Abstract
This study presents a drug delivery system of poly (Ɛ-caprolactone) (PCL) ribbons to optimize the pharmaceutical action of tramadol for the first time according to our knowledge. PCL ribbons were fabricated and loaded with tramadol HCl. Ribbons were prepared by slip casting technique and coated with dipping technique with β-cyclodextrin. The chemical integrity and surface morphology of the ribbons were confirmed using FTIR and SEM coupled with EDX. In addition, thermodynamic behavior of the fabricated ribbons was investigated using DSC/TGA. Tramadol loading into PCL ribbons, biodegradation of ribbons and tramadol release kinetics were studied in PBS.The results revealed that the formulated composition did not affect the chemical integrity of the drug. Furthermore, SEM/EDX confirmed the inclusion of tramadol into the PCL matrix in homogenous distribution pattern without any observation of porous structure. The particle size of loaded tramadol was found to be in the range of (2–4 nm). The formulated composition did not affect the chemical integrity of the drug and should be further investigated for bioavailability. Tramadol exhibited controlled release behavior from PCL ribbons up to 45 days governed mainly by diffusion mechanism. The fabricated ribbons have a great potentiality to be implemented in the long term subcutaneous delivery of tramadol.
Keywords: Tramadol, Polycaprolcatone, Subcutaneous membrane, Ribbons, β-Cyclodextrin, Controlled release
1. Introduction
Tramadol HCl is an atypical opioid analgesic. It provides analgesia through a dual mechanism of action; the main compound acts primarily to block reuptake of serotonin and norepinephrine while its primary metabolite (+)-o-desmethyltramadol acts as a mu opioid receptor (Mu μ receptor is responsible for endorphin action and its pharmacologic primary agonist is morphine and opioid actions are due to its activation) agonist with mild action (Enggaard et al., 2006). Tramadol is used orally, intramuscularly or intravenously in management of moderate to moderately severe pain including postoperative, gynecologic, obstetric, and cancer pain (Lee et al., 1993, Dayer et al., 1994). Nonetheless, tramadol HCl has less abuse potential than prototypic opioid agonists. It is commercially available in many dosage forms; immediate-release tabletsor capsules (Ultram, Contramal, Tramal) to be taken orally in doses 50–100 mg every 4–6 h as needed for pain, extended-release (ER) capsules (ConZip) (150 mg) or ER tablets (100, 200, 300 mg tablets) (Ryzolt, Ultram ER) to be taken once daily, injection (Anadol, Dolonil 100 mg ampoules), and suppositories (100 mg Anadol). In Egypt, only immediate or extended release tablets are produced locally (‘Tramadol’, n.d.). However, oral administration has many drawbacks and of low patient compliance. Preparations that reduce tolerability help patients with diseases of chronic pain such as cancer and autoimmune disease. Besides, toxicityis a significant concern that may result in poor pain control (Miaskowski et al., 2001). In a previous study, implantable drug delivery system reduced the opioid reported toxicity to the half (Smith et al., 2005).
Research is proceeding to produce a sustainable and effective drug formulation for such compound to help relieve chronic pain patients with minimal side effects. Various new oral formulations for tramadol have been developed with a maximum release duration of 48 h (Obaidat and Obaidat, 2001, Gonjari et al., 2009, Raghavendra et al., 2009, Jeevana and Sunitha, 2009, Deore et al., 2010, Chaurasia et al., 2011, Kakar et al., 2013). Tramadol was also encapsulated into polyhydroxybutyrate (PHB) microspheres for epidural injections where tramadol was released for seven days (Salman et al., 2003).
Implantable biodegradable systems resemble a good alternative for pharmaceutical dosage forms. Biodegradable materials are either natural or synthetic and are degraded in vivo into safe biocompatible by-products that are eliminated through normal metabolic pathways. The main benefits of the controlled and sustained release formulations depend on their ability to prolong the effective therapeutic times by controlling drug concentration in blood (Souto and Doktorovova, 2009). Tramadol containing subcutaneous batch was prepared from Eudragit RL-100: HPMC (8:2) and showed around 80% release within 12 h (Shinde et al., 2008). Chitosan biodegradable naturally occurring polymer was also used to prepare implants of tramadol that sustained the release up to 17 days (Iqbal et al., 2012). However, the main disadvantage of chitosan based polymer is the lack of mechanical stability and the risk of dissolution of the system, due to a highly pH-sensitive swelling (Berger et al., 2004).
PCL is an aliphatic polyester and is semicrystalline with a low glass transition temperature (Tg) of approximately −60 °C and a low melting point (Tm) of approximately 60 °C; hence, it is always rubbery state at room temperature. Another interesting property of PCL is that it has a low tensile modulus of approximately 0.4 GPa and a high elongation to break of approximately 80%. It shows slow degradation rate in vivo due to its high crystallinity and hydrophobicity (Allen et al., 1999). Manipulation of the composition of PCL containing systems can be used to modify water permeability and the degree of hydration of a copolymer matrix. However, the disadvantage is a reduction of the mechanical strength, as a result of decrease in the crystallinity of the material (Ratajska & Boryniec, 1998). PCL has been used as a biomaterial for different biomedical systems (Sinha et al., 2004, Woodruff and Hutmacher, 2010). Complexation of PCL with other biocompatible polymers has shown promise in expanding the applications of PCL in pharmaceutical field (Woodruff and Hutmacher, 2010).
The present study aimed at formulating tramadol HCl in a biocompatible subcutaneous implant that can control the release for more than 30 days and help reducing its side effects. Up to our knowledge, the use of the long acting biodegradable polymer PCL to formulate tramadol have not been introduced before, and its release could not be sustained through subcutaneous implants for more than 17 days. Therefore, this study was focused on the preparation and evaluation of properties of the long term tramadol controlled delivery ribbons.
2. Material and methods
2.1. Material
Tramadol hydrochloride (>98% HPLC) was generously supplied by Sigma pharmaceuticals, Egypt. poly (Ɛ-caprolactone) (PCL) (MW = 80,000 g/mol), Dichloromethane (DCM), PVA (Mw = 67,000), β-cyclodextrin (BCD) (Wacker, Burghausen were purchased from Sigma–Aldrich (Germany). Any other used chemical was of high analytical grade and purchased from Sigma Co., USA.
2.2. Synthesis and preparation of PCL ribbons and drug loading
Slip casting solvent evaporation technique was applied in the preparation procedures. Briefly, 250 mg of PCL powder was dissolved in dichloromethane to obtain 2.5% w/v solution. Thereafter, the resulting solution was casted onto a ribbon shaped mould with dimensions of (5 × 15mm) and were allowed to dry overnight at room temperature. The obtained ribbons were kept as control samples. Tramadol hydrochloride was dispersed in the prepared PCL solution (2.5% w/v) in two different quantities (350 and 650 mg). Thereafter, the resultant mixtures were casted onto ribbon shaped moulds with dimensions of (5 × 15mm) and were allowed to dry overnight at room temperature. Five ribbons of each concentration were obtained (T350 and T650).
2.3. Synthesis of modified tramadol-loaded ribbons by dipping technique
Tramadol-loaded ribbons were prepared as described above then the obtained ribbons were dip coated in tramadol containing PVA and β-cyclodextrin solution. The coating solution was prepared by dissolving both PVA (250 mg) and β-cyclodextrin (250 mg) in 10 ml of methanol-distilled water co-solvent at 70 °C. Afterwards, tramadol (350 mg) was dispersed in the PVA and β-cyclodextrin solution. The coated ribbons were allowed to dry overnight at room temperature.
2.4. Chemical integrity of the prepared ribbons
FT-IR spectra were measured for all ribbons in order to investigate the effect of the tramadol on the functional group determination using a Perkin Elmer Spectrum 2000 FT-IR spectrometer, employing a single-reflection diamond MIRTGS detector (PerkinElmer Spectrum 100, Llantrisant, Wales, UK). All samples were analyzed by a universal FTIR spectrum series at a resolution of 4 cm−1. The samples were small pellets, of 0.5 cm diameter, obtained by pressing the ribbon powder with KBr.
2.5. Thermodynamic behavior of the tramadol-loaded ribbons
The thermal behavior of the prepared ribbons before and after tramadol-loading was tested by Differential Scanning Calorimeter (DSC) and thermal gravimetric analysis (TGA) using a computerized SETARAM labsys™ TG-DSC thermal analysis system. Samples (100 ± 1 mg) were placed in platinum crucible (30 ml in volume) with heating range of 25–500 °C with a heating rate of 10 °C/min.
2.6. Morphology of the tramadol-loaded ribbons
The internal morphology and surface area of the tramadol-loaded ribbons before and after immersion in PBS was investigated using Scanning Electron Microscope (SEM). SEM analysis was undertaken using a (JEOL JXA-840A, Electronprobe micro-analyzer, Japan) at 15 kV. Samples were rendered electrically conductive before analysis through gold-sputter coating (SPI Module™ Sputter Coater, SPI Supplies, PA) and were attached to the SEM stub using adhesive carbon tape.
2.7. Particle size determination
In addition, TEM images were recorded for tramadol to determine its particle size. Tramadol sample was prepared by dispersion of 10 mg of tramadol in 10 ml of acetone, afterwards, cupper grade was submersed into the tramadol suspension and was further dried at room temperature before the TEM image capturing process.
2.8. In vitro drug release
Actual loaded tramadol amount in the prepared ribbons was determined by grinding tramadol-loaded ribbons and extracting tramadol in methanol by gentle heat over 12 h. The solution was then filtered, diluted with PBS and analysed by UV spectrophotometry. All measurements were conducted in triplicate and the percentage of tramadol loading and encapsulation efficiency was calculated using Eqs. (1), (2).
| (1) |
| (2) |
Moreover, the in vitro tramadol release was measured in PBS (pH 7.4; 37 °C) at different time intervals spectrophotometrically at 271 nm using UV–vis spectroscopy (Lambda 25 UV/Vis Spectrophotometer, PerkinElmer, MA, USA).
2.9. Weight loss (%) and micro-environmental pH variation analysis
The weight loss (%) of tramadol loaded-ribbons was performed in PBS at the same conditions for the in vitro release study. At each time period a sample was collected and washed several times in deionized water to ensure removal of the adsorbed ions and subsequently dried at 21 °C. Weight loss (%) of the ribbons was calculated using Eq. (3). Three specimens from each sample were measured at each time intervals. The micro-environmental pH variation in the PBS at different time intervals was measured with a pH meter in order to predict the effect of tramadol (%) in tramadol loaded-ribbons on the release behavior. The results are demonstrated as an average value ± standard deviation (N = 3).
| (3) |
where the ribbon weight before soaking in PBS is (W0) and ribbon weight after specific soaking time is (Wt).
2.10. In vitro tramadol release kinetics
In order to predict the physical mechanism of the tramadol in vitro release behavior in PBS the release data were compared with the mathematical models of zero order, diffusion and Korsmeyer–Peppas, using the following equation.
| (4) |
where Mt/M∞ is the fraction of drug released at time t, k is the rate constant and n is the release exponent. In case of quasi-Fickian diffusion the value of n < 0.5, Fickian diffusion = 0.5, non-Fickian or anomalous transport n = 0.5–1.0 and Case II transport n = 1.0.
3. Results
3.1. Chemical integrity of the prepared ribbons
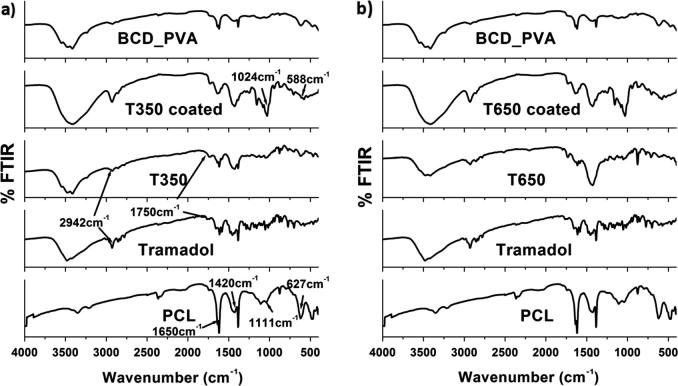
The FT-IR spectra of tramadol-loaded ribbons are illustrated in Fig. 1. First, the characteristic bands of the basic polymers were observed as follows; the main characteristic bands of PCL were observed at 1625 cm−1 corresponding to carbonyl stretching, bands at 1420 and 1111 cm−1indicating C—C and C—O stretching in PCL. PVA possess a stretching vibration band of —OH at 3300 cm−1. An important peak was also observed at 1150 cm−1 due to —C—O stretching, which is used for structural investigation of PVA. The C—O stretching vibration of BCD primary hydroxyl at 1030 cm−1, the C—O stretching vibration of BCD secondary hydroxyl at 1079 cm−1.
Fig. 1.
FTIR spectrum of the tramadol-loaded ribbons with reference to the native components and polymers.
Tramadol showed major characteristic bands as follows (2942, 1580–1750 and 1429 cm−1) corresponding respectively to, aliphatic C—H stretching and bending vibrations, the vibration stretching of carboxylic acid groups C O and C—H bending vibration. Furthermore, the PVA/BCD mixture demonstrated a slight shift (1035 cm−1) for the C—H stretching vibration of CH2 next to tertiary amino group and a remarkable shift (1120 cm−1) for the C—O stretching vibration of BCD primary and secondary hydroxyl groups, suggesting that hydrogen bonding between PVA and BCD has the form of BCD—O—H⋯N(CH3)2—PVA.
3.2. Thermodynamic behavior of the tramadol-loaded ribbons
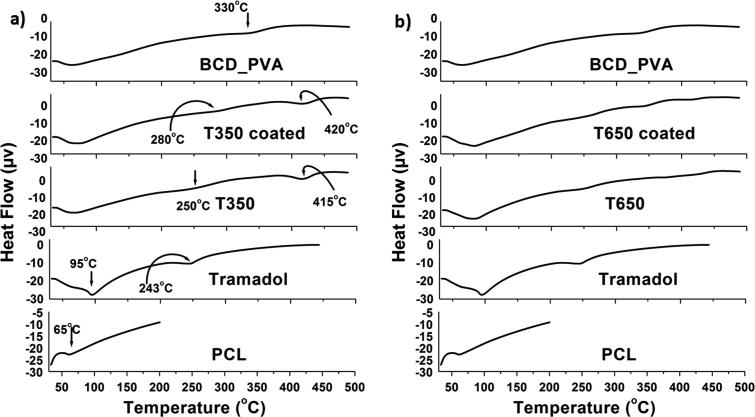
The thermal behavior of tramadol-loaded ribbons was determined by DSC as shown in Fig. 2. The native polymer; PCL showed one characteristic peak at 60 °C corresponding to Tm of PCL as early reported (Mabrouk et al., 2016). Tramadol possessed characteristic peaks at 95 °C and 243 °C corresponding to Tm and Tg of tramadol as previously mentioned (Boumraha et al., 2016).
Fig. 2.
Thermal behavior of the tramadol-loaded ribbons by DSC.
Tramadol-loaded ribbons showed remarkable changes compared to separate components as follows; important peak was observed at (415 °C), this peak was more boarded and shifted to (420 °C) in the coated ribbons due to the presence of BCD_PVA coating layer. The decomposition of BCD was reported to be between 172 °C and 264 °C (Li et al., 2017). Endothermic peak of BCD_PVA that was observed at 330 °C suggested higher Tg for the obtained mixture.
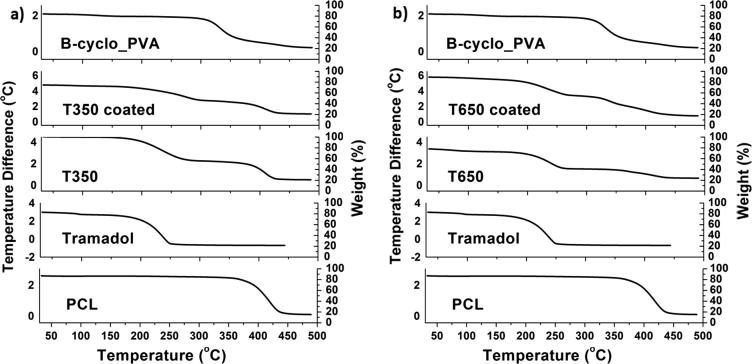
In addition to the DSC results, TGA of the tramadol-loaded ribbons with reference to the native components was recorded as shown in Fig. 3. Generally, TGA thermograms of all tramadol-loaded ribbons with reference to the native components exhibited downward shift which indicated loss of mass (due to both moisture loss and degradation) upon heating, but PCL exhibited only a degradation downward shift due to it hydrophobicity.
Fig. 3.
The thermal behavior of the tramadol-loaded ribbons by the TGA.
In details, PCL native polymer showed a degradation downward shift (85%) at range of (380–425 °C). Tramadol pure drug exhibited two downward shifts, the first one was (20%) at the range of (95–125 °C) due to moisture loss, the other shift (50%) was recorded at range of (220–235 °C) due to degradation.
Moreover, the tramadol-loaded ribbons exhibited a combined effect for both PCL and tramadol with little decrements in the heating temperature. Tramadol degradation downward shift (55%) was observed at range of (190–235 °C) and PCL degradation downward shift (40%) at range of (360–415 °C), thus confirmed the inclusion of tramadol into the tramadol-loaded ribbons as well as the drug loading efficiency. The BCD_PVA coating layer on the tramadol-loaded ribbons was confirmed by third degradation downward shift (35%) at range of (260–215 °C).
3.3. Morphology of the tramadol-loaded ribbons
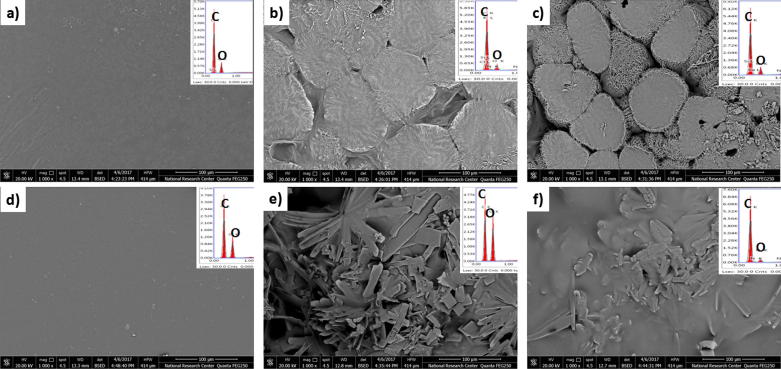
The tramadol-loaded ribbons morphology and elemental analysis with reference to tramadol-free ribbons were investigated using SEM coupled with EDX as illustrated in Fig. 4. Tramadol-free ribbons exhibited amorphous smooth surfaces (PCL and BCD_PVA) (Fig. 4a and d), while tramadol-loaded ribbons showed a close island like morphology and rough surfaces, this phenomena was highly obvious with increased concentrations of tramadol (Fig. 4b and c). The island like morphology phenomena was not recognized upon the coating with BCD_PVA containing tramadol.
Fig. 4.
The SEM images and their corresponding EDX of (a) PCL, (b) T350, (c) T650, (d) BCD_PVA, (e) T350 Coated and (f) T650 Coated.
3.4. Particle size determination
The particle size for loaded tramadol was measured using TEM analysis. TEM results revealed that loaded tramadol existed in nanoscale (2–5 nm) as illustrated in Fig. 5.
Fig. 5.
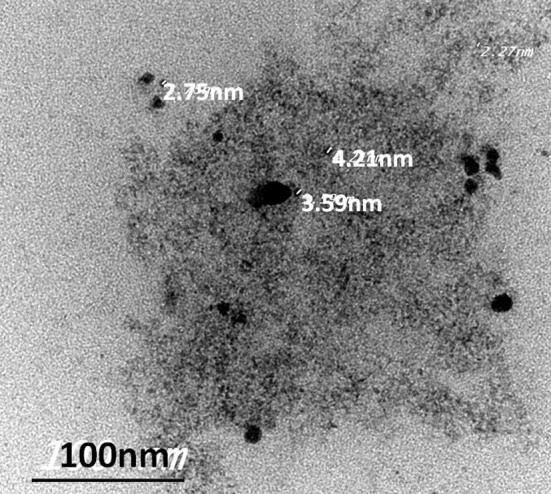
TEM image of loaded tramadol.
3.5. Drug loading efficiency and in vitro drug release
The entrapment efficiency values ranged from 97.00 ± 0.70% for non-coated ribbons to 85.00 ± 0.82% due to the presence of BCD_PVA coating. The cumulative drug release (%CDR) profiles of tramadol-loaded ribbons are presented in Fig. 6. Tramadol exhibited controlled release behavior from PCL ribbons up to 1080 h. Tramadol showed high release percentage from ribbon T350 (94.14%) while ribbon T650 was of lower percentage (48.20%). On the other hand, the presence of BCD_PVA coating layer on T650Coated and T350Coated ribbons had decreased the initial release percentage (26.90 and 38.40%) compared to the non-coated ribbons, that resulted in total tramadol decreased release rate at the end immersion time.
Fig. 6.
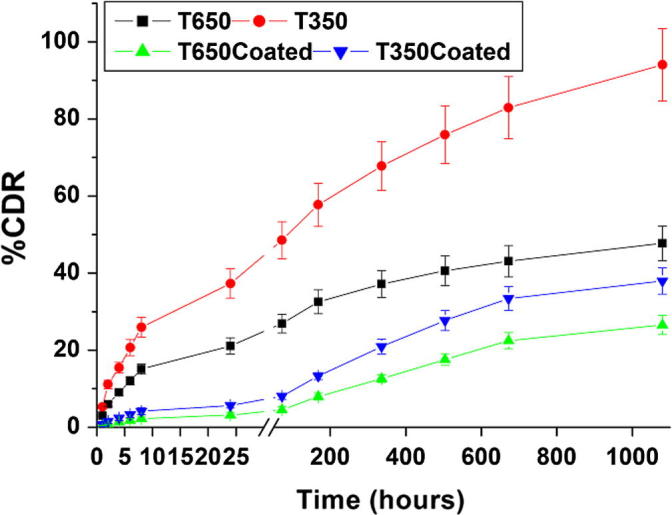
Percentage cumulative drug release of tramadol-loaded ribbons in PBS.
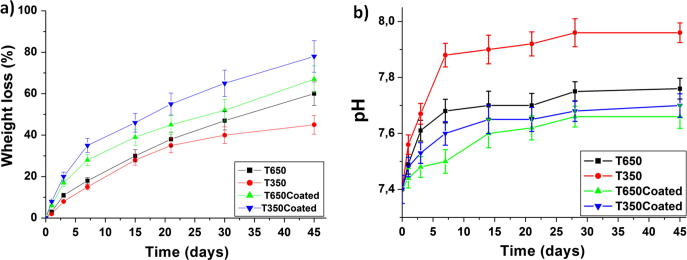
3.6. Weight loss (%) and micro-environmental pH variation analysis
Coated ribbons showed higher weight loss where T350Coated exhibited 78.17% loss and T650Coated showed 66.99% compared to non-coated ribbons T350 (45.08%) and T650 (60.12%). Results are demonstrated in Fig. 7a. Fig. 7b shows the pH changes of the PBS solution after soaking of tramadol-loaded ribbons for different time intervals. The results revealed that the pH values for tramadol-loaded ribbons were increased from 7.40 to 7.67, 7.88, 7.50 and 7.60 for T650, T350, T650Coated and T350Coated, respectively during the first week. This could be attributed to the release of the alkaline drug, tramadol, from ribbons into PBS. Thereafter, the pH values for all ribbons were relatively stable during the remaining immersion time.
Fig. 7.
Weight loss of the ribbons (a) and pH changes of the PBS solution (b) after soaking of tramadol-loaded ribbons for 45 days.
3.7. In vitro tramadol release kinetics
In vitro tramadol release from tramadol-loaded ribbons was fitted using different mathematical models and the kinetic parameters are listed in Table 1. It is evident from the release figure that the plots are curvilinear suggesting that the release process is not zero-order in nature. In general, when a solid drug is dispersed in an insoluble matrix (PCL polymer in this case), the drug release rate would be related to the drug diffusion rate.
Table 1.
Release kinetics parameters of different tramadol-loaded samples.
| Formula code | R2-value† |
Korsmeyer-Peppas model |
n | K* | RE0–1080h‡ (%) | |||
|---|---|---|---|---|---|---|---|---|
| Zero-order | Diffusion | Korsmeyer–Peppas | t50** (h) | t90*** (h) | ||||
| T650 | 0.733 | 0.909 | 0.920 | 26.978 | 49.084 | 0.343 | 5.272 | 47.745 |
| T350 | 0.786 | 0.941 | 0.937 | 171.497 | 792.511 | 0.356 | 9.089 | 94.030 |
| T650Coated | 0.958 | 0.984 | 0.986 | 160.083 | 288.477 | 0.544 | 0.573 | 26.527 |
| T350Coated | 0.932 | 0.990 | 0.982 | 90.469 | 163.124 | 0.518 | 1.063 | 37.953 |
n: diffusion exponent, k*: release rate constant, t50** time required for 50% of the drug to be released t90*** time required for 90% drug release, ‡RE0–1080h release efficiency of drug from 0 to 1080 h. †R2-value Regression co-efficient.
The mathematical calculations revealed that the release of tramadol from T650 and T650Coated are following Ritger-Peppas models for non-sewellable matrix, this means that the drug release followed diffusion controlled mechanism. Typically, the tramadol release from PCL polymer involves the simultaneous penetration of the surrounding liquid, dissolution of the drug, and leaching out of the drug through interstitial channels or pores. On the other side, T350 and T350Coated exhibited relatively different release behavior due to low tramadol concentration which was found to be following the diffusion mechanism as confirmed by R2 values.
3.8. Microstructural features of the tramadol-loaded ribbons after immersion in PBS
In order to confirm the suggested release kinetics for tramadol-loaded ribbons by the applied mathematical models, SEM images were recorded for all the ribbons under investigation after 45 days of immersion in PBS. SEM images exhibited micro channels and pores that were developed on all the ribbon surfaces by the PBS as demonstrated by black arrows in Fig. 8. This result confirmed the suggested release kinetics by the applied mathematical models, that the tramadol release from the PCL matrix involves simultaneous penetration of the surrounding liquid, dissolution of the drug, and leaching out of the drug through interstitial channels or pores. Moreover, some mineral particles were observed, that were precipitated from PBS onto the ribbons surface as demonstrated by red arrows in Fig. 8. In addition BCD_PVA layer residuals were also observed on the coated ribbons surface as demonstrated by blue arrows in Fig. 8. This observation suggested the layer by layer degradation for coated ribbons, thus would maintain tramadol controlled release behavior over long time.
Fig. 8.
SEM images of (a) T350, (b) T650, (c) T350Coated and (d) T650Coated after immersion in PBS for 45 days.
4. Discussion
PCL polymer represented a good candidate for implantable ribbons, where the obtained integrity was intact and tramadol release was sustained for more than 700 h. Moreover, the presented complex did not affect the chemical integrity or stability of the drug. The polymer proved its biodegradation and weight loss during the experimentation period.
The in vitro study suggested that the proposed system can be valid for sustaining the drug for at least 45 days in vivo. Tramadol exhibited controlled release behavior from PCL ribbons up to 1080 h. This can be explained by the non-soluble nature of PCL which limits PBS interaction with polymeric PCL ribbons. However, it was noted that tramadol release behavior was concentration dependent and was affected with the presence of BCD_PVA coating. Tramadol release behavior in non-coated ribbons can be attributed to the blockade of dissolution channels with higher tramadol concentrations and in coated ribbons by the low tramadol concentration in the coating layer. Besides, the presence of BCD_PVA coating extended the drug diffusion path from the PCL ribbon to the PBS medium, so the initial burst release was avoided and the drug release period was prolonged. These results were in the same line with the reported results for polycaprolactone/zein core/shell nanofiber membranes loaded with metronidazole by (He et al., 2017).
Coated ribbons showed higher weight loss compared to non-coated ribbons as the coating layer possesses a relative hydrophilicity compared to the core PCL polymer, which is completely hydrophobic (Mabrouk et al., 2015). Evidently, controlling tramadol concentration and the presence of BCD_PVA coating layer facilitate the ribbons degradation in PBS.
The layer by layer degradation of coated ribbons would maintain tramadol controlled release behavior over long time and suggest a better controlled delivery system (Wan et al., 2015, Irani et al., 2017, Rocha-García et al., 2017).
Moreover, stable pH values suggested stable drug concentrations in PBS. The relation between the weight loss (%) and pH of the physiological medium was highlighted in a similar research study that was previously reported for mesoporous calcium magnesium silicate cement (Cao et al., 2017). It is worthy noted that both weight loss (%) and pH are correlated with the tramadol release behavior.
5. Conclusions
PCL ribbons were successfully fabricated using slip casting solvent evaporation technique and loaded with tramadol HCl as confirmed by FTIR, DSC/TGA and SEM coupled with EDX. Long term tramadol controlled delivery ribbons showed concentration dependent release kinetics and was affected with the presence of BCD_PVA coating. Introducing polycaprolactone to implantable systems can provide new avenue for drug delivery and enhance the physical properties of such delivery systems. Bioavailability and biocompatibility need to be evaluated for full picture analysis of this system.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Acknowledgment
This work was supported by a grant from National Research Centre NRC. #11010133.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mostafa Mabrouk, Email: mostafamabrouk.nrc@gmail.com.
Hanan H. Beherei, Email: hanan_274@yahoo.com.
Shaimaa ElShebiney, Email: shaimaaelshebiney@gmail.com.
Masaru Tanaka, Email: masaru_tanaka@ms.ifoc.kyushu-u.ac.jp.
References
- Allen C., Eisenberg A., Maysinger D. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf., B. 1999;16(1–4):3–27. [Google Scholar]
- Berger J., Reist M., Mayer J.M., Felt O., Peppas N.A., Gurny R. Structure and interactions in covalently and ionicallycrosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004;57(1):19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- Cao L., Weng W., Chen X., Zhang J., Zhou Q., Cui J., Wang L., Shin J.-W., Su J. Effects of mesoporous calcium magnesium silicate on setting time, compressive strength, apatite formation, degradability and cell behavior to magnesium phosphate based bone cements. RSC Adv. 2017;7:870–879. [Google Scholar]
- Dayer P., Collart L., Desmeules J. The pharmacology of tramadol. Drugs. 1994;47(Suppl 1):3–7. doi: 10.2165/00003495-199400471-00003. [DOI] [PubMed] [Google Scholar]
- Deore R., Kavitha K., Tamizhmani T. Preparation and evaluation of sustained release matrix tablets of tramadol hydrochloride using glycerylpalmitostearate. Trop. J. Pharm. Res. 2010;9(3):275–281. [Google Scholar]
- Enggaard T.P., Poulsen L., Arendt-Nielsen L., Brosen K., Ossig J., Sindrup S.H. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth. Analg. 2006;102:146–150. doi: 10.1213/01.ane.0000189613.61910.32. [DOI] [PubMed] [Google Scholar]
- Gonjari I., Karmarkar A.B., Hosmani A.H. Evaluation of in vitro dissolution profile comparison methods of sustained release tramadol hydrochloride liquisolid compact formulations with marketed sustained release tablets. Digest J. Nanomater. Biostruct. 2009;4(4):651–661. [PubMed] [Google Scholar]
- He M., Jiang H., Wang R., Xie Y., Zhao C. Fabrication of metronidazole loaded poly (Ɛ-caprolactone)/zein core/shell nanofiber membranes via coaxial electrospinning for guided tissue regeneration. J. Colloid Interface Sci. 2017;490:270–278. doi: 10.1016/j.jcis.2016.11.062. [DOI] [PubMed] [Google Scholar]
- Iqbal M.M., Gupta S., Sagar S., Ibrahim M. Design and evaluation of subcutaneous implantable drug delivery system of tramadol using natural biodegradable polymer. Ann. Phytomed. 2012;1(2):30–38. [Google Scholar]
- Irani M., Sadeghi G.M.M., Haririan I. The sustained delivery of temozolomide from electrospun PCL-Diol-b-PU/gold nanocompsitenanofibers to treat glioblastoma tumors. Mater. Sci. Eng., C. 2017;75:165–174. doi: 10.1016/j.msec.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Jeevana J., Sunitha G. Development and evaluation of gelatin microspheres of tramadol hydrochloride. J. Young Pharm. 2009;1(1):24. [Google Scholar]
- Kakar S., Singh R., Shah M. Formulation and evaluation of orodispersible tablets of tramadol hydrochloride. FABAD J. Pharm. Sci. 2013;38(2):73–81. [Google Scholar]
- Chaurasia D., Kaushik K., Bhardwaj P., Chaurasia H., Jain S.K., Shobhna S. Development and in vitro characterization of floating microspheres bearing tramadol HCl. Acta Pol Pharm. 2011;68:795–801. [PubMed] [Google Scholar]
- Lee C.R., McTavish D., Sorkin E.M. Tramadol: a preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs. 1993;46:313–340. doi: 10.2165/00003495-199346020-00008. [DOI] [PubMed] [Google Scholar]
- Ratajska M., Boryniec S. Physical and chemical aspects of biodegradation of natural polymers. React. Funct. Polym. 1998;38(1):35–49. [Google Scholar]
- Mabrouk M., Bijukumar D., Mulla J.A., Chejara D.R., Badhe R.V., Choonara Y.E., Kumar P., du Toit L.C., Pillay V. Enhancement of the biomineralization and cellular adhesivity of polycaprolactone-based hollow porous Microspheres via dopamine bioactivation for tissue engineering applications. Mater. Lett. 2015;161:503–507. [Google Scholar]
- Mabrouk, M., Choonara, Y.E., Kumar, P., du Toit L.C., Pillay, V., 2016. Effect of lyophilized emugel silica microspheres on the physicomechanical properties. In: Vitro Bioactivity and Biodegradation of a Novel Ciprofloxacin-Loaded PCL/PAA blend Scaffold, Polymers, 8, 232; doi: 10.3390/polym8060232. [DOI] [PMC free article] [PubMed]
- Boumraha Y., Bouzahia I., Bouanani S., Khimeche K., Dahmani A. Thermodynamic and analytical studies of drugs binary systems of paracetamol mixed with pseudoephedrine. HCl, dextropropoxyphene. HCl and tramadol. HCl. Thermochim. Acta. 2016;20:48–56. [Google Scholar]
- Li J.-M., Hu C.-S., Shao J.-M., Li H.-J., Li P.-Y., Li X.-C., He W.-D. Fabricating ternary hydrogels of P(AM-co-DMAEMA)/PVA/β-CD based on multiple physical crosslinkage. Polymer. 2017;119:152–159. [Google Scholar]
- Miaskowski C., Dodd M.J., West C., et al. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J. Clin. Oncol. 2001;19:4275–4279. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- Obaidat A.A., Obaidat R.M. Controlled release of tramadol hydrochloride from matrices prepared using glycerylbehenate. Eur. J. Pharm. Biopharm. 2001;52(2):231–235. doi: 10.1016/s0939-6411(01)00173-4. [DOI] [PubMed] [Google Scholar]
- Raghavendra R.N., Gandhi S., Patel T. Formulation and evaluation of sustained release matrix tablets of tramadol hydrochloride. Int. J. Pharm. Phar. Sci. 2009;1(1):60–69. [Google Scholar]
- Rocha-García D., Guerra-Contreras A., Reyes-Hernández J., Palestino G. Thermal and kinetic evaluation of biodegradable thermo-sensitive gelatin/poly(ethylene glycol) diaminecrosslinked citric acid hydrogels for controlled release of tramadol. Eur. Polym. J. 2017;89:42–56. [Google Scholar]
- Salman M.A., Sahin A., Onur M.A., Öge K., Kassab A., Aypar Ü. Tramadol encapsulated into polyhydroxybutyrate microspheres: in vitro release and epidural analgesic effect in rats. Acta Anaesthesiol. Scand. 2003;47(8):1006–1012. doi: 10.1034/j.1399-6576.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- Shinde A., Garala K., More H. Development and characterization of transdermal therapeutics system of tramadol hydrochloride. Asian J. Pharm. 2008;2(4):265. [Google Scholar]
- Sinha V.R., Bansal K., Kaushik R., Kumria R., Trehan A. Poly-∊-caprolactone microspheres and nanospheres: an overview. Int. J. Pharm. 2004;278(1):1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Smith T.J., Coyne P.J., Staats P.S., Deer T., Stearns L.J., Rauck R.L., Pool G.E. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM) Ann. Oncol. 2005;16(5):825–833. doi: 10.1093/annonc/mdi156. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Doktorovova S. Chapter six-solid lipid nanoparticle formulations: pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol. 2009;464:105–129. doi: 10.1016/S0076-6879(09)64006-4. [DOI] [PubMed] [Google Scholar]
- Wan T., Stylios G.K., Giannoudi M., Giannoudis P.V. Investigating a new drug delivery nano composite membrane system based on PVA/PCL and PVA/HA(PEG) for the controlled release of biopharmaceuticals for bone infection, Injury. Int. J. Care Injured. 2015;46(S8):S39–S43. doi: 10.1016/S0020-1383(15)30053-X. [DOI] [PubMed] [Google Scholar]
- Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer–polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. [Google Scholar]
Further reading
- Tramadol. (n.d.). Drugs.com. Retrieved September 9, 2017, from <https://www.drugs.com/international/tramadol.html>.