Abstract
Purpose
Randomized trials on the effect of external beam radiotherapy (EBRT) with or without vaginal brachytherapy (VBT) for endometrial carcinoma are very few. In view of this, the current study was conducted with the hypothesizes: whether the escalated dose of 26 Gy (VBT alone) in comparison with various major international trials (PORTEC-2) has any difference in rates of disease-free and overall survival with fewer adverse effects in low resource setting like India.
Material and methods
An open-labeled, non-inferiority, randomized control trial was undertaken at a regional cancer center among patients with stage IA or IB high-intermediate risk endometrial carcinoma. A total of 50 patients were divided equally among two arms of combined EBRT with VBT (arm I) and VBT alone (arm II). A dose of 50-50.4 Gy in 25-28 fractions of EBRT with 2 fractions of VBT 6.5 Gy each were delivered to patients in arm I and 4 fractions of VBT 6.5 Gy each to patients in arm II, and were followed up for 60 months.
Results
During the median follow-up of 36.5 months, two patients developed loco-regional recurrence in arm II, three (arm II), and one (arm I) developed distant metastasis. The 5-year survival rates for arms I and II were 96.0% vs. 92.0% overall, and 88.0% vs. 84.0% disease-free, respectively, and were not found to be statistically significantly different. Dermatological, gastro-intestinal toxicities, and cystitis were lower in the VBT group compared to combined group.
Conclusions
VBT alone is as effective as EBRT+VBT in ensuring loco-regional control and achieving comparable survival rates, with fewer toxic effects for patients with stage I intermediate- and high-risk endometrial carcinoma. The dose escalation did not make a difference in the survival rates and was like in the other major trials (PORTEC-2).
Keywords: endometrial cancer, EBRT, vaginal brachytherapy
Purpose
Cancer of the corpus uteri ranks fifth among cancers in women worldwide, and accounts for nearly 5% of all cancers excluding non-melanoma skin cancer in women [1]. Endometrial cancer (EC) is the most common gynecological cancer in developed countries where its prevalence rate is increasing, primarily affects post-menopausal women over the age of 60 years [2]. The age-standardized rate across all geographic estimates remains 8.2 per 100,000 female populations [1]. The incidence and prevalence of carcinoma uterus in India, as reported in GLOBOCAN 2012, are 12,325 and 44,980 cases, respectively [1]. Vaginal brachytherapy (VBT) boost is often performed in patients who are known to benefit from external beam radiotherapy (EBRT) but have a higher risk of vaginal failure. However, there are no randomized trials comparing EBRT+VBT with VBT alone. The knowledge about the efficacy of these two lines of treatment can guide cost-based judgements, which is critical in low resource settings like India [3]. In different trials, like PORTEC-2 [4], VBT with the dose of 7 Gy × 3 fractions alone did not make a significant difference in the overall or disease-free survival when compared to patients treated with EBRT only. Hence, in the current study, based on trials published before, the dose of VBT was escalated (within the maximum tolerance of vaginal cuff) and compared for any reduction in the loco-regional recurrences, improvement in the overall or disease-free survival with minimal toxicities. The doses delivered were prescribed considering the different dose schedules used in other trials and after considering an expert’s opinion [4,5].
Material and methods
This was an open-labeled, non-inferiority, randomized control trial. The study was conducted for a period of 60 months from August 2011 to July 2016 in the Department of Radiation Oncology, Gujarat Cancer Research Institute (GCRI), Ahmedabad. Patients aged between 18-70 years, who underwent the surgical procedure (as mentioned below) with FIGO stage I intermediate- and high-risk endometrial carcinoma, consisting of stage Ia (grade II, grade III), stage Ib (grade I, grade II) and stage Ib (grade III), respectively, with no lymphovascular and peri-neural invasions; and patients who had never been treated with chemotherapy or radiotherapy for pelvic malignancies with normal hematopoietic parameters, KPS ≥ 70, adequate liver and renal functions, and who were willing to come for the follow-up were included in the study.
Furthermore, patients with history of other malignancy of pelvic organs interfering with the treatment, incompletely surgically staged patients, histology other than adenocarcinoma, and delay of more than 6 weeks for initiation of radiotherapy after surgery were excluded from the study. Detailed blood profile for complete blood count, blood chemistry profile (serum creatinine, serum electrolytes, serum total proteins, albumin) were done. Urine routine and microscopic examination, chest X-ray, ultrasonography of abdomen and pelvis were done before starting the treatment; CT scan or MRI of abdomen and pelvis were done only when necessary.
Sampling and sample size calculation
Based on the inclusion and exclusion criteria, a total of 50 patients were enrolled in the study after obtaining written informed consent from patients and ethical committee clearance from the institutional ethical committee board. First patient was randomly allocated among two arms using a coin toss with heads falling to arm I, receiving pelvic EBRT followed by VBT, and tails falling to arm II, receiving VBT alone. After the selection of the first patient, following patients were allocated systematically to consecutive arms I and II.
In the previous studies, true hazard ratio (relative risk) of control subjects relative to experimental subjects in terms of loco-regional recurrence was ≈ 0.4 [4], and median survival time (follow-up time) on the control and experimental arm was 45 months. We planned a study with 1 control per experimental subject, with an accrual interval of 25 months, and additional follow-up after the accrual interval of 35 months. We needed to study 24 experimental subjects and 24 control subjects to be able to reject the null hypothesis that the difference in experimental and control loco-regional recurrences are equal with probability (power) of 0.8. The type I error probability associated with this test of null hypothesis is 0.05 [6].
Procedures
Surgery and histo-pathological examination
Medically fit patients underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection of 12-14 in number, para-aortic lymph node dissection, and omental sampling. The samples were sent for histo-pathological examination (HPE). The staging was done according to FIGO 2009 [7] and was investigated for any lymphovascular and peri-neural invasions.
Radiotherapy among both the arms
External beam radiotherapy (EBRT)
Patients were simulated in supine position with custom made thermoplastic cast. Pelvic EBRT was administered using megavoltage beam on linear accelerator by four field boxes with 2D technique to cover the vaginal vault and bilateral pelvic lymph nodes including common iliac lymph nodes. A midplane dose of 50 Gy in 25 fractions or 50.4 Gy in 28 fractions were delivered with 5 fractions per week [8].
Vaginal brachytherapy
Per-vaginal examination was done before the initiation of treatment to assess the vaginal diameter and length. Then cylinders of maximum diameter were inserted into vagina and assembly was locked. VBT was delivered using Nucletron MicroSelectron high-dose-rate (HDR) unit. In arm I, VBT was given after a period of 7-10 days of completion of EBRT. Two fractions of VBT with a gap of 1 week were delivered with a dose of 6.5 Gy per fraction. In arm II, four fractions of VBT were delivered with a dose of 6.5 Gy per fraction, with a 1 week of interval between the fractions. These doses were prescribed at a depth of 0.5 cm from the surface of applicators using 2D technique, to cover the vaginal vault and upper half of vagina. After the completion of the treatment, the applicators were removed, and patient was discharged immediately.
Monitoring and assessment
During the process of treatment
All patients were regularly monitored at weekly intervals or SOS basis to document the performance status and tolerance of the treatment till the completion of treatment. Complete blood counts and renal function test was done every week.
Follow-up after the treatment
The patients were followed up in the OPD regularly with physical examination monthly for the first 6 months, every two months for the next 6 months, every three months for the next 1 year, and every six months afterwards, till the completion of the study in July 2016. PAP smears and abdomen and pelvis USG were done every three months, then every 6 months, till the end of the study, and chest X-ray was done every 6 months till the end of the study period. CT scan was done whenever necessary, and hence the response to treatment was assessed for progression of the disease by assessing loco-regional recurrence/relapse and distant metastases.
Operational definitions
Primary end point was disease-free survival, i.e. till the development of loco-regional recurrence (vaginal or pelvic lymph nodes) and distant metastases: 1. Secondary end points were overall survival and treatment related toxic effects; 2. Local recurrence: vaginal vault recurrences only; 3. Regional recurrence: positivity of regional draining lymph nodes (pelvic); 4. Distant metastasis: the recurrences secondary to the primary tumor bed other than loco-regional recurrence was considered as distant metastasis; 5. All the acute toxicities following the treatment were assessed using RTOG toxicity grading scale [9], and late toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTAEC) version 4.0 scale [10].
Statistical analyses
The data was tabulated in Microsoft Excel sheet. Time to event (loco-regional recurrence, distant metastasis) analyses was completed using log rank tests and Cox proportional hazards regression models, with date of randomization as a starting point. Data for patients who were alive and free of disease were censored at date of last follow-up. The Kaplan-Meier method was used for overall and disease-free survival. Patient and tumor characteristics were compared with χ² statistics or Fisher’s exact test for categorical variables, and t test for continuous variables. The analysis was done using a SPSS version 18.0. Statistical tests were considered significant at 5% significance level.
Results
Mean age of the patients in arm I was 57.40 (± 6.6 years), and arm II was 54.96 (± 8.3 years), respectively. Majority of the patients in arm I (17; 68.0%) and II (13; 52.0%) were stage Ib. Most of the patients in arm I (23; 92.0%) and all of them in arm II belonged to intermediate-risk group. Majority of the patients (28; 56.0%) had grade II endometrial carcinoma, among whom 53.6% were in arm II and 46.4% were in arm I. The difference between the patient and tumor characteristics among both the arms was insignificant (p > 0.05). The mean difference in the duration of treatment in arm II (22.08 ± 2.29 days) compared to arm I (58.28 ± 7.81 days) was statistically significant (p < 0.05) (Table 1).
Table 1.
Patient and tumor characteristics
| Particulars (n = 50) | Arm-I (%) | Arm-II (%) | Total | χ2 | p value |
|---|---|---|---|---|---|
| Age | 1.67 | 0.19 | |||
| ≤ 60 | 16 (64.0) | 21 (84.0) | 37 (74.0) | ||
| > 60 | 9 (36.0) | 4 (16.0) | 13 (26.0) | ||
| FIGO stage | 0.75 | 0.38 | |||
| Ia | 8 (32.0) | 12 (48.0) | 20 (40.0) | ||
| Ib | 17 (68.0) | 13 (52.0) | 30 (60.0) | ||
| Grade | 1.22 | 0.54 | |||
| I | 6 (24.0) | 7 (28.0) | 13 (26.0) | ||
| II | 13 (52.0) | 15 (60.0) | 28 (56.0) | ||
| III | 6 (24.0) | 3 (12.0) | 9 (18.0) | ||
| Risk group* | 0.48 | ||||
| High | 2 (8.0) | 0 (0.0) | 2 (4.0) | ||
| Intermediate | 23 (92.0) | 25 (100.0) | 48 (96.0) |
Fisher’s exact test applied
During the median follow-up of 36.5 months, only 2/25 (8.0%) in arm II developed regional lymph nodal recurrence, none had local (vault) recurrence. In total, four patients (3 from arm II and 1 from arm I) developed distant metastasis, and 3 patients from arm I and 2 from arm II were lost to follow-up (Table 2).
Table 2.
Comparison of both the arms in terms of recurrence and death
| Particulars | EBRT+VBT (arm I) (n = 25) | VBT (arm II) (n = 25) | p value |
|---|---|---|---|
| Local recurrence | 0 (0.0) | 2 (8.0) | 0.15 |
| Distant metastasis | 1 (4.0) | 3 (12.0) | 0.30 |
| Death | 2 (8.0) | 4 (16.0) | 0.38 |
(Figures in the parenthesis indicates percentage); EBRT – external beam radiation therapy, VBT – vaginal brachytherapy
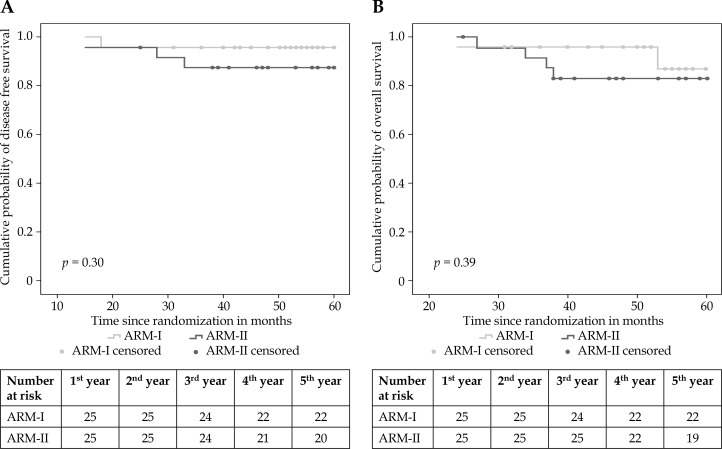
In total, during the study period, six patients died, 2 from arm I and 4 from arm II. Of those who died in arm I, one (4%) patient died from a cardiac disease, and one (4%) due to a recurrence. Of the 4 patients who died in arm II, three (12%) died due to a recurrence followed by its progression, and one (4%) died from second malignancy in lung. Overall and disease-free survival at 5 years were 96.0% (95% CI: 77.0-99.0) and 88.0% (95% CI: 68.0-97.0), respectively, in arm I, and 92.0% (95% CI: 72.0-98.0) and 84.0% (95% CI: 63.0-95.0), respectively, in arm II (Figure 1).
Fig. 1.
Kaplan-Meier survival curve. A) Disease-free survival, B) overall survival
Among the study subjects, there was no significant difference in the disease-free survival in both the arms, though the hazard ratio was > 1, (HR 2.30; 95% CI: 0.23-23.05; p = 0.48) indicating that patients in both the arms had same probability of having disease-free survival irrespective of treatment received (p > 0.05). The difference in the overall survival was not statistically significant in both the arms, though hazard ratio was > 1 (HR 1.97; 95% CI: 0.36-10.86; p = 0.44) indicating that patients in both the arms had same probability of overall survival irrespective of the treatment received (Table 3).
Table 3.
Comparison of disease-free survival and overall survival among both the arms
| Survival | Events/total | Estimated 5-yr (%, 95% CI) | Hazard ratio (CI)* | Log-rank, p value* |
|---|---|---|---|---|
| Disease-free survival | 0.30 | |||
| Arm I | 1/25 | 96% (77.0-99.0) | 1.00 | |
| Arm II | 3/25 | 88% (68.0-97.0) | 2.30 (0.23-23.05) | |
| Overall survival | 0.39 | |||
| Arm I | 2/25 | 92% (72.0-98.0) | 1.00 | |
| Arm II | 4/25 | 84% (63.0-95.0) | 1.97 (0.36-10.86) |
Adjusted for FIGO staging and grades (FIGO – International Federation of Gynecology and Obstetrics)
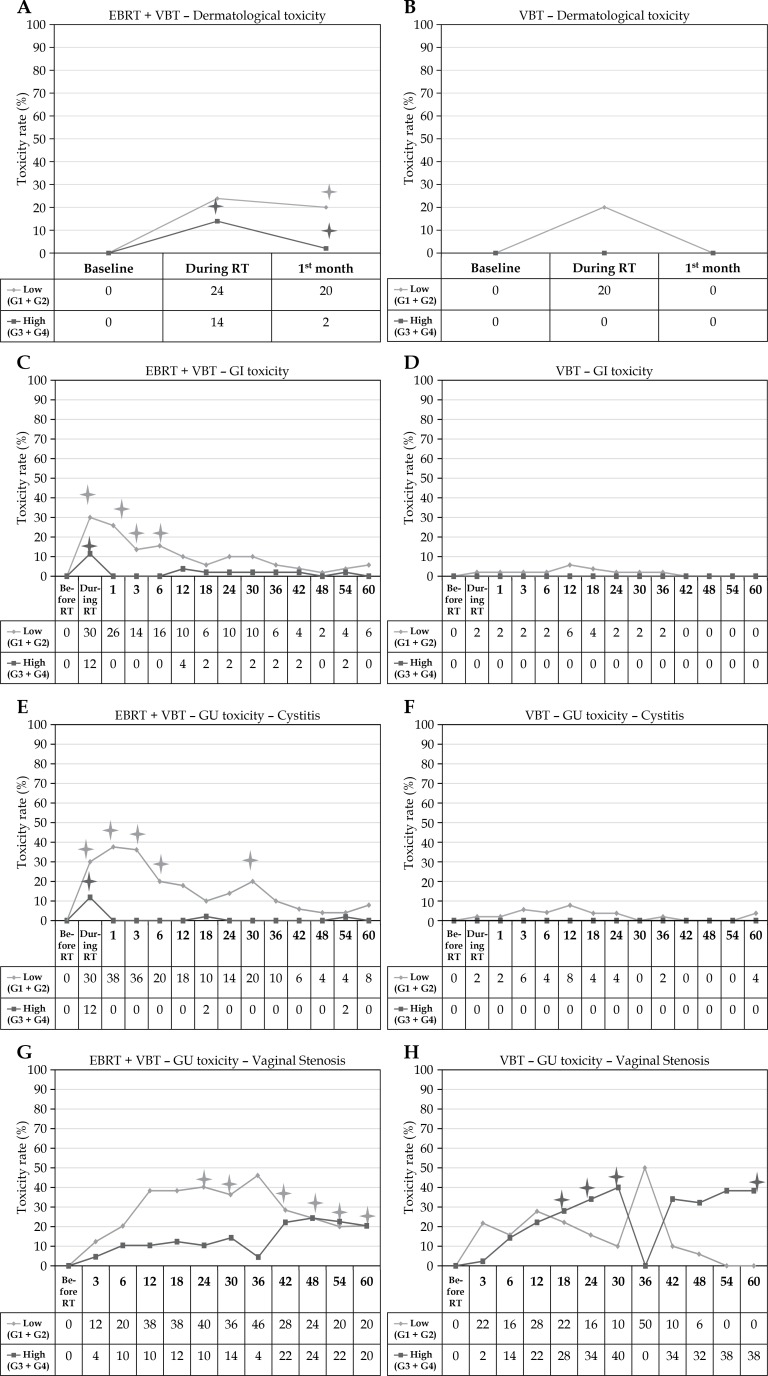
Dermatological toxicities (Figures 2 A and B)
Fig. 2.
A-B) Dermatological toxicity in arm I and II. C-D) Gastro-intestinal toxicity in arm I and II. E-F) Genito-urinary toxicity (cystitis) in arm I and II G-H) Genito-urinary toxicity (vaginal toxicity) in arm I and II
Toxicity rate at every follow-up time point represents the number of patients with toxicity as a percentage of the total number of patients who have reached that follow-up time point. EBRT – external beam radiotherapy, VBT – vaginal brachytherapy, RT – radiotherapy
*Time points showing significant (p < 0.05) difference between arm I and arm II
In arm I, 24.0% and 20.0% of patients developed low-grade skin toxicities during the treatment and at first month follow-up, respectively, whereas only 20.0% developed low-grade skin toxicities during the treatment and none developed at first month follow-up, respectively, in arm II. In arm I, 14.0% and 2.0% of patients developed high-grade skin toxicities during the treatment and at first month follow-up, respectively, whereas none of them developed high-grade skin toxicities during the treatment and at first month follow-up in arm II.
The high-grade toxicity was significantly higher during and at first month after treatment in arm I compared to arm II (p < 0.05). However, the low-grade toxicity was significantly higher at first month after the treatment in arm I compared to arm II (p < 0.05).
Gastro-intestinal (early and late) toxicities (Figures 2 C and D)
In arm I, 30.0% of patients developed low-grade GI toxicities, whereas only 2.0% developed low-grade toxicities during the treatment in arm II. In arm I, 12.0% of patients developed high-grade GI toxicities, whereas none of them developed high-grade toxicities during the treatment in arm II. During the follow-up, higher percentages of high- and low-grade GI toxicities persisted in arm I compared to arm II. However, the difference in proportions were significantly higher during and at 1st, 3rd, and 6th month after the treatment for low-grade GI toxicities in arm I compared to arm II and the proportion of patients having high-grade toxicity was significantly higher in arm I during the treatment compared to arm II (p < 0.05).
Genito-urinary (cystitis and vaginal) toxicities (Figures 2 E, F, G, and H)
Cystitis
During the treatment in arm I, 30.0% of patients developed low-grade GU toxicities, whereas only 2.0% developed low-grade toxicities in arm II. In arm I, 12.0% of patients developed high-grade GU toxicities, whereas none of them developed high-grade toxicities in arm II. During the follow-up, higher percentages of high- and low-grade GU toxicities persisted in arm I compared to arm II. However, the difference in proportions were significantly higher during and at 1st, 3rd, 6th, and 30th month after the treatment for low-grade GU toxicities in arm I compared to arm II, and the proportion of patients having high-grade toxicity was significantly higher in arm I during the treatment compared to arm II (p < 0.05).
Vaginal toxicity
During the follow-up, higher percentages of low-grade vaginal toxicities persisted in arm I compared to arm II, except at 3rd and 36th month of treatment. However, higher percentages of high-grade vaginal toxicities persisted in arm II compared to arm I, except at 3rd and 36th month of treatment. The difference in proportions remained significantly higher at 24th month till the end, except at 36th month after treatment for low-grade vaginal toxicities in arm I compared to arm II (p < 0.05). However, the proportion of patients having high-grade toxicity was significantly higher at 18th month till 30th month after treatment, and it lost its significance after 30th month and again gained significance at the end of the follow-up in arm II compared to arm I (p < 0.05).
Discussion
The role of adjuvant radiotherapy has been proved previously by three major randomized trials: GOG-99, PORTEC-1, and ASTEC [8,11,12]. These trials have demonstrated that adjuvant radiotherapy after surgery for carcinoma endometrium has reduced the rate of local recurrence from 12-14% to 3-4% with radiotherapy, and without radiotherapy – benefit in overall survival. The VBT dose delivery in arm I was done with 6.5 Gy x 2 per fraction (13 Gy) after a period of 7-10 days of completion of EBRT and in arm II, 6.5 Gy x 4 per fraction (26 Gy) were prescribed at a depth of 0.5 cm. However, in various studies, VBT dose ranges from 5 Gy x 3 fractions (15 Gy) to 10 Gy x 4 fractions (40 Gy) [5]. In a study conducted by Atahan et al., a total dose of 27.5 Gy at 0.5 cm was prescribed, the dose of which is approximately the same as in the current study (26 Gy) [13]. As mentioned before, considering the different dose schedules used in other trials and after an expert’s opinion, the dose of VBT was escalated (within the maximum tolerance of vaginal cuff) and compared for any difference in the overall or disease-free survival in the different arms in reference to other trials (PORTEC-2) [4,5,13]. During the median follow-up of 36.5 months, the loco-regional control in VBT arm is almost as good as in combined EBRT and VBT arm (p > 0.05), which is in accordance with the PORTEC-2 trial findings [4].
The overall or disease-free survival did not differ significantly in both the arms, which are like the corroborative evidences noted in studies PORTEC-2 and Sorbe et al. [14] (Table 4).
Table 4.
Comparison of overall survival, disease-free survival, loco-regional relapse, and distant failure along with the total EQD2 doses of various international trials with the present study
| Trial name | Treatment arms | Overall survival (%) | Disease-free survival (%) | Loco-regional relapse (%) | Distant failure (%) | EQD2 |
|---|---|---|---|---|---|---|
| Present study | EBRT (50 Gy) + VBT (6.5 Gy x 2 fractions) | 92.0 | 96.0 | 0.0 | 1.0 | 67.9 Gy |
| VBT (6.5 Gy x 4 fractions) | 84.0 | 88.0 | 2.0 | 3.0 | 35.8 Gy | |
| Sorbe et al. [14] | EBRT (46 Gy median dose) + VBT | 89.0 | 86.7 | 1.5 | 4.6 | Total dose of brachytherapy given ≈ 19.5-23.5 Gy and EBRT ≈ 6-50 Gy |
| VBT alone (3 to 20 Gy in 6-1 fractions) | 90.0 | 86.2 | 5.0 | 6.5 | ||
| PORTEC-2 trial [4] | EBRT (46 Gy in 23 fractions) | 79.6 | 78.0 | 2.1 | 5.7 | 46 Gy |
| VBT only (21 Gy in 3 fractions with HDR or 30 Gy with LDR) | 84.8 | 82.0 | 5.1 | 8.3 | 29.8 Gy | |
| Alektiar et al. [15] | VBT (median HDR-IVRT dose ranged from 6-21 Gy) | 93.0 | 97.0 | – | – | 6-29.8 Gy |
| Laliscia et al. [16] | VBT (21 Gy in 3 fractions) ± adjuvant chemotherapy | 93.0 | 88.0 | 7.9 | 1.6 | 29.8 Gy |
| Draghini et al. [17] | VBT (3-7 fractions x 5-8 Gy ≈ in VBT and 46-50 Gy in EBRT | 77.0 | 63.0 | 18.0 | – | 20-44 Gy for patients receiving only VBT |
EBRT – external beam radiation therapy, VBT – vaginal brachytherapy, EQD2 – equivalent dose at 2 Gy
A meta-analysis has shown that in few of the high-quality trials, adjuvant pelvic external beam radiotherapy (EBRT) reduces the risk of loco-regional recurrence; however, there was no difference in distant recurrence rates. The improvement was not seen in terms of overall survival (HR = 0.99; 95% CI: 0.82-1.20) or endometrial cancer-specific survival (HR = 0.96; 95% CI: 0.72-1.28) [15]. In the current study, the loco-regional recurrence and distant recurrence rates were lesser in combined EBRT and VBT arm; however, it was not significant, which shows the importance of VBT as an alternative to EBRT with or without VBT.
The rates of acute grade 1/2 gastrointestinal toxicities were significantly lower in the VBT group than in the EBRT group at completion of radiotherapy (12.6% vs. 53.8%) in PORTEC-2 trial [4]. In the present study in combined EBRT+VBT group, 30.0% of them developed low-grade GI toxicities, whereas only 2.0% developed low-grade toxicities during the treatment in VBT group. Similarly, during the follow-up, higher percentages of high- and low-grade GI toxicities persisted in combined EBRT and VBT group compared to VBT group. Late grade 3 gastrointestinal toxic effects were reported in 2% patients receiving EBRT and in < 1% receiving VBT, as noted in PORTEC-2 trial [4]. This is in concordance with the present study, where late (low- and high-grade) toxicities were noted more in patients receiving combined EBRT and VBT when compared to patients in VBT group, where the toxicity remained at the baseline. Draghini et al. observed acute toxicities among 12.0% patients after administration of VBT. Among those, grade 2 nausea and proctitis (6.0%), grade 2 diarrhea and proctitis (6.0%), and grade 1 late rectal bleeding (12.0%) were noted. No grade 3-4 iatrogenic toxicities were observed in their study, like in the current study. However, these findings by Draghini et al. were noted among inoperable cases and differences among the toxicity percentages were observed because of the different brachytherapy techniques used [16]. Sekii et al. noted grade 2 rectal bleeding among 5.0% of all patients who underwent HDR brachytherapy for initial recurrence as a salvage option in the vagina for endometrial cancer after definitive surgery with or without EBRT, and there was no grade 3 or higher late complications experienced, which is similar to present study findings in arm II with VBT alone [17].
Comparing the trend of vaginal toxicities among both the arms, patients treated with VBT alone had higher incidence of high-grade vaginal toxicities during the follow-up of 60 months. Low-grade vaginal toxicities were higher in arm II in the initial 3 months of treatment, however, the reported toxicities were significantly higher from 24th month of follow-up in arm I compared to arm II, except at 36 months. On the other hand, high-grade vaginal toxicities were higher in arm II compared to arm I, except at 36 months, and it was significant during 18th month to 30th month and at 60th month of follow-up. During the initial 36 months, the patients underwent multiple dilatations using vaginal dilators, because of which the vaginal toxicity has dropped to baseline at around 36th month, after which the dilatations did not make much of a difference. Grade 1/2 mucosal atrophy (vaginal toxicity) increased from 6 months onwards and the increase was significantly more with grade 2 atrophy after VBT than after EBRT [4]. Grade 3 atrophy (marked atrophy with or without shortening or narrowing) was reported in < 1% patient receiving EBRT and 2% receiving VBT in the PORTEC-2 trial [4]. However, in the present study, high-grade vaginal toxicity (grade 3 and grade 4) was significantly higher in VBT group, which could be due to the difference in the total dose delivered in VBT. The most common side effects of HDR intravaginal brachytherapy in a retrospective study by Sourav et al. were mucosal telangiectasia, atrophy, stricture, or adhesions, similar the findings in this study [18]. Laliscia et al. also found the most common acute toxicities as vaginal inflammation, dyspareunia, fibrosis, and telangiectasias, like the current study. These findings were noted among cases with VBT with or without adjuvant chemotherapy [19]. However, there were no statistical differences in terms of acute or late vaginal toxicities between patients who underwent adjuvant chemotherapy and those who did not. The decrease in the vaginal toxicity compared to our study is because of the usage of hyaluronic acid for all the patients.
During the treatment, 30.0% and 2.0% of patients developed low-grade GU toxicities in combined EBRT and VBT group and VBT alone group, respectively. 12.0% of patients developed high-grade GU toxicities in combined group and none developed high-grade toxicities during the treatment in the other group. In a study by Sourav et al., late radiation-induced bladder toxicity was noted in 27.3% of cases and 3.5% patients had experienced grade 3 adverse events. However, in the study by Sourav et al., the findings were assessed for both endometrial and cervical cancers [18]. Sekii et al. noted grade 2 hematuria among 10.8% of all patients who underwent HDR brachytherapy for initial recurrence as a salvage option in the vagina for endometrial cancer after definitive surgery with or without EBRT, and there was no grade 3 or higher late complications experienced, as similar to present study findings in arm II with VBT alone [17].
The skin and mucosal toxicities were higher in arm I compared to arm II. Skin reactions are inherent findings following 2D technique of EBRT. PORTEC-1, GOG-99, and ASTEC/EN5 trials have also noted that the use of pelvic irradiation caused unacceptable increase in toxicity. Retrospective studies on vaginal brachytherapy (VBT) have shown much lower toxicities among early endometrial cancer patients.
Quality of life in women after adjuvant EBRT decreased because of significant levels of urinary and bowel toxicities [4,11,20,21]. Though grade 1 and grade 2 vaginal toxicities were higher in arm I, grade 3 vaginal toxicities were higher in arm II compared to arm I, as higher dose is delivered to vaginal mucosa in VBT group than in combined EBRT and VBT group (Table 5).
Table 5.
Comparison of late toxicities of various international trials with the present study
| Trials | Treatment arms | GI toxicity | Vaginal toxicity | Urological toxicity | |||
|---|---|---|---|---|---|---|---|
| LG (%) | HG (%) | LG (%) | HG (%) | LG (%) | HG (%) | ||
| Present study (at the end of 5 years) | EBRT + VBT | 6.0 | 0.0 | 20.0 | 20.0 | 8.0 | 0.0 |
| VBT | 0.0 | 0.0 | 0.0 | 38.0 | 4.0 | 0.0 | |
| Sorbe et al. [14] (> 3 months) | EBRT + VBT | 12.7 | 1.8 | 13.4 | – | 33.3 | 1.9 |
| VBT alone | 2.7 | – | 4.9 | 0.8 | 22.9 | 0.8 | |
| PORTEC-2 trial [4] (at the end of 3 years) | EBRT | 20.2 | – | 17.2 | 0.5 | – | – |
| VBT only | 8.5 | < 1.0 | 35.2 | 1.4 | – | – | |
| Alektiar et al. [15] (late toxicity at 5 years) | VBT | – | – | – | < 1.0 | – | < 1.0 |
| Laliscia et al. [16] (> 6 months) | VBT | – | – | 23.0 | 0.0 | – | – |
| Draghini et al. [17] (at 53 months of follow-up) | EBRT ± VBT | 12.0 | 0.0 | – | 0.0 | – | – |
EBRT – external beam radiation therapy, VBT – vaginal brachytherapy, LG – low grade, HG – high grade
What this study adds?
Though vaginal brachytherapy continues to evolve as one of the integral modalities in the post-operative management of endometrial cancer, different studies on VBT demonstrate significantly reduced low rates of high-grade vaginal complications by the use of lower dose per fraction regimens. Since the studies with dose prescribed in the present study (6.5 Gy x 4 fractions) are few, we also have attempted to observe if escalation of dose makes any difference in the survival of patients. The same has been done in a limited resource setting using 2D technique, however, the toxicity profiles are comparatively acceptable as per the observation in the current study.
The difference in toxicity profile is one of the most important advantages in conduction of regional studies, based on international trials. This difference in toxicities depends on multiple factors. The impact of these factors can be studied by data from multiple small trials like the current ones. In this study, the patients received 6.5 Gy per fraction in VBT but still had toxicity comparable to other international trials. The cause for this will have to be evaluated with further studies. In endometrium carcinoma, only vaginal brachytherapy to the stage I intermediate-risk group improves the patient’s quality of life and compliance for the treatment. Unlike in developed countries with extensive support systems for patients, in a developing country like India, the avoidance of EBRT and treating with VBT alone without compromising on the treatment results is more important.
Limitations of the study
The present study is an open labelled RCT, where blinding could not be adopted because of some feasibility issues. The patients in this study were staged based on FIGO 2009 staging. Though, FIGO staging has been revised in 2010, it is unlikely to affect the trial results. Because of the limitation of the resources at the center, all the patients could only be treated using 2D technique and hence, the dose delivered to the surrounding organs at risk could not be assessed. The recent studies also suggest that if pelvic lymph nodes are negative on imaging then the retroperitoneal lymph node dissection is not necessary, however in the current study, all patients have undergone retroperitoneal lymph node dissection, which may differ the outcome of the study. The study was initially designed to recruit patients for both high- and intermediate-risk group but only 2 patients of high-risk group were accrued; hence, the study could not analyze the treatment outcome in high-risk groups separately. Though, the minimum sample size has been calculated, the study lacks generalizability to all the ethnic groups, therefore, the study needs to be conducted in larger group of patients.
Conclusions
VBT is as effective as combined EBRT and VBT in ensuring loco-regional control and in achieving the same rates of overall survival and disease-free survival in patients with endometrial carcinoma of high- and intermediate-risk. VBT has fewer side effects and better quality of life in terms of toxicity profiles compared to combined EBRT and VBT. However, the benefit of vaginal brachytherapy in terms of survival did not outweigh the combined technique, comparing toxicity profile of the two treatment arms, VBT alone far outweighed the combined VBT and EBRT technique. Hence, VBT alone can probably be the treatment of choice for adjuvant treatment of patients with high- and intermediate-risk endometrial carcinomas. On the other hand, the escalated dose in the current study compared to the other major trials did not make any difference in the disease-free and overall survival [4,5].
Disclosure
The authors report no conflict of interest.
References
- 1.WHO. GLOBOCAN 2012 Estimated cancer incidence, mortality and prevalence worldwide in 2012. [Internet] 2017 [cited 2017 June 23]. Available from: http://globocan.iarc.fr/Default.aspx.
- 2.Munro MG, Southern California Permanente Medical Group’s Abnormal Uterine Bleeding Working Group Investigation of women with postmenopausal uterine bleeding: clinical practice recommendations. Perm J. 2014;18:55–70. doi: 10.7812/TPP/13-072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harkenrider MM, Block AM, Alektiar KM, et al. American Brachytherapy Task Group Report: Adjuvant vaginal brachytherapy for early-stage endometrial cancer: A comprehensive review. Brachytherapy. 2017;16:95–108. doi: 10.1016/j.brachy.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 5.Small W, Beriwal S, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy. 2012;11:58–67. doi: 10.1016/j.brachy.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Dupont WD, Plummer WD. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 7.Lewin SN. Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol. 2011;54:215–218. doi: 10.1097/GRF.0b013e3182185baa. [DOI] [PubMed] [Google Scholar]
- 8.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 9.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) version 4.0. National Cancer Institute; 2009. May 28, (09-5410) [Google Scholar]
- 11.Creutzberg CL, Van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post-operative radiation therapy in endometrial carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 12.ASTEC study group. Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ATEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atahan IL, Ozyar E, Yildiz F, et al. Vaginal high dose rate brachytherapy alone in patients with intermediate to high risk stage I endometrial carcinoma after radical surgery. Int J Gynecol Cancer. 2008;18:1294–1299. doi: 10.1111/j.1525-1438.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 14.Sorbe BG, Horvath G, Andersson H, et al. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma: A prospective, randomized study–quality-of-life analysis. Int J Gynecol Cancer. 2012;22:1281–1288. doi: 10.1097/IGC.0b013e3182643ba0. [DOI] [PubMed] [Google Scholar]
- 15.Laliscia C, Delishaj D, Fabrini MG, et al. Acute and late vaginal toxicity after adjuvant high-dose-rate vaginal brachytherapy in patients with intermediate risk endometrial cancer: is local therapy with hyaluronic acid of clinical benefit? J Contemp Brachytherapy. 2016;8:512–517. doi: 10.5114/jcb.2016.64511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draghini L, Maranzano E, Casale M, et al. Definitive three-dimensional high-dose-rate brachytherapy for inoperable endometrial cancer. J Contemp Brachytherapy. 2017;9:118–123. doi: 10.5114/jcb.2017.67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–1634. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 18.Sekii S, Murakami N, Kato T, et al. Outcomes of salvage high-dose-rate brachytherapy with or without external beam radiotherapy for isolated vaginal recurrence of endometrial cancer. J Contemp Brachytherapy. 2017;9:209–215. doi: 10.5114/jcb.2017.67755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sau S, Ghosh S, Mitra S, et al. A retrospective six years analysis of survival and late morbidity of post-operative gynaecological malignancy treated with external radiotherapy followed by brachytherapy in Medical College & Hospitals, Kolkata. J Obstet Gynaecol India. 2013;63:128–134. doi: 10.1007/s13224-012-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellas-Sleczka S, Wojcieszek P, Białas B. Adjuvant vaginal brachytherapy as a part of management in early endometrial cancer. J Contemp Brachytherapy. 2012;4:247–252. doi: 10.5114/jcb.2012.32560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly ED, Rakhra S, Helenowski I, et al. Dosimetry and toxicity outcomes in postoperative high-dose-rate intracavitary brachytherapy for endometrial carcinoma. J Contemp Brachytherapy. 2012;4:135–140. doi: 10.5114/jcb.2012.30679. [DOI] [PMC free article] [PubMed] [Google Scholar]