.
Acknowledgments
Dr. Solomon was supported by National Institutes of Health (NIH) grants R01MH106518 and R01MH103284; Dr. Nordahl was supported by R01MH104438. Dr. Amaral was supported by R01MH103371. Dr. Reinhardt was supported by 5T32MH073124.
Statistical Support: Ana-Maria Iosif, Ph.D. provided statistical support as part of the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125).
Footnotes
Conflict of Interest and Disclosures: Drs. Solomon, Iosif, Reinhardt, Libero, Nordahl, Ozonoff, and Rogers report no biomedical financial interests or conflicts of interest. Dr. Amaral is on the Scientific Advisory Board of Stemina Biomaker Discovery and Axial Biotherapeutics.
Presentation Information: Poster presented at the American College of Neuropsychopharmacology 49th Annual Meeting, Hollywood, Florida, December 4–8, 2016.
References
- Achenbach TM, Rescorla L. ASEBA school-age forms & profiles. Aseba Burlington 2001 [Google Scholar]
- Achenbach TM, Rescorla LA. ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families; 2000. [Google Scholar]
- Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2013 doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. doi:2007-11558-008 [pii] [DOI] [PubMed] [Google Scholar]
- Anderson DK, Maye MP, Lord C. Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. Am J Intellect Dev Disabil. 2011;116(5):381–397. doi: 10.1352/1944-7558-116.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Oti RS, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. J Abnorm Child Psychol. 2009;37(7):1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdadli A, Assouline B, Sonie S, Pernon E, Darrou C, Michelon C, Pry R. Developmental trajectories of adaptive behaviors from early childhood to adolescence in a cohort of 152 children with autism spectrum disorders. J Autism Dev Disord. 2012;42(7):1314–1325. doi: 10.1007/s10803-011-1357-z. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, Prevention Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LC, Ho HH. The very early identification of autism: outcome to age 4 1/2-5. J Autism Dev Disord. 2004;34(4):367–378. doi: 10.1023/b:jadd.0000037414.33270.a8. [DOI] [PubMed] [Google Scholar]
- Elliot CD. Differential Ability Scales. 2. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Farmer C, Golden C, Thurm A. Concurrent validity of the differential ability scales, second edition with the Mullen Scales of Early Learning in young children with and without neurodevelopmental disorders. Child Neuropsychol. 2016;22(5):556–569. doi: 10.1080/09297049.2015.1020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D, Barton M, Eigsti IM, Kelley E, Naigles L, Schultz RT, Tyson K. Optimal outcome in individuals with a history of autism. J Child Psychol Psychiatry. 2013;54(2):195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernell E, Hedvall A, Westerlund J, Hoglund Carlsson L, Eriksson M, Barnevik Olsson M, Gillberg C. Early intervention in 208 Swedish preschoolers with autism spectrum disorder. A prospective naturalistic study. Res Dev Disabil. 2011;32(6):2092–2101. doi: 10.1016/j.ridd.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Flanagan HE, Smith IM, Vaillancourt T, Duku E, Szatmari P, Bryson S, Georgiades S. Stability and Change in the Cognitive and Adaptive Behaviour Scores of Preschoolers with Autism Spectrum Disorder. J Autism Dev Disord. 2015;45(9):2691–2703. doi: 10.1007/s10803-015-2433-6. [DOI] [PubMed] [Google Scholar]
- Fountain C, Winter AS, Bearman PS. Six developmental trajectories characterize children with autism. Pediatrics. 2012;129(5):e1112–1120. doi: 10.1542/peds.2011-1601. doi:peds.2011-1601 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades S, Boyle M, Szatmari P, Hanna S, Duku E, Zwaigenbaum L Pathways in, A. S. D. S. T. Modeling the phenotypic architecture of autism symptoms from time of diagnosis to age 6. J Autism Dev Disord. 2014;44(12):3045–3055. doi: 10.1007/s10803-014-2167-x. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. doi:S0959-4388(07)00011-6 [pii] [DOI] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Sepeta L, Wang Y, Marshall S, Gomez L, Sigman M, Hutman T. Early childhood predictors of the social competence of adults with autism. J Autism Dev Disord. 2012;42(2):161–174. doi: 10.1007/s10803-011-1222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Trajectories of autism severity in children using standardized ADOS scores. Pediatrics. 2012;130(5):e1278–1284. doi: 10.1542/peds.2011-3668. doi:peds.2011-3668 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Gray K, Keating C, Taffe J, Brereton A, Einfeld S, Tonge B. Trajectory of behavior and emotional problems in autism. Am J Intellect Dev Disabil. 2012;117(2):121–133. doi: 10.1352/1944-7588-117-2.121. [DOI] [PubMed] [Google Scholar]
- Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert M, Fein D. Can children with autism recover? If so, how? Neuropsychol Rev. 2008;18(4):339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of autism and developmental disorders. 2014;44(10):2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir SL, Saemundsen E, Asmundsdottir G, Hjartardottir S, Asgeirsdottir BB, Smaradottir HH, Smari J. Follow-up of children diagnosed with pervasive developmental disorders: stability and change during the preschool years. J Autism Dev Disord. 2007;37(7):1361–1374. doi: 10.1007/s10803-006-0282-z. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends Cogn Sci. 1998;2(10):389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early Autism Spectrum Disorder: subtypes and short-term outcomes. J Child Psychol Psychiatry. 2015 doi: 10.1111/jcpp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- Lord C, Bishop S, Anderson DK. Developmental trajectories as autism phenotypes. Am J Med Genet C Semin Med Genet. 2015;169(2):198–208. doi: 10.1002/ajmg.c.31440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. doi:63/6/694 [pii] [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule: ADOS-2. Western Psychological Services; 2012. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Kanne SM. Friendship and internalizing symptoms among children and adolescents with ASD. J Autism Dev Disord. 2010;40(12):1512–1520. doi: 10.1007/s10803-010-1014-y. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Kanne SM, Miles JH. Predicting improvement in social–communication symptoms of autism spectrum disorders using retrospective treatment data. Research in Autism Spectrum Disorders. 2012;6(1):535–545. [Google Scholar]
- Miller JN, Ozonoff S. The external validity of Asperger disorder: lack of evidence from the domain of neuropsychology. J Abnorm Psychol. 2000;109(2):227–238. [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. AGS Circle Pines, MN; 1995. [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Elizabeth K, Abbott R. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am J Ment Retard. 2008;113(6):439–452. doi: 10.1352/2008.113-439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund KL, Asparoutiov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling-a Multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- Pellicano E. Do autistic symptoms persist across time? Evidence of substantial change in symptomatology over a 3-year period in cognitively able children with autism. Am J Intellect Dev Disabil. 2012;117(2):156–166. doi: 10.1352/1944-558-117.2.156. [DOI] [PubMed] [Google Scholar]
- Pickles A, Anderson DK, Lord C. Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J Child Psychol Psychiatry. 2014;55(12):1354–1362. doi: 10.1111/jcpp.12269. [DOI] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J Am Acad Child Adolesc Psychiatry. 1996;35(4):523–529. doi: 10.1097/00004583-199604000-00019. doi:S0890-8567(09)63523-1 [pii] [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland-II adaptive behavior scales. AGS Publishing; 2005. [Google Scholar]
- Stevens MC, Fein DA, Dunn M, Allen D, Waterhouse LH, Feinstein C, Rapin I. Subgroups of children with autism by cluster analysis: a longitudinal examination. J Am Acad Child Adolesc Psychiatry. 2000;39(3):346–352. doi: 10.1097/00004583-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Szatmari P. Complexity and Parsimony in Natural History Studies of Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(8):636–638. doi: 10.1016/j.jaac.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Bryson SE, Boyle MH, Streiner DL, Duku E. Predictors of outcome among high functioning children with autism and asperger syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44(4):520–528. doi: 10.1111/1469-7610.00141. [DOI] [PubMed] [Google Scholar]
- Thurm A, Manwaring SS, Swineford L, Farmer C. Longitudinal study of symptom severity and language in minimally verbal children with autism. J Child Psychol Psychiatry. 2015;56(1):97–104. doi: 10.1111/jcpp.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LM, Stone WL, Pozdol SL, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism. 2006;10(3):243–265. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T, Haltigan JD, Smith I, Zwaigenbaum L, Szatmari P, Fombonne E, Georgiades S. Joint trajectories of internalizing and externalizing problems in preschool children with autism spectrum disorder. Development and Psychopathology. 2016:1–12. doi: 10.1017/S0954579416000043. [DOI] [PubMed] [Google Scholar]
- Venker CE, Ray-Subramanian CE, Bolt DM, Weismer SE. Trajectories of Autism Severity in Early Childhood. Journal of Autism and Developmental Disorders. 2014;44(3):546–563. doi: 10.1007/s10803-013-1903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JC, Rommelse NNJ, Lappenschaar M, Servatius-Oosterling IJ, Greven CU, Buitelaar JK. Variation in the Early Trajectories of Autism Symptoms Is Related to the Development of Language, Cognition, and Behavior Problems. J Am Acad Child Adolesc Psychiatry. 2017;56(8):659–668. doi: 10.1016/j.jaac.2017.05.022. [DOI] [PubMed] [Google Scholar]