Abstract
Previous attempts to improve growth and development of the intrauterine growth-restricted (IUGR) foetus during pregnancy have not worked or caused harm. Our research identifies tissue-specific mechanisms underlying foetal growth restriction and then tests strategies to improve growth and ameliorate many of the metabolic problems before the infant is born. The goal of our studies is to reduce the impact of foetal growth restriction at critical stages of development on the lifelong complications of IUGR offspring.
Conclusion
Defining specific mechanisms that cause growth restriction in the foetus might identify specific nutrients and hormones that could be given to the mother to improve foetal growth and reduce metabolic complications, using strategies first tested in our IUGR animal model.
Keywords: Amino acids, Glucose, Insulin, Intrauterine growth restriction, Oxygen
INTRODUCTION
Intrauterine growth restriction (IUGR) of the placenta and the foetus affects 5–8% of all pregnancies (1,2), is a primary cause of stillbirth (3) and leads to 10-fold higher rates of neonatal morbidity and mortality (4). Ischaemic placental disease (which includes IUGR) accounts for well over half of all indicated preterm deliveries (5). The consequences of IUGR in the foetus, or foetal growth restriction specifically, reach far beyond the perinatal period. Growth-restricted foetuses suffer from lifelong reductions in muscle mass (sarcopenia) (6) and are at risk for visceral adiposity, insulin resistance and diabetes (7). IUGR commonly develops in pregnancies complicated by placental insufficiency (PI), which chronically limits nutrient and oxygen supply to the foetus and reduces foetal anabolic hormone concentrations. In response to such deficiencies, mechanisms are initiated in the PI-IUGR foetus, such as dilation of the ductus venosus (8), that shunt oxygenated blood towards the heart and brain. The middle cerebral artery also dilates to maximise blood flow to the brain. The end result of PI-IUGR is asymmetrical foetal growth that spares brain growth relative to overall foetal weight and linear growth. This asymmetrical foetal growth restriction pattern is in contrast to symmetrically grown small for gestational age (SGA) neonates who are the result of normal but slower than average rates of foetal growth, such as those who are constitutionally small (9,10).
These adaptive adaptations also allow normal cellular oxidative metabolism to continue, maintaining foetal viability at the expense of growth. The successful growth-restricted foetal phenotype involves several adaptations that are successful for foetal survival. Increased capacity for foetal tissue and cellular glucose uptake (increased glucose and insulin sensitivity) sustains normal cellular metabolism. Increased capacity for foetal hepatic glucose production (HGP) maintains glucose concentrations to support cellular metabolism of critical organs such as the brain and the heart. Decreased foetal pancreatic development and insulin secretion match anabolic hormone concentrations to the reduced levels of energy and protein supply. Decreased capacity for amino acids to be synthesised into foetal protein slows cellular growth to maintain normal cellular energy metabolism (9,10).
Unfortunately, such foetal adaptations also appear to produce problems that are maladaptive to conditions immediately after birth and in later childhood and adult periods of life. These include the acute problems that growth-restricted foetuses suffer at birth (e.g. asphyxia, hypoglycaemia). They also include later life problems associated with foetal growth restriction, such as brain under growth and directly associated neurodevelopmental and cognitive and behavioural deficiencies, short stature, underdeveloped lungs, cardiomyocyte deficiency and propensity to heart failure, skeletal myocyte deficiency and associated muscle weakness and reduced glucose disposal capacity, and insulin resistance and excessive glucose production leading to the metabolic syndrome (including obesity, type 2 diabetes and cardiovascular disorders such as hypertension, stroke and myocardial infarctions) (9,10).
Despite these many problems during foetal life and throughout the lifespan, no one has been able to do very much about improving growth and development of the growth-restricted foetus once diagnosed in pregnancy that might prevent the development of these disorders. Many previous attempts at enhancing nutrition and oxygenation of the pregnant mother with an IUGR pregnancy (e.g. maternal oxygen and bed rest, augmented maternal nutrition, a variety of medications) have either not worked or caused harm, producing worse IUGR and even foetal death (9–12). As a result, current management of IUGR pregnancies involves foetal surveillance and delivery of the foetus when adverse physiology develops, even if preterm, hoping that the growth-restricted foetus can be treated better outside the uterus as a growth-restricted neonate, clearly a less-than-satisfactory strategy that continues to produce growth-restricted preterm neonates with considerable pathology. Understanding how IUGR develops, but also understanding how the growth-restricted foetus responds to corrections of placental insufficiency, is essential to find ways to improve restricted foetal growth before it produces adverse outcomes. Animal models must be optimised first to study the impact of adding nutrients or blood transfusions or anabolic hormones directly to the foetus before considering, let alone testing, future efforts to promote foetal growth and development in human IUGR pregnancies.
MODEL OF PLACENTAL INSUFFICIENCY AND IUGR IN FOETAL SHEEP
Our research group has been developing and testing strategies to see whether we could improve growth and development of the restricted foetus before its adaptations to under nutrition, hypoxia and anabolic hormone deficiencies become permanently programmed.
Our first goal, conducted in established late gestation growth-restricted foetuses in our unique model of placental insufficiency and IUGR in pregnant sheep, has been to study selected organ function following increased supply of nutrients and anabolic hormones. These studies have focused on (i) the pancreas and insulin secretion; (ii) the liver and glucose production; (iii) skeletal muscle and myocyte growth and development; and (iv) the brain and neuronal growth and development. Our second goal has been to start replacements of these factors much earlier in gestation, during early phases of IUGR development, before programming has produced permanent changes. Our aim was to find a period when adaptive mechanisms in the growth-restricted foetus remain plastic enough that we could prevent or repair relatively unfixed, less permanent programming of molecular, cellular and physiological responses of the growth restriction to placental insufficiency.
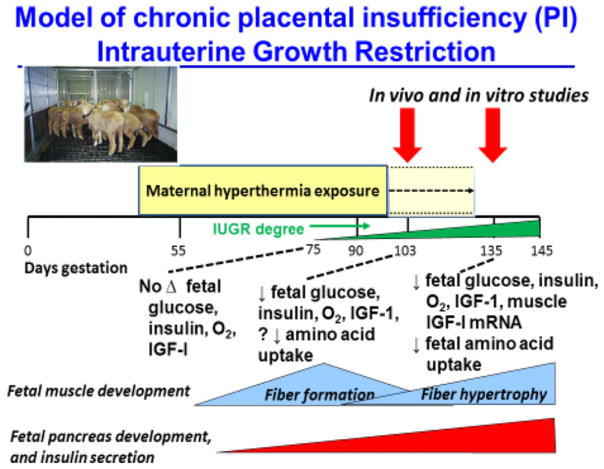
With our unique model of IUGR in pregnant sheep, we are able to carefully control short- and long-term supplements of energy (glucose), protein (amino acids) and anabolic hormones (insulin). We are able to provide these replacements at graded levels and during critical stages of gestation that allow us to determine how they produce beneficial or adverse effects, what can be done to prevent or correct adverse effects, and when during the development of IUGR, there is sufficient plasticity of adaptations to foetal under nutrition that correction is successful. Our studies have characterised the progressive decline of foetal plasma insulin, IGF-I, oxygen and foetal amino acid uptake in our ovine model of placental insufficiency and IUGR in relation to muscle fibre formation and pancreatic development (Fig. 1) (13). These direct foetal studies then could lead to more effective treatments via the mother that could be tested in our model before adapting such treatments for trials in human pregnancies.
Figure 1.
Our experimental model of placental insufficiency IUGR (PI-IUGR) produced by exposing pregnant sheep to daily 12 hours of hyperthermia, adapted from naturally occurring placental and foetal growth restriction in pregnant sheep that carry their pregnancies in the hot summer months We are able to measure different developmental responses (glucose and oxygen and amino acid supply and foetal plasma concentrations of oxygen, nutrients and anabolic hormones) of the foetus to reduced nutrient supply from the smaller, insufficient placenta at different gestational ages, and then conduct physiological studies in which we manipulate the supplies of oxygen, glucose, amino acids, insulin and IGF to help determine when the IUGR foetus can still respond with increased nutrient metabolism and growth to improved oxygenation and nutrition, or when programming already has developed and responses are limited to adverse consequences. Figure adapted from Brown et al. (13).
Our model of IUGR (Table 1) is a ‘natural’ model in pregnant sheep of placental insufficiency (PI-IUGR) produced by exposing pregnant ewes to elevated ambient temperatures for 70–80 days during mid-gestation (control ewes are housed at normal ambient temperatures and fed a diet matched to the PI-IUGR ewes (14). Maternal hyperthermia and IUGR are natural in sheep that, uncharacteristically, carry pregnancies in the hotter summer months, producing a smaller placenta with nutrient and oxygen transport defects (15). In our model of placental insufficiency-induced IUGR, the placental insufficiency is the result of reduction in both placental size and placental transport capacity of glucose, amino acids and oxygen. The placental insufficiency, therefore, under-nourishes the foetus, producing a growth-restricted foetal phenotype that is identical to other ovine models of placental insufficiency, despite very different approaches to producing placental insufficiency (16–19). The model also is one of the asymmetric foetal growth, such that foetal weight and length measurements during late gestation are more reduced than brain weight. The key feature in all of these models of IUGR from placental insufficiency is that the foetus receives less than normal amounts of nutrients and oxygen. The principal advantage of our model is that it most closely mimics the human condition, representing a unique model for understanding foetal physiology in utero that cannot be accomplished in any other species, particularly in small animals.
Table 1.
Several important characteristics of the ovine placental insufficiency model of IUGR are very similar to characteristics in human placental insufficiency IUGR (67).
| Class | Physical/physiological variable | Human IUGR | Ovine PI-IUGR |
|---|---|---|---|
| Vasculature | Umbilical blood flow | Decreased | Decreased |
| Pulsatility index in artery | Increased | Increased | |
| Placental oxygenation at term | Hypoxic | Hypoxic | |
| Endocrinology | Progesterone | Decreased | Decreased |
| Placental lactogen | Decreased | Decreased | |
| Growth hormone (expression) | Decreased | Decreased | |
| IGF-I concentration | Decreased | Decreased | |
| Morphology | Placental Weight | Decreased | Decreased |
| Foetal weight | Decreased | Decreased | |
| Asymmetrical growth | Yes | Yes | |
| Metabolism | Maternal–foetal [glucose] gradient | Increased | Increased |
| Foetal glucose concentration at term | Hypoglycaemic | Hypoglycaemic | |
| Foetal lactate concentration | Increased | Increased | |
| Foetal amino acid concentration | Decreased | Decreased |
PANCREAS AND INSULIN SECRETION
Human IUGR infants and essentially all animal models of IUGR have consistently shown progressive reductions in pancreatic β-cell numbers and function when placental insufficiency reduces nutrient supply to the foetus over the bulk of gestation (20). Our studies have shown that decreased supply of these essential nutrients is primarily responsible for the adverse changes that occur in the growth-restricted foetus in β-cell development and insulin secretion at the physiological (decreased in vivo and in vitro β-cell function), morphological (decreased β-cell mass, mitosis, islet size and impaired vascular development) and protein and mRNA (decreased cell growth factors) levels (21–25). Our studies now are aimed at normalising the foetal nutrient supply and anabolic hormone concentrations to promote β-cell development and insulin secretion (26–29).
Prior to testing these manipulations in growth-restricted foetuses, we have routinely tested the role of these nutrients and hormones in normally grown foetuses. Chronically decreasing or increasing foetal glucose supply inhibits glucose-stimulated insulin secretion (GSIS) and decreases β-cell mass and/or increases β-cell apoptosis (26,30). However, increasing foetal glucose supply in a pulsatile pattern increases GSIS in normal foetuses (30). Increasing the amino acid supply in normal control foetuses potentiates foetal glucose-stimulated insulin secretion, although there is no impact on islet structure (27). A foetal insulin infusion increases foetal insulin and insulin-like growth factor 1 (IGF-1) concentrations, but inhibits glucose-stimulated insulin secretion and has no impact on β-cell mass (28). With respect to experiments in growth-restricted foetuses, we have completed studies in which we normalised foetal glucose concentrations with a continuous glucose infusion in IUGR foetuses. The glucose infusion had no impact on β-cell mass, but further impaired insulin secretion (29). Disappointingly, correction of foetal glucose concentrations in growth-restricted foetuses causes hypoxia, acidosis and decreased insulin secretion (29). These findings highlight the need to test prospective therapeutic strategies for foetal growth restriction in animal models where adverse foetal responses can be measured.
We also aim to understand the fundamental organ and cellular defects resulting in impaired foetal islet structure and function in IUGR. For example, an underlying cause of the impaired vascular development and islet insulin secretion may be reduced hepatocyte growth factor (HGF) production by the PI-IUGR islet endothelial cells and reduced islet vascular endothelial growth factor A (VEGFA) (23,31). Data resulting from this line of investigation demonstrate (i) the importance of HGF and VEGFA in foetal islets, (ii) the specificity of HGF in endothelial cell-conditioned media for stimulating islet function and (iii) impaired production of these hormones in IUGR foetal islets and islet-derived endothelial cells (23).
We also are testing how oxygen may play a fundamental role in the suppression of insulin secretion and insulin action by a mechanism of hypoxia-induced catecholamine secretion (32). Furthermore, hypoxia-generated increased cortisol production may promote gluconeogenesis and protein breakdown. These outcomes are likely, based on evidence from our model of IUGR that insulin concentrations increase when foetuses are treated with alpha- and beta-adrenergic receptor blockers (33,34). An extension of these studies into the neonatal period, when catecholamine concentrations are expected to be lower (normally), might lead to increased insulin secretion, which will secondarily lead to hypoglycaemia (35). Our studies have uniquely shown that IUGR islets have increased fractional insulin secretion capacity, which supports the in vivo characteristics of increased responsiveness following adrenergic suppression (24). Our studies, therefore, may have provided evidence for how IUGR newborn infants can initially have hyperglycaemia, but later on have low glucose concentrations that appear to be due to relative hyperinsulin-like conditions (36).
SKELETAL MUSCLE GROWTH RESPONSES
Our model of IUGR demonstrates reduced transplacental transport of nearly all amino acids, particularly leucine, an essential amino acid that has unique regulatory effects on muscle protein synthesis (37,38). In addition, foetal tissue uptake of leucine is reduced in IUGR foetuses (Fig. 3) (39). Thus, skeletal muscle growth is particularly vulnerable in the IUGR foetus, with both reduced amino acid supply from the placenta and further downregulation of tissue amino acid uptake. The latter problem indicates that there is potential to promote muscle amino acid utilisation independently of amino acid supply from the placenta by directly infusing amino acid into the foetus (38). Our current studies are designed to determine the mechanisms responsible for reduced foetal uptake of amino acid and restricted muscle growth in the growth-restricted foetus.
Figure 3.
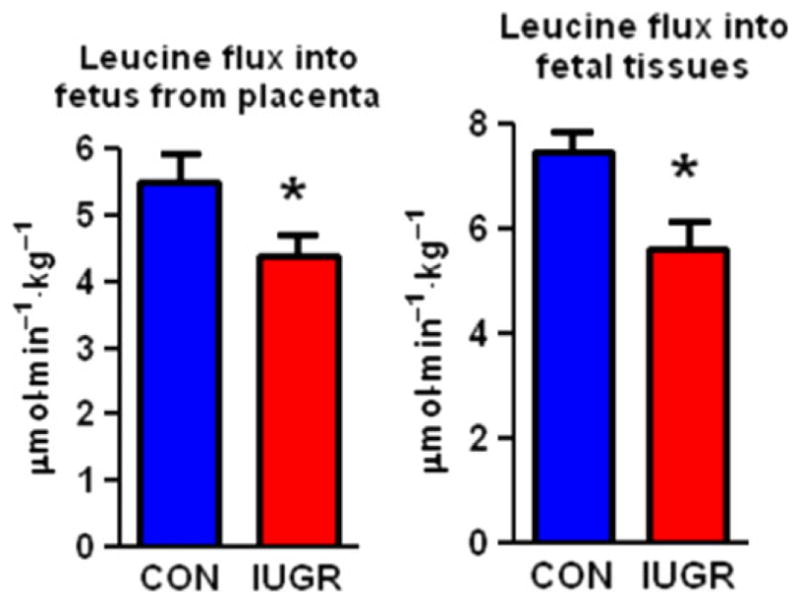
Decreased leucine flux in the IUGR foetus in late gestation Blue bars represent control foetuses and red bars indicate growth-restricted foetuses. *p < 0.05 Reproduced with permission from Brown et al. (38).
Chronic foetal under nutrition has the potential to disrupt normal myogenesis because several studies have shown that muscle fibre number is set at birth (40–42). Early in foetal development, myoblasts proliferate and differentiate into skeletal myofibres (43). With decreased nutrient and growth factor availability, myogenesis is reduced. For example, in a variety of species, maternal nutrient restriction during pregnancy limits foetal myoblast cell cycle activity, reduces myonuclei per myofibre and reduces myofibre number in offspring (44–47). Similarly, placental insufficiency independent of maternal nutrient intake lowers the proliferative capacity of foetal myoblasts, because mRNA expression of several genes that regulate cell cycle progression is reduced and reflects the in situ situation with less proliferating cell nuclear antigen (PCNA)-positive cells (39,48). Furthermore, myoblasts harvested from growth-restricted foetal sheep and cultured in vitro show slower rates of replication (39).
Myofibre hypertrophy is the primary mechanism of muscle growth late in gestation and in postnatal life (13). Foetal skeletal muscle weight is disproportionately reduced in relation to limb length in the growth-restricted foetus (56). Furthermore, we have found that smaller muscle fibre area associates with the foetal growth restriction in late gestation (0.9 of gestation; Fig. 2) (39,49). Decreased leucine flux into foetal tissues (shown in Fig. 3) reflects decreased capacity for amino acid uptake that might be driven by skeletal muscle (38). Protein breakdown pathways might also be activated, as is the case in adults during catabolic states such as starvation, cancer and burn injury, for supplying amino acids to organs such as the heart, liver and brain (50).
Figure 2.
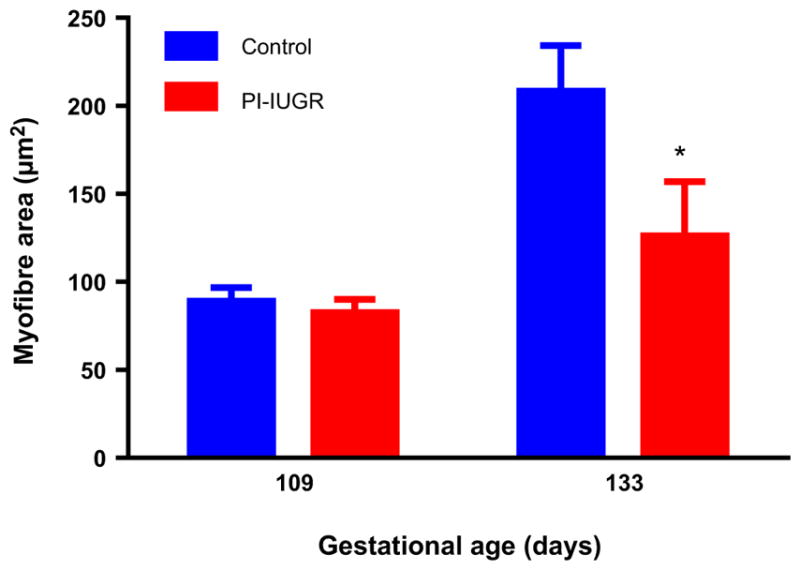
Decreased myofibre size in PI-IUGR foetal skeletal muscle in late gestation. *p < 0.05 Figure adapted from Yates et al. (39).
Our recent studies have evaluated the effect of increasing foetal amino acid delivery to late gestation control and growth-restricted foetal sheep. Normally growing, control foetal sheep demonstrate preferential oxidation of leucine by the foetus over glucose in response to increased amino acid delivery, as measured using leucine tracers and the glucose–oxygen quotient to estimate glucose oxidation. This effect was observed when amino acids were given to the pregnant ewe (51) or when given directly to the foetus, both acutely (three hours) (42) and chronically (10–14 days) (52). However, acute amino acid supplementation directly to the growth-restricted foetus for three hours suppressed protein breakdown rates and only minimally increased leucine oxidation, which produced increased foetal protein accretion rates (38) (Fig. 4). These observations have demonstrated that whereas excess amino acids in the normal foetus were oxidised, supplemental amino acids given for short periods of time in the growth-restricted foetus were used to suppress protein breakdown and thereby promote net protein accretion.
Figure 4.
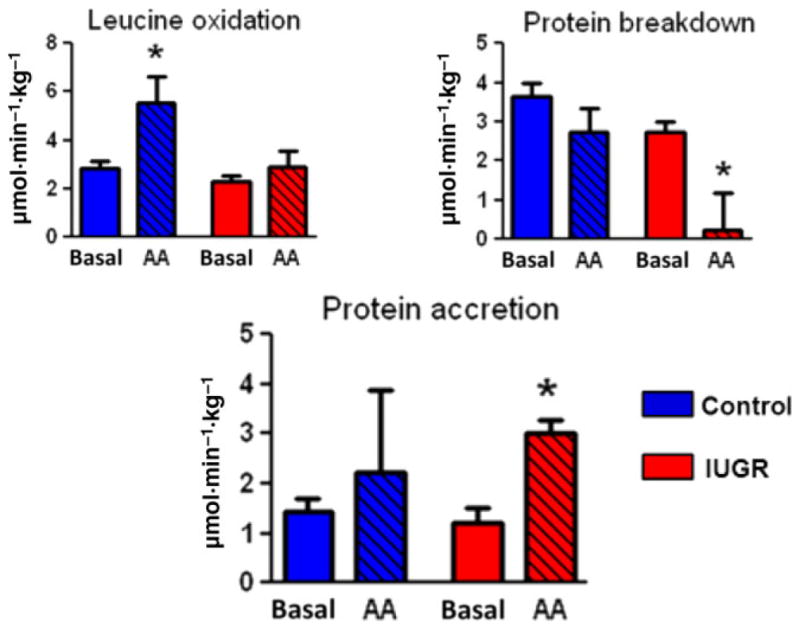
Acute amino acid supplementation of normally growing foetuses (blue bars) leads to increased amino acid oxidation, with no significant increase in protein accretion In contrast, the same amino acid supplementation of growth-restricted foetuses (red bars) does not increase amino acid oxidation, but does increase protein accretion by reducing protein breakdown. *p < 0.05 Reproduced with permission from Brown et al. (38).
We also have tested the effects of growth factor supplementation on myoblast proliferation and growth. Myoblasts harvested and cultured in vitro from control and growth-restricted foetal muscle show increased proliferation in the growth-restricted foetus in response to insulin stimulation compared with control (53). Our studies in normally growing foetuses suggest that both insulin and amino acids are required for the activation of protein synthetic pathways (54); thus, restoration of myoblast proliferation might be possible during foetal life in the growth-restricted foetus, but with appropriate growth factor stimulation as well as with supplemental amino acids.
LIVER AND GLUCOSE PRODUCTION
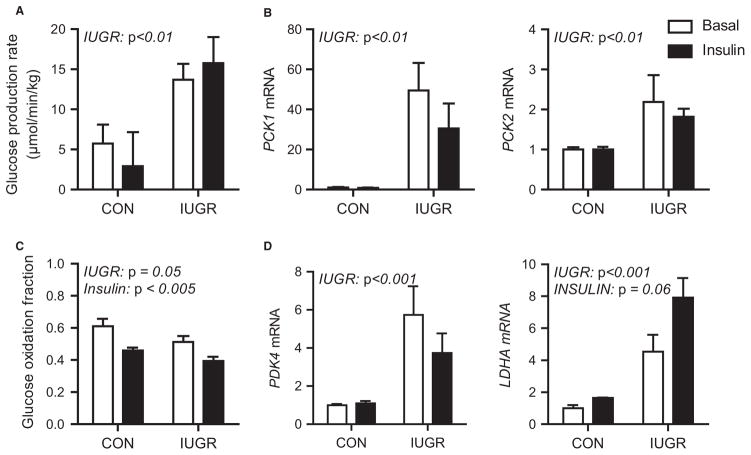
Foetal hepatic glucose production is normally absent due to transplacental maternal glucose supply (55). In animal models of IUGR, early developmental shifts in foetal glucose metabolism include increased hepatic gluconeogenic gene expression, HGP and decreased pancreatic insulin secretion (9,24,56,57). Specifically, foetal sheep with PI-IUGR demonstrate increased HGP rates and hepatic gluconeogenic gene expression (phosphoenolpyruvate carboxykinase 1 cytosolic form [PCK1], phosphoenolpyruvate carboxykinase 2 mitochondrial form [PCK2] and glucose-6-phosphatase, catalytic subunit [G6PC]) (Fig. 5A,B) (57). Increased HGP, hepatic gluconeogenic gene expression and growth restriction are also present in foetal sheep exposed to chronic (>8 week) hypoglycaemia (58). Increased expression of PCK1 is found in the nutrient-restricted foetal baboon liver (59) and in the IUGR neonatal rodent liver (60).
Figure 5.
Glucose metabolism in late gestation control (CON) and growth-restricted (IUGR) foetal sheep during basal and hyperinsulinemic clamp (insulin) periods (A) Foetal glucose production rate (B) Expression of gluconeogenic genes, PCK1 and PCK2, in foetal liver samples (C) Glucose oxidation rate (D) Expression of PDK4 and LDHA in foetal liver Means ± SE are shown for CON and growth-restricted (IUGR) foetuses studied during basal (white bars) and insulin clamp periods (black bars). Significant effects from two-way ANOVA are indicated. Adapted from Thorn et al., (57) and Brown et al., (62).
While these adaptations may be beneficial to sustain foetal viability, they also can lead to pathological conditions later in life, including type 2 diabetes (9). The early mechanisms responsible for these metabolic reprogramming adaptations in the PI-IUGR foetal liver are largely unknown. We have found increased nuclear levels of phosphorylated forkhead box protein O1 (FOXO1) protein in IUGR livers under basal and insulin-stimulated conditions (57). We speculate that aberrant FOXO1 regulation as a result of hypoxia may contribute to increased hepatic glucose production and insulin resistance. Despite reduced nutrient supply and hypoxia, the IUGR fails to activate key nutrient sensors like AMP-activated protein kinase (AMPK) (61). Thus, impaired nutrient sensing may be another therapeutic target for liver metabolism in the IUGR foetus.
We also have found increased expression of several key hypoxia target genes in the IUGR foetus. These include increased pyruvate dehydrogenase kinase 4 (PDK4), in both liver and muscle, which limits glucose oxidation and is consistent with reduced glucose oxidation in the IUGR foetus (Fig. 5C, D) (56,62). Lactate dehydrogenase A (LDHA) expression also is increased in the growth-restricted foetal liver (Fig. 5D), but not muscle, suggesting intrahepatic lactate production, which might provide carbon for HGP (62). These data support a role of hypoxia for producing HGP in the growth-restricted foetus.
Our recent data also support that coordinated changes in hepatic amino acid metabolism support the early activation of HGP. In response to foetal hypoglycaemia for 5 days, the foetal liver transitioned from net glucose uptake to net output (63). Hepatic total net amino acid uptake increased twofold, along with significantly increased concentrations of cortisol and glucagon. The increased net hepatic amino acid uptake included increased uptakes of both essential and nonessential amino acids. Interestingly, concentrations of branched chain essential amino acids, which increased by 20–40% on day 1 and day 5, were positively related to HGP. Thus, increased amino acid supply might support insulin production and muscle anabolism in the growth-restricted foetus, while also increasing foetal HGP that would support basal metabolism and provide energy for the anabolic conditions that amino acids and insulin might generate.
INCREASING FOETAL BLOOD RED BLOOD CELL AND BLOOD OXYGEN CONTENT BY BLOOD TRANSFUSIONS
Oxygen is fundamental for growth as well as normal cellular metabolism. Insufficient oxygen might be critically important in restricting foetal growth in PI-IUGR, either directly by inhibiting the synthesis of amino acids into net protein balance, or indirectly by restricting anabolic hormone production, principally insulin. This could occur by increased production of cortisol or catecholamines, both of which have the capacity to reduce insulin secretion and action (32,64,65). Our previous studies have shown that not only is the growth-restricted foetus relatively hypoxic, but gets even more hypoxic as glucose is infused, compounded by hypoxia-induced excessive catecholamine secretion. The normal foetus responds to hypoxia with increased red blood cell production via activated erythropoietin production (66). Our goal is to develop a rigorous protocol for improving foetal oxygenation. One approach under current investigation is to transfuse red blood cells into growth-restricted foetuses and measure their blood oxygen content, nutrient substrate and hormonal concentrations, metabolic rate (oxygen consumption) and specific cell metabolic functions, including glucose- and amino acid-stimulated insulin secretion, amino acid- and insulin-stimulated skeletal muscle myocyte replication and hypertrophy, glucose and amino acid effects on neuronal development, and hepatic glucose output. This novel technique would mimic the increase in RBC production that normally happens in foetuses exposed to low oxygen supply and prevents growth restriction from hypoxia. Alternatively, we could infuse recombinant erythropoietin, which might stimulate foetal RBC production. All growth-restricted foetuses from placental insufficiency experience reduced oxygen supply and hypoxia, which might interfere with nutrient and anabolic hormone supplement treatments, particularly via their stimulation of catecholamine secretion, which will inhibit insulin production (32,64). Catecholamines contribute to about 50% of the growth restriction; thus, correcting oxygen deficiency is fundamental also in reducing the secretion of catecholamines and thus their negative impact on foetal growth.
SUMMARY
Our studies are showing potential for improving the growth of the growth-restricted foetus before term gestation and birth. In some cases, this can be done early in the development of IUGR by simply replacing nutrients and oxygen. In others, anabolic hormone replacements might offer unique improvements in cell proliferation alone, as well as hypertrophy of cells when administered with nutrients (amino acids, principally). Key to all of these approaches with nutrients and anabolic hormones is to learn how to enhance foetal blood oxygen content to optimise energy utilisation for protein synthesis and protein balance. The studies in our ovine IUGR model indicate that there is much to be learned about how and when foetal growth restriction develops in response to placental insufficiency. Such studies should be performed first and then tested for the capacity to correct such foetal growth restriction in the same model to help determine which strategies might be successfully developed and applied in human cases of IUGR, since at this stage of clinical treatment, many of the strategies (foetal RBC transfusion, foetal intravenous nutrient and anabolic hormone infusions) are not yet possible. As such strategies become more realistic for adapting to human clinical cases of IUGR, it will be even more important for obstetricians to develop approaches for earlier diagnosis of IUGR (placental and foetal) during gestation and more accurate assessment of changes in growth that might occur in response to such potential treatments. It also is possible that outcomes of our studies might inform clinicians about how to better feed and oxygenate preterm infants to prevent or ameliorate postnatal growth restriction, but that would be for future studies to determine.
Key Notes.
We have developed research strategies in growth-restricted foetal sheep to determine approaches that would more successfully promote foetal growth.
These strategies might potentially limit the severity of foetal growth restriction prior to birth.
Limiting the severity of growth restriction at critical stages of foetal development might reduce the impact of foetal growth restriction on the lifelong complications that it continues to produce.
Acknowledgments
All authors participated in the writing and editing of the manuscript. PJR, LDB, SRW, SWL and WWH conducted the experiments. WWH was supported by NIH T32 HD007186 (PI and PD) and NIH K12 HD068372 (PD); PJR was supported by NIH R01 DK088139 and K08 HD060688 (PI); LDB was supported by NIH Building Interdisciplinary Careers in Women’s Health K12 HD057022 (Scholar), the University of Colorado Center for Women’s Health Research and NIH R01HD079404 (PI). SRW was supported by NIH R03 DK102972 and K01 DK090199 (PI); SWL was supported by NIH R01 DK084842 (PI).
References
- 1.Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Tuuli MG, Cahill A, Stamilio D, Macones G, Odibo AO. Comparative efficiency of measures of early fetal growth restriction for predicting adverse perinatal outcomes. Obstet Gynecol. 2011;117:1331–40. doi: 10.1097/AOG.0b013e31821ae239. [DOI] [PubMed] [Google Scholar]
- 3.Froen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand. 2004;83:801–7. doi: 10.1111/j.0001-6349.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 4.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–8. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 5.Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol. 2014;38:151–8. doi: 10.1053/j.semperi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–32. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchirikov M, Schroder HJ, Hecher K. Ductus venosus shunting in the fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet Gynecol. 2006;27:452–61. doi: 10.1002/uog.2747. [DOI] [PubMed] [Google Scholar]
- 9.Thorn SR, Rozance PJ, Brown LD, Hay WW., Jr The Intrauterine Growth Restriction (IUGR) phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med. 2011;29:225–36. doi: 10.1055/s-0031-1275516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozance PJ, Brown LD, Thorn SR, Anderson MS, Hay WW., Jr . Intrauterine growth restriction and the small-for-gestational-age infant. In: MacDonald MG, Seshia MMK, editors. Avery’s Neonatology: Pathophysiology and Management of the Newborn. 7. Philadelphia: Wolters Kluwer Health-Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 11.Say L, Gülmezoglu AM, Hofmeyr GJ. Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000148. [DOI] [PubMed] [Google Scholar]
- 12.Rush D, Stein Z, Susser M. Controlled trial of prenatal nutrition supplementation defended. Pediatrics. 1980;66:656–8. [PubMed] [Google Scholar]
- 13.Brown LD. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J Endocrinol. 2014;221:R13–29. doi: 10.1530/JOE-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol. 1987;9:17–29. [PubMed] [Google Scholar]
- 15.Regnault TR, Orbus RJ, Battaglia FC, Wilkening RB, Anthony RV. Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency. J Endocrinol. 1999;162:433–42. doi: 10.1677/joe.0.1620433. [DOI] [PubMed] [Google Scholar]
- 16.de Boo HA, van Zijl PL, Smith DE, Kulik W, Lafeber HN, Harding JE. Arginine and mixed amino acids increase protein accretion in the growth-restricted and normal ovine fetus by different mechanisms. Pediatr Res. 2005;58:270–7. doi: 10.1203/01.PDR.0000169977.48609.55. [DOI] [PubMed] [Google Scholar]
- 17.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology. 2007;148:1350–8. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- 18.Wallace JM, Milne JS, Aitken RP, Hay WW., Jr Sensitivity to metabolic signals in late-gestation growth-restricted fetuses from rapidly growing adolescent sheep. Am J Physiol Endocrinol Metab. 2007;293:E1233–41. doi: 10.1152/ajpendo.00294.2007. [DOI] [PubMed] [Google Scholar]
- 19.Lang U, Baker RS, Khoury J, Clark KE. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R53–9. doi: 10.1152/ajpregu.2000.279.1.R53. [DOI] [PubMed] [Google Scholar]
- 20.Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205:211–24. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW., Jr Reductions in insulin concentrations and beta-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab. 2013;304:E516–23. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 23.Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, et al. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–64. doi: 10.2337/db14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–97. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Rozance PJ, Hay WW, Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood) 2012;237:524–9. doi: 10.1258/ebm.2012.011375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavezzi JR, Thorn SR, O’Meara MC, LoTurco D, Brown LD, Hay WW, Jr, et al. Increased fetal insulin concentrations for one week fail to improve insulin secretion of beta-cell mass in fetal sheep with chronically reduced glucose supply. Am J Physiol, Reg Int. 2013;304:R50–8. doi: 10.1152/ajpregu.00413.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gadhia MM, Maliszewski AM, O’Meara MC, Thorn SR, Lavezzi JR, Hay WW, Jr, et al. Increased amino acid supply potentiates glucose stimulated insulin secretion but does not increase beta cell mass in fetal sheep. Am J Physiol Endocrinol Metab. 2013;304:E352–62. doi: 10.1152/ajpendo.00377.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews SE, Brown LD, Thorn SR, Limesand SW, Davis M, Hay WW, Jr, et al. Increased adrenergic signaling is responsible for decreased glucose-stimulated insulin secretion in the chronically hyperinsulinemic ovine fetus. Endocrinology. 2015;1:367–76. doi: 10.1210/en.2014-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW., Jr Glucose replacement causes hypoxia, acidosis, and decreased insulin secretion in a sheep model of intrauterine growth restriction. Pediatr Res V. 2009;65(1):72–8. doi: 10.1203/PDR.0b013e318189358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frost MS, Zehri AH, Limesand SW, Hay WW, Jr, Rozance PJ. Differential effects of chronic pulsatile versus chronic constant maternal hyperglycemia on fetal pancreatic beta-cells. J Pregnancy. 2012;2012:812094. doi: 10.1155/2012/812094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozance PJ, Hay WW., Jr Pancreatic islet hepatocyte growth factor and vascular endothelial growth factor A signaling in growth restricted fetuses. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, et al. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol. 2012;590:5439–47. doi: 10.1113/jphysiol.2012.237347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–8. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macko AR, Yates DT, Chen X, Green AS, Kelly AC, Brown LD, et al. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0. 7 of gestation. J Dev Orig Health Dis. 2013;4:402–10. doi: 10.1017/S2040174413000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Green AS, Macko AR, Yates DT, Kelly AC, Limesand SW. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am J Physiol Endocrinol Metab. 2014;306:E58–64. doi: 10.1152/ajpendo.00517.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley CA, Rozance PJ, Thornton PS, De Leon DD, Harris D, Haymond MW, et al. Re-evaluating “transitional neonatal hypoglycemia”: mechanism and implications for management. J Pediatr. 2015;166:1520–5. doi: 10.1016/j.jpeds.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol. 1996;270:E491–503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- 38.Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW., Jr Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–64. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yates DT, Clarke DS, Macko AR, Anderson MJ, Shelton LA, Nearing M, et al. Myoblasts from intrauterine growth-restricted sheep fetuses exhibit intrinsic deficiencies in proliferation that contribute to smaller semitendinosus myofibres. J Physiol. 2014;592:3113–25. doi: 10.1113/jphysiol.2014.272591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe RW, Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. J Anat. 1969;104:519–30. [PMC free article] [PubMed] [Google Scholar]
- 41.Stickland NC, Widdowson EM, Goldspink G. Effects of severe energy and protein deficiencies on the fibres and nuclei in skeletal muscle of pigs. Br J Nutr. 1975;34:421–8. doi: 10.1017/s0007114575000487. [DOI] [PubMed] [Google Scholar]
- 42.Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat. 1983;137(Pt 2):235–45. [PMC free article] [PubMed] [Google Scholar]
- 43.Yan X, Zhu MJ, Dodson MV, Du M. Developmental programming of fetal skeletal muscle and adipose tissue development. J Genomics. 2013;1:29–38. doi: 10.7150/jgen.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayol S, Jones D, Goldspink G, Stickland NC. The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. Br J Nutr. 2004;91:331–9. doi: 10.1079/BJN20031070. [DOI] [PubMed] [Google Scholar]
- 45.Costello PM, Rowlerson A, Astaman NA, Anthony FE, Sayer AA, Cooper C, et al. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008;586:2371–9. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fahey AJ, Brameld JM, Parr T, Buttery PJ. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J Anim Sci. 2005;83:2564–71. doi: 10.2527/2005.83112564x. [DOI] [PubMed] [Google Scholar]
- 47.Fahey AJ, Brameld JM, Parr T, Buttery PJ. Ontogeny of factors associated with proliferation and differentiation of muscle in the ovine fetus. J Anim Sci. 2005;83:2330–8. doi: 10.2527/2005.83102330x. [DOI] [PubMed] [Google Scholar]
- 48.Blake AC, Rozance PJ, Thorn SR, Brown LD. Increased expression of insulin receptor-β (IR-β) in myoblasts isolated from IUGR fetal sheep E-PAS2013:3829.472. Pediatric Academic Societies Annual Meeting; 2013; Poster Presentation. [Google Scholar]
- 49.Blake AC, Rozance PJ, Isenberg N, Thorn S, Hay WW, Jr, Brown LD. Myoblasts maintain proliferative capacity despite decreased muscle growth in the intrauterine growth restricted fetal sheep. E-PAS2015:2350.5. Pediatric Academic Society Annual Meeting; 2015; Platform Presentation. [Google Scholar]
- 50.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87:3378–84. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 51.Rozance PJ, Crispo MM, Barry JS, O’Meara MC, Frost MS, Hansen KC, et al. Prolonged maternal amino acid infusion in late-gestation pregnant sheep increases fetal amino acid oxidation. Am J Physiol Endocrinol Metab. 2009;297:E638–46. doi: 10.1152/ajpendo.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maliszewski AM, Gadhia MM, O’Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab. 2012;302:E1483–92. doi: 10.1152/ajpendo.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soto SM, Rozance PJ, Thorn SR, Hay WW, Jr, Friedman JE, Brown LD. Insulin stimulates proliferation in ovine IUGR but not control myoblasts E-PAS2011:1414184. Pediatric Academic Societies Annual Meeting; 2011; Poster Presentation. [Google Scholar]
- 54.Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW., Jr Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab. 2009;296:E56–63. doi: 10.1152/ajpendo.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hay WW., Jr Recent observations on the regulation of fetal metabolism by glucose. J Physiol. 2006;572:17–24. doi: 10.1113/jphysiol.2006.105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2007;293:E1716–25. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 57.Thorn SR, Brown LD, Rozance PJ, Hay WW, Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes. 2013;62:65–73. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carver TD, Hay WW., Jr Uteroplacental carbon substrate metabolism and O2 consumption after long-term hypoglycemia in pregnant sheep. Am J Physiol. 1995;269:E299–308. doi: 10.1152/ajpendo.1995.269.2.E299. [DOI] [PubMed] [Google Scholar]
- 59.Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588:1349–59. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. Increased hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology. 2002;143:2486–90. doi: 10.1210/endo.143.7.8898. [DOI] [PubMed] [Google Scholar]
- 61.Thorn SR, Regnault TRH, Brown LD, Rozance PJ, Keng J, Roper M, et al. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces hepatic and skeletal muscle mRNA translation initiation and nutrient sensing. Endocrinology. 2009;150:3021–30. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW, Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2015;309:R920–8. doi: 10.1152/ajpregu.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Houin SS, Rozance PJ, Brown LD, Hay WW, Jr, Wilkening RB, Thorn SR. Coordinated changes in hepatic amino acid metabolism and endocrine signals support hepatic glucose production during fetal hypoglycemia. Am J Physiol Endocrinol Metab. 2015;308:E306–14. doi: 10.1152/ajpendo.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milley JR. Ovine fetal metabolism during norepinephrine infusion. Am J Physiol. 1997;273:E336–47. doi: 10.1152/ajpendo.1997.273.2.E336. [DOI] [PubMed] [Google Scholar]
- 65.Milley JR. Effects of increased cortisol concentration on ovine fetal leucine kinetics and protein metabolism. Am J Physiol. 1995;268:E1114–22. doi: 10.1152/ajpendo.1995.268.6.E1114. [DOI] [PubMed] [Google Scholar]
- 66.Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol. 1989;256:R1348–54. doi: 10.1152/ajpregu.1989.256.6.R1348. [DOI] [PubMed] [Google Scholar]
- 67.Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–30. doi: 10.1053/j.semperi.2007.11.004. [DOI] [PubMed] [Google Scholar]