Abstract
Background
Eradication rate for Helicobacter pylori (H. pylori) has decreased due to antibiotic resistance. Therefore, new strategies are needed to enhance H. pylori eradication, especially for H. pylori with high antibiotic resistance. The objective of this study was to evaluate anti-H. pylori activities of constituents from key lime (Citrus aurantifolia) and their possible inhibitory effects on urease activity of H. pylori.
Methods
Helicobacter pylori strain ATCC 43526 and triple drug resistant (TDR) H. pylori strains were used in this study. Urease activities of H. pylori strains were measured by ammonia colorimetrical quantification using ELISA reader. Minimum inhibitory concentrations were determined by agar dilution method for antibiotics and by modified media dilution method for each constituent of Citrus aurantifolia (C. aurantifolia).
Results
Citrus aurantifolia extract decreased the number of colonies of H. pylori strain ATCC 43526 and TDR H. pylori stains. An increasing concentration of C. aurantifolia extract attenuated urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strains. Among constituents of C. aurantifolia, citral and 4-hexen-3-one were found to be able to inhibit the growth of H. pylori strain ATCC 43526 and TDR H. pylori strains. Furthermore, citral and 4-hexen-3-one inhibited urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strains in a dose-dependent manner.
Conclusion
Citrus aurantifolia has antimicrobial effect on TDR H. pylori strains, suggesting that C. aurantifolia might have therapeutic potential to control antibiotic-resistant H. pylori strains that cause eradication failure using other antibiotics.
Background
Helicobacter pylori (H. pylori) is known to be a major pathogen in the development of gastritis, peptic ulcer disease, gastric adenocarcinoma, and mucosa associated lymphoid tissue (MALT) lymphoma [1, 2]. Therefore, H. pylori eradication is important for the management of these diseases. However, eradication rate for H. pylori has decreased due to antibiotic resistance of H. pylori. Its resistance rates to clarithromycin and metronidazole in East Asia and Europe have been reported to be 17–34 and 28–65%, respectively [3–7]. Therefore, new alternatives or adjuvant approaches are needed for H. pylori eradication, especially in the area where there is high antibiotic resistance rate of H. pylori. Previous study has reported that foods or components of foods have anti-H. pylori activities by facilitating penetration of antibiotics to H. pylori by damaging cell membrane, inhibiting urease activity of H. pylori, inhibiting H. pylori adhesion to gastric mucosa, and interfering with cell division process of H. pylori [8]. Among these mechanisms, inhibiting urease activity of H. pylori can help eradicate H. pylori by altering optimal pH and inhibiting colonization of H. pylori [9–11].
A recent meta-analysis has shown that the intake of citrus fruits can reduce the incidence of gastric cancer in the area with high prevalence of H. pylori [12]. Another report has shown that phytochemical constituents of citrus peels possess biological activities, including anticancer, immunostimulation, and antigenotoxic effects [13]. Oranges, lemons, limes, grapefruit, and tangerines are well-known examples of citrus fruits. Citrus aurantifolia (C. aurantifolia), also known as key lime, is one of widely consumed citrus fruits in many cultural cuisines and juice production. It has antibacterial activities against Mycobacterium tuberculosis, Staphylococcus aureus, and others. Among various constituents of C. aurantifolia, citral, 4-Hexen-3 one, oleic acid, and palmitic acid have been found to possess antibacterial activities [14–19]. However, it is currently unclear whether C. aurantifolia and its constituents have anti-H. pylori activities. Therefore, the objective of this study was to evaluate anti-H. pylori activities of C. aurantifolia and its constituents and their possible inhibitory effects on urease activity of H. pylori.
Methods
Key lime (C. aurantifolia) extraction
Slices of C. aurantifolia were dried in a constant drying oven (VS-4150ND, VISION SCIENTIFIC, Daejeon, Korea) at temperature of 50 °C. Dried C. aurantifolia slices were mixed with liquid nitrogen and ground into fine powders using a mortar and pestle. Powders of C. aurantifolia (1 g) were then dissolved in 30 ml of sterile distilled water and incubated at room temperature for 24 h. Dissolved C. aurantifolia was filtered using a 0.45 µm pore syringe filter (Corning, NY 14831-001, USA). Twofold serial dilutions of C. aurantifolia extract (original concentration, 34 mg/ml) were made with distilled water (1:1 to 1:1024).
We used 4-hexen-3 one, oleic acid, and palmitic acid as constituents of C. aurantifolia to determine their antimicrobial activities and inhibitory effects on urease activity of H. pylori [14, 19]. For each constituent [citral (Sigma-Aldrich #W230316, USA), 4-hexen-3 one (Sigma-Aldrich #H13001, USA), oleic acid (Sigma-Aldrich #O1008, USA), and palmitic acid (Sigma-Aldrich #P0500, USA)], we prepared the following concentrations: 1, 2, 5, 10, 50, 100, 200, 400, 500, and 1000 µg/ml.
Helicobacter pylori strain ATCC 43526 and triple drug resistant (TDR) H. pylori strains
We used standard H. pylori strain (ATCC® CRL-43526™, USA) and TDR H. pylori strains isolated from gastric antrum and body from 18 patients with gastric epithelial neoplasm. Methods of isolation and culture for H. pylori were the same as those described in our previous study [20].
Antimicrobial susceptibility testing
We stored H. pylori strains at − 80 °C. After thawing and culture of standard H. pylori strain and 18 TDR H. pylori strains, we measured minimum inhibitory concentrations (MICs) by agar dilution method for antibiotics and by modified media dilution method for C. aurantifolia extract and each constituent of C. aurantifolia. We made agar plates using Muller Hinton agar containing 5% sheep blood (Hanilcomed, Korea), 1% IsoVitalex (BD Biosciences), and one of the following drug concentrations for MIC assay: 2–32 µg/ml of metronidazole, 0.25–4 µg/ml of clarithromycin, 0.125–2 µg/ml of amoxicillin and levofloxacin, and 1–16 µg/ml of tetracycline. All antibiotics used in this investigation were purchased from Sigma (St. Louis, MO, USA) except clarithromycin which was obtained from Abbott Laboratories (Abbott Park, IL, USA). We added 10 ml of agar solution into 100 π plate and then cooled down. H. pylori strain ATCC 43526 (Manassas, VA USA,) was used as a quality control organism. Antibiotic concentrations used in this study were based on cutoff levels related to Laboratory Standards Institute (CLSI) clinical breakpoints for resistance. All MICs were interpreted using CLSI breakpoints. Antibiotic resistance was defined as follows: amoxicillin, MIC ≥ 0.5 µg/ml; clarithromycin, MIC > 1.0 µg/ml; metronidazole, MIC > 8 µg/ml; tetracycline, MIC > 4 µg/ml; and levofloxacin, MIC > 1 µg/ml.
We tested MIC for C. aurantifolia and four constituents of C. aurantifolia. We mixed 6 × 108 CFU/ml H. pylori in twofold serial dilutions of C. aurantifolia extract (34 mg/ml–33.2 µg/ml, 1:1 to 1:1024) or in serial concentrations of its four constituents (1–1000 µg/ml), respectively. These mixtures of H. pylori with C. aurantifolia extract or its four constituents (5 µl each) were dropped immediately onto agar plates. We determined MIC levels of C. aurantifolia extract and each constituent based on invisible H. pylori colony on the agar plate after 7 days of incubation.
Urease activity inhibition test
We harvested H. pylori in 0.9% saline and then prepared mixtures of 6 × 108 colony forming units (CFU)/mL of H. pylori with two-fold serially diluted solution of C. aurantifolia extract (1:1 to 1:1024). We prepared 6 × 108 CFU/ml of H. pylori with citral, 4-hexen-3-one, oleic acid, or palmitic acid in the following concentrations: 10, 50, 100, 200, 400, 500, and 1000 µg/ml. H. pylori strains with each constituent were incubated at room temperature for 10 min. We used 0.9% saline as a control. We added each H. pylori strain (6 × 108 CFU/ml) in 5 into 200 µl of the following mixture: 1.5% urea (Bioshop, Canada Inc.) and 0.1% EDTA with 0.02% cresol red solution (Bioshop, Canada Inc.). The mixture ratio was 2:1. The reaction was incubated at room temperature for 20 min. After that, we measured urease activity at absorbance of 590 nm using a VersaMax™ ELISA reader (MOLECULAR DEVICES, Silicon Valley, CA, USA) [21, 22]. Urease inhibition test for each H. pylori strain was repeated three times.
Statistical analysis
Analysis of variance (ANOVA) was used to determine whether there were any statistically significant differences in urease activity depending on the concentration of C. aurantifolia extract and its constituents. Urease activities were shown as mean ± standard deviation (SD). All reported P values were two-sided and P < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS software, version 23 (IBM Corp, Armonk, NY, USA).
Results
TDR H. pylori strains
According to MIC data of clarithromycin, metronidazole, and levofloxacin for H. pylori in our previous study [20], TDR H. pylori strains were all resistant to clarithromycin, metronidazole, and levofloxacin. Results are summarized in Table 1.
Table 1.
Triple drug resistant Helicobacter pylori and antimicrobial activities of four components of Citrus aurantifolia
| Strain no. | MIC (μg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| Citral | 4-Hexen-3-one | Oleic acid | Palmitic acid | Clarithromycin | Metronidazole | Levofloxacin | |
| TDR 1 | 5–10 | 20–50 | R | R | R | R | R |
| TDR 2 | 10–20 | 50–100 | R | R | R | R | R |
| TDR 3 | 5–10 | 20–50 | R | R | R | R | R |
| TDR 4 | 10–20 | 100–200 | R | R | R | R | R |
| TDR 5 | 10–20 | 50–100 | R | R | R | R | R |
| TDR 6 | 10–20 | 50–100 | R | R | R | R | R |
| TDR 7 | 100–200 | 100–200 | R | R | R | R | R |
| TDR 8 | 5–10 | 20–50 | R | R | R | R | R |
| TDR 9 | 10–20 | 20–50 | R | R | R | R | R |
| TDR 10 | 2–5 | 5–10 | R | R | R | R | R |
| TDR 11 | 5–10 | 50–100 | R | R | R | R | R |
| TDR 12 | 2–5 | 20–50 | R | R | R | R | R |
| TDR 13 | 10–20 | 20–50 | R | R | R | R | R |
| TDR 14 | 5–10 | 20–50 | R | R | R | R | R |
| TDR 15 | 10–20 | 20–50 | R | R | R | R | R |
| TDR 16 | 5–10 | 20–50 | R | R | R | R | R |
| TDR 17 | 2–5 | 20–50 | R | R | R | R | R |
| TDR 18 | 2–5 | 20–50 | R | R | R | R | R |
| ATCC 43526 | 2–5 | 20–50 | R | R | S | R | S |
No. number, MIC minimum inhibitory concentration, TDR triple drug resistant, R resistant, S sensitive, ATCC 43526 Helicobacter pylori strains ATCC 43526
Effect of C. aurantifolia extract on growth and urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strains
First, we evaluated the effect of C. aurantifolia extract on the growth of standard H. pylori strain ATCC 43526 and TDR H. pylori strains. We observed visible growth of H. pylori mixed with C. aurantifolia after twofold serial dilution (1:1 to 1:1024) on agar plate after 7 days of inoculation. The number of visible colonies of H. pylori strain ATCC 43526 and TDR H. pylori strains was decreased in the presence of C. aurantifolia extract compared to that in the control without the presence of C. aurantifolia extract (Fig. 1a). We measured urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strains mixed with C. aurantifolia extract at each dilution. Results for their inhibitory effects on urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strains at each concentration of C. aurantifolia extract are shown in Fig. 1b. With increasing concentration of C. aurantifolia extract, higher attenuation of urease activity of H. pylori was observed (P < 0.001, Fig. 1c, Table 2). H. pylori strains ATCC 43526 treated with C. aurantifolia extract at dilution of 1:64 showed 18.77 ± 1.74% of urease activity compared to that of the control whereas TDR H. pylori strains treated with C. aurantifolia extract at dilution of 1:128 showed 2.62 ± 0.05% of urease activity compared to that of the control (Table 2).
Fig. 1.
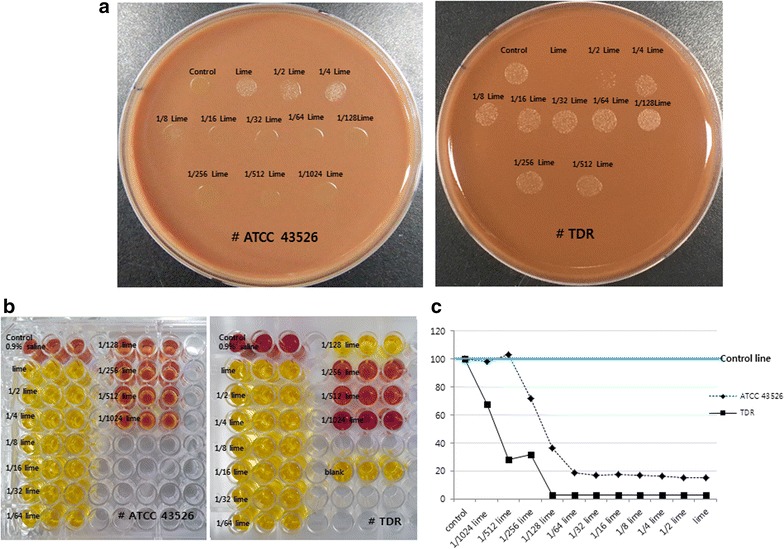
a Effect of C. aurantifolia extract on growth of standard H. pylori strain ATCC 43526 and triple drug resistant (TDR) H. pylori strain. The number of visible colonies of H. pylori strain ATCC 43526 or TDR H. pylori was decreased in the presence of C. aurantifolia extract compared to that in the control without the presence of C. aurantifolia extract. b Urease activity test of standard H. pylori strain ATCC 43526 and triple drug resistant (TDR) H. pylori strain. We determined inhibitory effects of C. aurantifolia extract at dilution of 1:1 to 1:64 on urease activity of H. pylori strain ATCC 43526 and at dilution of 1:1 to 1:128 on urease activity of TDR H. pylori. c Dose-dependent inhibitory effect of C. aurantifolia extract on urease activities of H. pylori strain ATCC 43526 and triple drug resistant H. pylori strain. An increasing concentration of C. aurantifolia extract showed higher attenuation of urease activity (P < 0.001)
Table 2.
Inhibition effect of Citrus aurantifolia extract on urease activities of Helicobacter pylori strains ATCC 43526 and triple drug resistant Helicobacter pylori
| Dilution titer of citrus aurantifolia | ATCC 43526 Urease activity (%) |
TDR Urease activity (%) |
|---|---|---|
| Control | 100 | 100 |
| 1/1024 | 98.3 | 67.6 |
| 1/512 | 103.0 | 28.0 |
| 1/256 | 71.8 | 31.5 |
| 1/128 | 36.6 | 2.6 |
| 1/64 | 18.8 | 2.7 |
| 1/32 | 17.0 | 2.7 |
| 1/16 | 17.6 | 2.6 |
| 1/8 | 16.9 | 2.6 |
| 1/4 | 16.1 | 2.7 |
| 1/2 | 15.2 | 2.7 |
| 1 | 14.9 | 2.8 |
ATCC 43526 Helicobacter pylori strains ATCC 43526, TDR triple drug resistant
Effect of citral, 4-hexen-3-one, oleic acid, and palmitic acid on growth and urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strains
We evaluated effects of constituents from C. aurantifolia on the growth of standard H. pylori strain ATCC 43526. We found visible growth of H. pylori colony treated with low dose of citral (≤ 2 μg/ml) and low dose of 4-hexene-3-one (≤ 20 μg/ml) on agar plate after 7 days of inoculation. Citral above concentration of 5 μg/ml persistently stopped the growth of H. pylori (MIC, 2–5 μg/ml, Fig. 2a). 4-hexen-3-one above concentration of 50 μg/ml persistently stopped the growth of H. pylori (MIC, 20–50 μg/ml, Fig. 2b). However, oleic acid or palmitic acid showed no effect on the growth of H. pylori strain ATCC 43526.
Fig. 2.
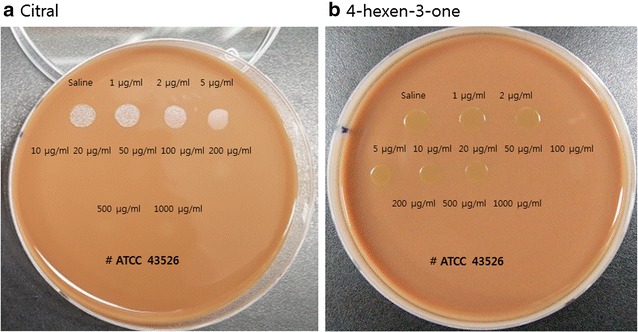
Effect of citral (a) and 4-hexen-3-one (b) on the growth of standard H. pylori strain ATCC 43526. Small spot without convexity and translucency is only trace of drop. Citral had inhibitory effect on standard H. pylori strain ATCC 43526, with MIC of 2–5 μg/ml. 4-Hexen-3-one also had inhibitory effect on standard H. pylori strain, with MIC of 20–50 μg/ml
We measured effects of citral, 4-hexen-3-one, oleic acid, and palmitic acid on urease activities of standard H. pylori strain ATCC 43526. With increasing concentration, 4-hexen-3-one showed higher attenuation effects on urease activity of H. pylori strain ATCC 43526 (P <0.001). H. pylori strain ATCC 43526 treated with 4-hexen-3-one at concentration of 10 µg/ml had urease activity of 37.11% compared to the control (P =0.006, Fig. 3a, Table 3). With increasing concentration of citral, higher attenuation of urease activity of H. pylori strain ATCC 43526 was achieved (P <0.001). H. pylori strain ATCC 43526 treated with citral showed decreased urease activity depending on the concentration used (P <0.001). H. pylori strain ATCC 43526 treated with citral at 100 µg/ml showed urease activity of 52.67% compared to the control (P =0.002, Fig. 3a, Table 3). However, palmitic acid or oleic acid showed no inhibitory effect on urease activity of H. pylori strain 43526 (Fig. 3a, Table 3).
Fig. 3.
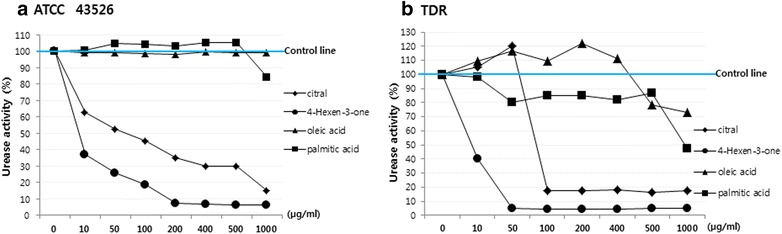
Effect of citral, 4-hexen-3-one, oleic acid, and palmitic acid on urease activities of standard H. pylori strain ATCC 43526 (a) and triple drug resistant (TDR) H. pylori strain (b). An increasing concentration of citral and 4-hexen-3-one attenuated more urease activities of H. pylori strain ATCC 43526 and TDR H. pylori strain (both P <0.001). However, palmitic acid and oleic acid showed no inhibition effects on urease activity (both P > 0.05)
Table 3.
Inhibition effect of citral, 4-hexen-3-one, oleic acid, and palmitic acid on urease activities of Helicobacter pylori strain ATCC 43526 and triple drug resistant Helicobacter pylori
| Concentration (μg/ml) | Citral Urease activity (%) |
4-Hexen-3-one Urease activity (%) |
Oleic acid Urease activity (%) |
Palmitic acid Urease activity (%) |
||||
|---|---|---|---|---|---|---|---|---|
| ATCC 43526 | TDR | ATCC 43526 | TDR | ATCC 43526 | TDR | ATCC 43526 | TDR | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 10 | 62.48 | 105.09 | 37.11 | 40.48 | 99.29 | 109.25 | 100.83 | 98.38 |
| 50 | 52.67 | 120.07 | 25.74 | 4.97 | 99.27 | 116.37 | 104.65 | 80.02 |
| 100 | 45.04 | 17.74 | 18.47 | 4.27 | 98.58 | 109.69 | 104.51 | 85.17 |
| 200 | 35.23 | 17.29 | 7.35 | 4.44 | 98.08 | 121.82 | 103.16 | 84.76 |
| 400 | 29.70 | 17.86 | 6.67 | 4.45 | 99.61 | 111.19 | 105.19 | 81.76 |
| 500 | 29.65 | 16.39 | 6.53 | 4.92 | 99.30 | 78.19 | 105.11 | 87.00 |
| 1000 | 14.75 | 17.26 | 6.38 | 5.14 | 98.90 | 72.97 | 84.51 | 47.34 |
ATCC 43526 Helicobacter pylori strains ATCC 43526, TDR triple drug resistant
Antimicrobial activities of four constituents from C. aurantifolia against TDR H. pylori strains are shown in Table 1. Oleic acid or palmitic acid showed no antimicrobial effect on TDR H. pylori strains. However, citral and 4-hexen-3-one inhibited the growth of TDR H. pylori strains.
We measured effects of citral, 4-hexen-3-one, oleic acid, and palmitic acid on urease activities of TDR H. pylori strains. With increasing concentration, 4-hexen-3-one showed higher attenuation effects on urease activity of TDR H. pylori strains (P <0.001). TDR H. pylori strains treated with 4-hexen-3-one at concentration of 10 µg/ml had urease activity of 40.48% compared to the control (P =0.007, Fig. 3b, Table 3). With increasing concentration of citral, higher attenuation of urease activity of TDR H. pylori strains was achieved (P < 0.001). TDR H. pylori strains treated with citral showed decreased urease activity depending on the concentration used (P < 0.001). TDR H. pylori strains treated with citral at concentration of 100 µg/ml showed urease activity of 17.74% compared to the control (P < 0.001, Fig. 3b, Table 3). However, palmitic acid or oleic acid showed no inhibitory effect on urease activities of TDR H. pylori strains (Fig. 3b, Table 3).
Discussion
Our present study showed that C. aurantifolia extracts could inhibit urease activity of antibiotic-susceptible H. pylori strain and TDR H. pylori strains in vitro in a dose-dependent manner. Among constituents of C. aurantifolia, citral and 4-hexen-3-one showed dose-dependent inhibition of urease activities of antibiotic-susceptible H. pylori strain and TDR H. pylori strains. Furthermore, citral and 4-hexen-3-one showed inhibitory effects on the growth of antibiotic-susceptible H. pylori strain and TDR H. pylori strains.
Helicobacter pylori eradication rates have decreased while their resistance rates to antibiotics have increased. To improve eradication rates of H. pylori, alternative treatments such as antibiotics combined with plant extracts, probiotics, curcumin, honey, and antioxidants have been suggested [8]. Previous study has shown that lime juice concentrates have good inhibitory effects on both Gram-negative and Gram-positive bacterial strains, with MIC in the range of 12.5–50 μg/ml [23]. Another study has demonstrated that hexane extract of fruit peels of C. aurantifolia exhibits inhibitory effect against antimicrobial resistant M. tuberculosis strains, with MIC in the range of 25–50 μg/ml [24]. Among constituents from C. aurantifolia, palmitic acid, linoleic acid, oleic acid, 4-hexen-3-one, and citral are active against M. tuberculosis strains [14, 25]. We selected four available constituents (palmitic acid, oleic acid, 4-hexen-3-one, and citral) from C. aurantifolia that showed antimycobacterial activity. In our study, C. aurantifolia extract decreased the number of H. pylori ATCC 43526 colonies and TDR H. pylori colonies. Constituents of C. aurantifolia also showed inhibitory effects against H. pylori strain ATCC 43526, with MIC of citral at 5–10 μg/ml and MIC of 4-hexen-3-one at 20–50 μg/ml. Furthermore, citral showed inhibitory effects against 18 H. pylori strains with triple drug resistance. Its MIC ranged from 2 to 100 μg/ml. In addition, 4-hexen-3-one showed inhibitory effects against 18 H. pylori strains with triple drug resistance. Its MIC was in the range of 20–200 μg/ml.
Biglar et al. have shown that C. aurantifolia can inhibit the activity of Jack-bean urease (IC50 = 28 μg/ml) [26]. Our study also showed that C. aurantifolia extract could inhibit urease activity of H. pylori at dilution of 1:64 to 1:1. Among constituents from C. aurantifolia, citral and 4-hexen-3-one showed dose-dependent inhibitory effects on urease activity of H. pylori. It is known that H. pylori can neutralize acid in its environment by producing urease which breaks down urea in the stomach to carbon dioxide and ammonia. These chemicals then react with strong acids in the gastric environment to produce a neutralized area around H. pylori [27]. Previous animal study has shown that H. pylori is unable to colonize at gastric mucosa with normal physiological pH in urease-negative mutant piglet [28]. Recently, another study has demonstrated that bacterial load is decreased within 5–7 days in a urease knockout infection mouse model [29]. Urease expression is required for establishing initial colonization and maintaining chronic infection [2, 29]. In the present study, C. aurantifolia extract and its constituents showed inhibitory effects on urease activity of H. pylori, suggesting that they might have potential as adjuvants to enhance H. pylori eradication.
In this study, we did not show the association between antibacterial effect and inhibition of urease activity. Bactericidal effect of C. aurantifolia may affect the growth of H. pylori colonies, leading to inhibition of urease activity and vice versa. Although low dose of C. aurantifolia extract showed no obvious effect on the growth of H. pylori, it showed inhibitory effect on urease activity of H. pylori. Further studies are needed to evaluate the mechanism involved in the antibacterial effect of C. aurantifolia and the causal association between its inhibition of urease activity and bactericidal effects.
In conclusion, C. aurantifolia and its constituents attenuated urease activities of H. pylori strains. Citral and 4-hexen-3-one had antimicrobial effects on H. pylori strains with triple drug resistance, suggesting that C. aurantifolia might have potential as a therapeutic agent to control H. pylori strains that cause eradication failure with other antibiotics. Future studies are needed to evaluate the efficacy and toxicity of C. aurantifolia in vivo.
Authors’ contributions
CHP: study concept and design; analysis and interpretation of data; drafting and finalizing the manuscript; study supervision. SML: Carrying out the experiment; analysis and interpretation of data; drafting the manuscript. SYP: Analysis of electronic medical records; analysis and interpretation of data; drafting the manuscript. MJK: Carrying out the experiment. EAC, CHJ, HSK, SKC and JSR: Patient recruitment and care. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
We allow the use of data and materials.
Consent for publication
All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Funding
Dr. Park is supported by a Grant (CRI13009-1) of Chonnam National University Hospital Biomedical Research Institute.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Su-Mi Lee, Email: ssumia@naver.com.
Seon-Young Park, Email: drpsy@naver.com.
Moon-Ju Kim, Email: 4_chance@naver.com.
Eun-Ae Cho, Email: cea202@hanmail.net.
Chung-Hwan Jun, Email: estevanj@naver.com.
Chang-Hwan Park, Phone: 82-62-220-6296, Email: p1052ccy@hanmail.net.
Hyun-Soo Kim, Email: dshskim@chonnam.ac.kr.
Sung-Kyu Choi, Email: choisk@chonnam.ac.kr.
Jong-Sun Rew, Email: jsrew@chonnam.ac.kr.
References
- 1.Backert S, et al. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2016;21(Suppl 1):19–25. doi: 10.1111/hel.12335. [DOI] [PubMed] [Google Scholar]
- 2.Periti P, et al. Managing Helicobacter pylori infection in the new millennium: a review. J Chemother. 1999;11(sup4):3–55. doi: 10.1080/1120009X.1999.11782276. [DOI] [Google Scholar]
- 3.De Francesco V, et al. Worldwide H. pylori antibiotic resistance: a systematic. J Gastrointestin Liver Dis. 2010;19(4):409–414. [PubMed] [Google Scholar]
- 4.Koletzko S, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55(12):1711–1716. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su P, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18(4):274–279. doi: 10.1111/hel.12046. [DOI] [PubMed] [Google Scholar]
- 6.Hunt R, et al. Helicobacter pylori in developing countries. World gastroenterology organisation global guideline. J Gastrointestin Liver Dis. 2011;20(3):299–304. [PubMed] [Google Scholar]
- 7.Lee JW, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18(3):206–214. doi: 10.1111/hel.12031. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi H, et al. Natural products and food components with anti-Helicobacter pylori activities. World J Gastroenterol. 2014;20(27):8971–8978. doi: 10.3748/wjg.v20.i27.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy P, et al. Helicobacter pylori containing only cytoplasmic urease is susceptible to acid. Infect Immun. 1998;66(11):5060–5066. doi: 10.1128/iai.66.11.5060-5066.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smoot DT, et al. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990;58(6):1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weeks DL, et al. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287(5452):482–485. doi: 10.1126/science.287.5452.482. [DOI] [PubMed] [Google Scholar]
- 12.Bae JM, Kim EH. Dietary intakes of citrus fruit and risk of gastric cancer incidence: an adaptive meta-analysis of cohort studies. Epidemiol Health. 2016;38:e2016034. doi: 10.4178/epih.e2016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diab KA. In vitro studies on phytochemical content, antioxidant, anticancer, immunomodulatory, and antigenotoxic activities of lemon, grapefruit, and mandarin citrus peels. Asian Pac J Cancer Prev. 2016;17(7):3559–3567. [PubMed] [Google Scholar]
- 14.Sandoval-Montemayor NE, et al. Chemical composition of hexane extract of Citrus aurantifolia and anti-Mycobacterium tuberculosis activity of some of its constituents. Molecules. 2012;17(9):11173–11184. doi: 10.3390/molecules170911173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyanova L. Comparative evaluation of the activity of plant infusions against Helicobacter pylori strains by three methods. World J Microbiol Biotechnol. 2014;30(5):1633–1637. doi: 10.1007/s11274-013-1589-5. [DOI] [PubMed] [Google Scholar]
- 16.Mahadwar G, et al. Swarm motility of Salmonella enterica serovar Typhimurium is inhibited by compounds from fruit peel extracts. Lett Appl Microbiol. 2015;60(4):334–340. doi: 10.1111/lam.12364. [DOI] [PubMed] [Google Scholar]
- 17.Matewele P. The effect of electromagnetic field on antimicrobial activity of lime oil. J Microbiol Methods. 2010;83(2):275–276. doi: 10.1016/j.mimet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Sumer Z, et al. Cytotoxic and antibacterial activity of the mixture of olive oil and lime cream in vitro conditions. Afr J Tradit Complement Altern Med. 2013;10(4):137–143. doi: 10.4314/ajtcam.v10i4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Heying E, Tanumihardjo SA. History, global distribution, and nutritional importance of citrus fruits. Compr Rev Food Sci Food Saf. 2012;11(6):530–545. doi: 10.1111/j.1541-4337.2012.00201.x. [DOI] [Google Scholar]
- 20.Park CS, et al. Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol. 2014;109(10):1595–1602. doi: 10.1038/ajg.2014.222. [DOI] [PubMed] [Google Scholar]
- 21.Mirshahi F, et al. Omeprazole may exert both a bacteriostatic and a bactericidal effect on the growth of Helicobacter pylori (NCTC 11637) in vitro by inhibiting bacterial urease activity. J Clin Pathol. 1998;51:220–224. doi: 10.1136/jcp.51.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David J, et al. Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J Bacteriol. 1999;181:7314–7322. doi: 10.1128/jb.181.23.7314-7322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang CS, Baik GH. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J Gastroenterol. 2014;20(18):5252–5262. doi: 10.3748/wjg.v20.i18.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oikeh EI, et al. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci Nutr. 2015;4(1):103–109. doi: 10.1002/fsn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camacho-Corona Mdel R, et al. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother Res. 2008;22(1):82–85. doi: 10.1002/ptr.2269. [DOI] [PubMed] [Google Scholar]
- 26.Biglar M, et al. A preliminary investigation of the jack-bean urease inhibition by randomly selected traditionally used herbal medicine. Iran J Pharm Res. 2012;11(3):831–837. [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton KA, et al. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59(7):2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton KA, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62(9):3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debowski AW, et al. Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection. PLoS Pathog. 2017;13(6):e1006464. doi: 10.1371/journal.ppat.1006464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We allow the use of data and materials.


