Abstract
Although rare, pleuroparenchymal fibroelastosis (PPFE) is a serious late‐onset complication of haematopoietic stem cell transplantation (HSCT). It remains unclear whether graft‐versus‐host disease (GVHD) is involved in the development of PPFE. We report the case of a patient with PPFE after HSCT. The patient experienced pneumothorax repeatedly despite surgical treatment. A surgical specimen demonstrated PPFE findings, without evidence of GVHD. In this case, development of PPFE was not associated with GVHD, and immunosuppressive therapy did not improve pulmonary function. Surgical biopsy is recommended for precise treatment and elucidation of pathogenesis in each suspected PPFE patient.
Keywords: Graft‐versus‐host disease, haematopoietic stem cell transplantation, pleuroparenchymal fibroelastosis, pneumothorax
Introduction
With increasing numbers of patients receiving haematopoietic stem cell transplantation (HSCT), more cases with pulmonary complications after HSCT are encountered; pulmonary complications affect 40−60% of such patients. Pleuroparenchymal fibroelastosis (PPFE) is one such complication; it is rare, occurring in about 0.3% of these patients 1. PPFE has a high mortality rate; Ishii et al. reported that all five patients who developed histologically proven PPFE after HSCT died of the disease 2. Although its aetiology is unclear, GVHD is assumed to be involved because most cases were reported after HSCT.
We experienced a case of a patient who developed post‐HSCT PPFE and who suffered from repeated pneumothorax. The patient was treated surgically, and the surgical specimen showed PPFE findings, without evidence of GVHD.
Case Report
The patient was diagnosed with aplastic anaemia at 11 years old; this progressed to myelodysplastic syndrome (MDS) when she was 35 years old. Although she was treated with tacrolimus (FK506) for 6 months, she developed refractory anaemia with excess blasts (RAEB‐I), for which she received a blood transfusion. She underwent chest X‐ray imaging (Fig. 1A, B) and pulmonary function tests to evaluate her eligibility for HSCT, and no abnormal findings other than small subpleural nodules were observed (VC:3.12 L, %VC: 97.5%, FEV1: 3.06 L, %FEV1: 94.2%). She was treated with HSCT under cyclophosphamide and 12‐Gy total body irradiation conditioning. Five years later, she first developed pneumothorax. When she developed a second episode of pneumothorax, the air leak was so intractable that she required a bullectomy. The surgical specimen showed PPFE findings but no evidence of GVHD (Fig. 2).
Figure 1.
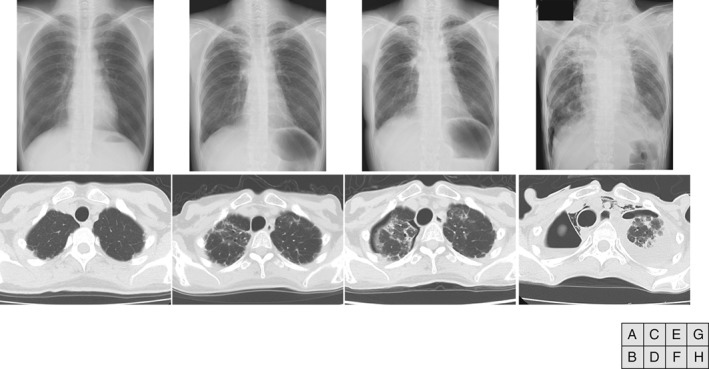
Chest radiography and computed tomography images showing bilateral upper lung volume loss with pleural thickening. (A, B) Before haematopoietic transplantation. No abnormal findings are observed. (C, D) Four years after haematopoietic stem cell transplantation (HSCT). Bilateral pleural thickening of the pulmonary apex is observed. (E, F) Five years after HSCT. Air space persists in the thoracic cavity. Full lung expansion cannot be achieved due to repeated pneumothorax and lung contraction. (G, H) Eight years after HSCT (1 week before death). Progressive lung contraction and mediastinal emphysema is observed.
Figure 2.
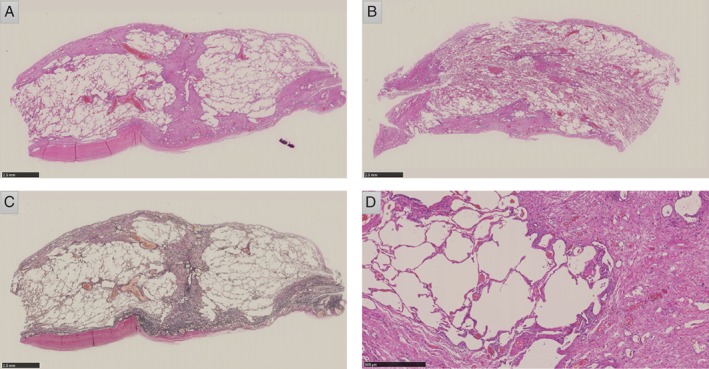
Pathological findings of the surgical sample obtained by the resection of pulmonary emphysema with bulla, demonstrating pleural thickening and subpleural alveolar collapse. (A, B) Haematoxylin and eosin staining in a low‐power field (LPF). Pleural thickening and patchy fibrotic lesions are observed. There are no findings suggesting obliterative bronchiolitis (BO). (C) Elastica van Gieson (EVG) staining in an LPF. Proliferation of elastic fibres is observed. (D) Haematoxylin and eosin staining in a high‐power field. There are no findings of lymphocytic inflammation or eosinophilic scarring suggestive of GVHD or obliterative bronchiolitis.
Although pneumothorax improved once, she required repeated admission for pneumothorax treatment (six times). Her condition gradually worsened (Fig. 1C−H), and she died of respiratory failure at 45 years old.
Discussion
In this case, the surgical specimen did not show GVHD findings, and immunosuppressive therapy did not improve pulmonary function. Those findings suggested an association with the conditioning treatment for HSCT rather than with GVHD.
The surgical specimen showed fibroelastosis with pleural thickening, which was consistent with PPFE (Fig. 2A–D). However, there were no findings of lymphocytic inflammation or eosinophilic scarring suggestive of GVHD or obliterative bronchiolitis (BO). The pathological finding of BO is considered a diagnostic feature of pulmonary chronic GVHD. In BO, lung biopsy shows unequivocal dense eosinophilic scarring of the bronchioles, resulting in some degree of luminal narrowing.
PPFE after HSCT has been thought to be a GVHD‐related disease because most cases developed PPFE post‐HSCT. Takeuchi et al. reported a retrospective review of 20 patients who underwent lung transplantation for pulmonary disease following HSCT 3. They suggested that PPFE may be a consequence of persistent intra‐alveolar organizing pneumonia, in addition to collapsed fibrosis resulting from GVHD‐related BO. Von der Thusen et al. also reported PPFE cases that were complicated by GVHD‐related BO. These reports support a relationship between PPFE and GVHD.
However, some cases develop PPFE after autologous bone marrow transplantation or after chemotherapy alone 4. Ishii et al. reported five cases of PPFE after HSCT, and the histological pulmonary specimens showed no findings of BO or GVHD 2. These reports suggest that the GVHD contribution to PPFE development differ across cases.
In this case, the patient received myeloablative conditioning with cyclophosphamide and 12‐Gy total body irradiation (TBI) for HSCT. PPFE has been associated with many factors, such as drugs, chronic hypersensitivity pneumonia, collagen vascular diseases, infections, and bone marrow transplantation. Previous reports have identified two distinct patterns of cyclophosphamide‐induced lung injury: 1) early onset pneumonitis that improved after cessation of the drug and 2) late‐onset pleuropulmonary fibrosis developing and progressing after cyclophosphamide was discontinued, which shows pathological findings similar to those of PPFE after HSCT 5.
In Figure 1B, subpleural nodules in the bilateral apices are shown. These could be early lesions of PPFE. It is possible that conditioning treatment, including cyclophosphamide and TBI, exacerbated idiopathic PPFE, suggesting that it is necessary to consider reduced intensity conditioning for HSCT when such findings are detected.
Although it is common that PPFE, irrespective of the aetiology, has few cellular infiltrates upon histology in the end stage, our patient experienced no respiratory distress after bullectomy. Additionally, the surgical specimen contained relatively normal alveolar tissue without cellular infiltrates. It is plausible that this histology did not represent end‐stage disease and that conditioning treatment for HSCT played an important role in the development of PPFE.
Secondly, immunosuppressive therapy did not improve pulmonary function in our patient. She had received FK506 and oral corticosteroid for 10 years for chronic liver and skin GVHD, which were under control. PPFE development did not correlate with the state of chronic GVHD in other organs.
In contrast to immunosuppressive/anti‐inflammatory therapy, most reported cases were not rescued without lung transplantation. In contrast, BO associated with HSCT responds to anti‐inflammatory therapy, including oral corticosteroid, inhaled corticosteroid, azithromycin, and montelukast. Some PPFE cases shows BO findings, while others do not. In idiopathic pulmonary fibrosis, nintedanib and pirfenidone reduce disease progression. These agents may prevent disease progression when no BO finding is observed. Although the chances of performing a surgical biopsy are limited, it may lead to precise treatment for patients with post‐HSCT PPFE. Therefore, surgical biopsy should be considered in cases of recurrent or chest drainage‐refractory pneumothorax.
In the case described here, the surgical specimen did not show GVHD findings, and immunosuppressive therapy did not improve pulmonary function. Some patients who develop PPFE after HSCT may thus not have GVHD, and PPFE after HSCT is therefore a heterogeneous condition, with the GVHD contribution to the PPFE development differing across cases. Surgical biopsy is therefore recommended to ensure precise treatment and elucidate the pathogenesis.
Disclosure Statement
Appropriate written informed consent was obtained for the publication of this case report and accompanying images.
Acknowledgments
We thank Editage (http://www.editage.jp) for English language editing. We wish to thank Dr. Ishikawa for commenting on the pathological findings.
Okimoto, T. , Tsubata, Y. , Hamaguchi, M. , Sutani, A. , Hamaguchi, S. and Isobe, T. (2018) Pleuroparenchymal fibroelastosis after haematopoietic stem cell transplantation without graft‐versus‐host disease findings. Respirology Case Reports, 6 (3), e00298. doi: 10.1002/rcr2.298.
Associate Editor: Kazuhisa Takahashi
References
- 1. Mariani F, Gatti B, Rocca A, et al. 2016. Pleuroparenchymal fibroelastosis: the prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn. Interv. Radiol. 22:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishii T, Bandoh S, Kanaji N, et al. 2016. Air‐leak syndrome by pleuroparenchymal fibroelastosis after bone marrow transplantation. Intern. Med. 55:105–111. [DOI] [PubMed] [Google Scholar]
- 3. Takeuchi Y, Miyagawa‐Hayashino A, Chen F, et al. 2015. Pleuroparenchymal fibroelastosis and non‐specific interstitial pneumonia: frequent pulmonary sequelae of haematopoietic stem cell transplantation. Histopathology 66:536–544. [DOI] [PubMed] [Google Scholar]
- 4. Beynat‐Mouterde C, Beltramo G, Lezmi G, et al. 2014. Pleuroparenchymal fibroelastosis as a late complication of chemotherapy agents. Eur. Respir. J. 44:523–527. [DOI] [PubMed] [Google Scholar]
- 5. Malik SW, Myers JL, DeRemee RA, et al. 1996. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am. J. Respir. Crit. Care Med. 154:1851–1856. [DOI] [PubMed] [Google Scholar]


