Abstract
Purpose
Breast cancer (BC) is a highly heterogeneous disease presenting a broad range of clinical and molecular characteristics. In the past years, a growing body of evidence demonstrated that immune response plays a significant role in cancer outcome. However, immune prognostic markers are not completely validated in clinical practice in BC patients.
Materials and methods
With the aim to characterize immune features, several parameters were analyzed in peripheral blood at diagnosis of 85 nonmetastatic BC patients between April 2011 and July 2014.
Results
With a median follow-up of 38.6 months, peripheral blood analysis of BC patients (stages I, II, and III) showed that total lymphocyte and T lymphocyte counts were augmented in nonrelapsed patients. Also, a higher neutrophil-to-lymphocytes ratio was associated with prolonged disease-free survival. Natural killer cell receptor analysis revealed that early activation receptor CD69 was associated with a better outcome.
Conclusion
This preliminary evidence is in accordance with the concept of immune surveillance. We suggest an “immune phenotype” that provides relevant prognostic information in early-stage BC patients and which could be useful in the decision-making process.
Keywords: breast neoplasm, prognostic factors, lymphocytes, neutrophil-to-lymphocyte ratio
Introduction
Breast cancer (BC) is a highly heterogeneous disease presenting a broad range of clinical and molecular characteristics. It is the most frequent cancer in women and the second most common cause of death.1 Patients with The Union for International Cancer Controls (UICC)-classified stage I–III disease require surgical resection with or without adjuvant chemotherapy. After definitive treatment, there is a likelihood of recurrence ranging from 10% to 40%, and this depends mainly on the underlying molecular tumor subtype.2
There is a growing body of evidence that immune response plays a significant role in cancer outcome.3 Both the innate and adaptive immune responses affect tumor growth and progression. Furthermore, the genetic instability of tumor cells contributes to tumor immune escape.4,5
In BC, tumor-infiltrating lymphocytes were found to be associated with better survival and better response to anthracycline-based chemotherapy.6 In peripheral blood (PB), other studies demonstrated an association between lower lymphocyte count and poor survival in several tumor models.7 Indeed, both PB neutrophils and lymphocytes are nonspecific acute parameters of inflammation. A higher neutrophil to lymphocyte ratio (NLR) has been associated with worse prognosis in numerous tumors.8–14 In BC, a higher NLR was associated with shorter overall survival, mostly in triple-negative BC patients.15,16
Innate immune natural killer (NK) cells, defined as CD3− CD56+ cells, are bone marrow-derived cells representing 5%–20% of circulating lymphocytes and actively participate in antiviral and antitumor responses.17 They express killer cell immunoglobulin receptors, cellular adhesion molecules, and multiple cytokine receptors (eg, receptors for IL12, IL15, and IL18).18 NK cells can produce several cytokines with antitumor effects (ie, IFNγ, TNFα, and MIP-1α) and cytolytic granules that contain perforin and granzymes.19 The function of NK cells depends on an intricate balance between activating and inhibitory receptors which can bind ligands present on target cells. Inhibitory receptors include the killer immunoglobulin-like receptors, CD94/NKG2A, and ILT2/CD85j.20 The primary activating receptors of NK cells are the natural cytotoxicity receptors (NCRs: NKp46, NKp30, NKp44, NKG2D, and DNAM-1).21 NK cells also express the FcγRIIIa (CD16) receptor, an intermediate-affinity activating receptor that recognizes the Fc region of IgG. This receptor is critical in mediating antibody-dependent cellular cytotoxicity against antibody-coated targets.22 The prognostic value of NK cells has been explored in solid tumors including BC.23,24 Peripheral NK cell counts differ among different BC phenotypes or subtypes.25 An analysis in early-stage BC of the activating NK cell receptor NKG2D ligand MIC-A/B and ULBP1-5 revealed that its expression increases relapse-free survival.26
Previously, in comparative analysis of NK cell receptor expression, we determined that most receptors were similarly expressed in healthy donors (HDs) and BC patients. However, NK cells from BC patients displayed higher dispersion in expression levels and a loss of the normal distribution typically observed for HD receptors. PB NK cells from BC patients tended to overexpress inhibitory receptors, such as killer immunoglobulin-like receptors CD158a/h, CD158b, and NKG2A, and underexpress activating receptors, such as NKp30, CD161, and DNAM-1.27 In the present study, looking for prognostic immune markers, we analyzed PB cells, including innate and adaptive populations and NK cell phenotype, from early-stage BC patients and investigated their correlation with clinical outcomes.
Materials and methods
Patients
Eighty-five BC patients treated at the Alexander Fleming Institute were recruited at diagnosis between April 2011 and July 2014. Blood samples were collected during surgery and before administration of any treatment. Patients were classified according to the American Joint Committee on Cancer staging classification or into four groups according to pathological tumor characteristics based on hormonal receptors, HER-2 overexpression, and Ki-67 index: luminal A, luminal B, Her2+, and triple negative (Table 1). All subjects gave written informed consent, and the study was approved by the Institutional Review Board (Comité de Ética en Investigación del Instituto Alexander Fleming – Disposition DI-2015-219-DGDOIN).
Table 1.
Baseline characteristics
| Characteristics | Number (%) |
|---|---|
| Number of patients | 85 |
| Follow-up (months)a | 38.6 (29.4–60.1) |
| Age (years)a | 56 (44–66) |
| Breast tumor size (cm)a | 1.7 (1.15–2.9) |
| Stage at diagnosis | |
| I | 49 (57.7) |
| II | 29 (34.1) |
| III | 7 (8.2) |
| Histologic subtypes | |
| Ductal | 67 (78.8) |
| Lobular | 12 (14.1) |
| Ductolobular | 5 (5.9) |
| Other | 1 (1.2) |
| Histologic grade | |
| 1 | 19 (22.4) |
| 2 | 45 (52.9) |
| 3 | 21 (24.7) |
| Hormonal receptors | |
| Estrogen receptora | 67 (43–88) |
| Progesterone receptora | 50 (5–70) |
| Ki67 (n:76)a | 9 (5–16) |
| High (>20) | 11 (14.5) |
| Lymphovascular invasion | 51 (59.3) |
| Tumor phenotype | |
| Luminal A | 50 (58.8) |
| Luminal B | 20 (23.5) |
| Luminal HER2 | 6 (7.1) |
| HER2 | 4 (4.7) |
| Triple negative | 5 (5.9) |
| Lymph nodes metastasis | 32 (37.6) |
| Postsurgical treatment | |
| Hormonotherapy | 70 (82.4) |
| Chemotherapy | 47 (55.3) |
Note:
Median number (interquartile range).
Complete blood cell count (CBC) and flow cytometry analysis of PB
Neutrophils, lymphocytes, and monocytes were evaluated by an automated CBC from PB samples. The number of samples differs in the various analyses. PB mononuclear cells (PBMCs) were isolated through Ficoll–Paque density gradient (GE Healthcare, Little Chalfont, UK). For T lymphocyte, NKT cell, and NK cell phenotyping, 2.0×105 PBMC were incubated with the appropriate Abs: FITC-conjugated anti-CD3 (Clone UCHT1), APC-conjugated anti-CD56 (Clone B159) and anti-CD69 (Clone FN50), PE-conjugated anti-NKG2D (Clone 1D11), anti-NKp30 (Clone p30-15), anti-NKp44 (Clone p44-8.1), anti-NKp46 (Clone 9E2/Nkp46), anti-CD16 (Clone 3G8), anti-DNAM-1 (Clone DX11), anti-CD94 (Clone HP-3D9), anti-CD161 (Clone DX12), anti-CD158a (Clone HP-3E4), anti-CD158b (Clone CH-L), and anti-CD85j (Clone GHI/75) (all purchased at BD PharMingen, Franklin Lakes, NJ, USA) or PE-conjugated anti-NKG2A (Clone131411) (R&D Systems, Minneapolis, MN, USA) for 30 min at 4°C. The percentage of T lymphocyte, NKT cell, and NK cell population was selected based on the CD3 and CD56 expression of gated lymphocytes, according to forward-side scatter, as indicated in Figure S1. The total number of cells of these populations was extrapolated combining CBC and flow cytometry data. All samples were acquired on a BD FACS Calibur using Cellquest Pro software (BD Biosciences) and analyzed with FlowJo 7.6.2 software (Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
Comparisons of continuous variables between clinical groups (relapsed and nonrelapsed) were performed using Wilcoxon rank sum test or Student’s t-test depending on sample distribution. χ2 test was used to estimate the association between categorical variables. Uni- and multivariate analyses were carried out to assess the effect of prognostic variables on relapse. Disease-free survival (DFS) was defined as the interval between the date of surgery of BC to the first locoregional failure and/or distant relapse, second invasive primary, or death. Kaplan–Meier graphs were used to estimate DFS, and univariate differences in DFS were compared with log rank test. In all figures, data are represented using the median ± interquartile range. Reported p-values are two-tailed, and p<0.05 was considered significant. Statistical analysis was performed using Stata14 (StataCorp LLC, College Station, TX, USA) and GraphPad v5.00 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patient characteristics and outcome
Patients were enrolled between April 2011 and July 2014. Eighty-five patients fulfilled the eligibility criteria as described in Table 1. Univariate analysis showed that the clinicopathological factors associated with recurrence were positive lymph nodes (p=0.014), lymphovascular invasion (LVI) (p=0.007), and TNM stage (p=0.024) (Table 2).
Table 2.
Univariate analysis of clinical and pathological characteristics associated with recurrence
| Characteristic | RR (95% CI) | p-value |
|---|---|---|
| Positive axillary lymph nodes | 3.53 (1.19–10.43) | 0.014 |
| Lymphovascular invasion | 8.25 (1.12–60.76) | 0.007 |
| TNM stage (2–3 vs 1) | 3.23 (1.09–9.60) | 0.024 |
| Tumor size (≤2 cm vs >2 cm) | 2.08 (0.78–5.59) | 0.14 |
| Histologic grade (3 vs 1–2) | 1.78 (0.66–4.82) | 0.26 |
| Luminal phenotype | 1.33 (0.33–5.38) | 0.68 |
| Ki67 (≥20% vs <20%) | 1.81 (0.56–5.85) | 0.39 |
Abbreviations: CI, confidence interval; RR, relative risk.
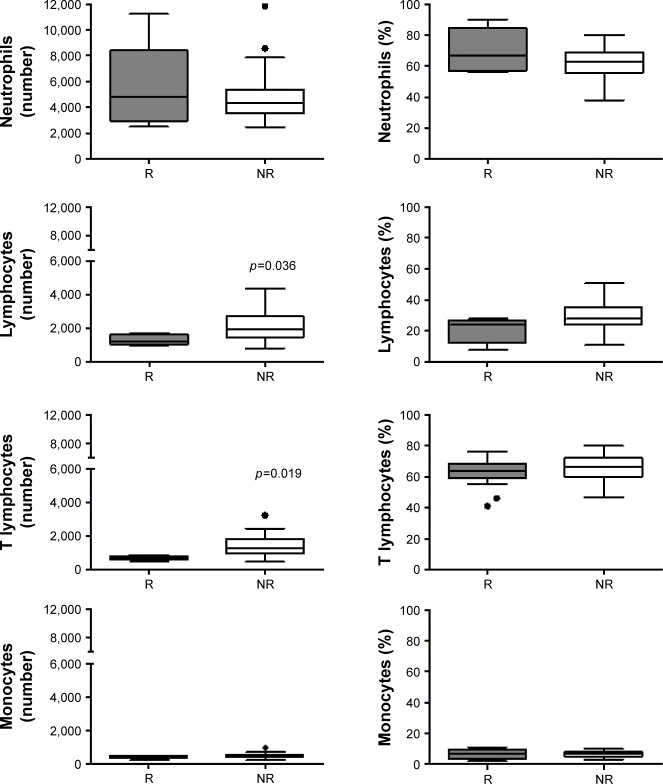
Nonrelapsed patients present higher total lymphocyte and T lymphocytes number
CBC was performed in only 41 patients. As is shown in Figure 1, nonrelapsed (NR) patients had a higher absolute count of total lymphocytes than relapsed (R) patients (R median 1,204 vs NR median 1,948; p=0.036), but not a higher percentage. Moreover, T lymphocyte absolute count, not frequency, was also augmented in NR patients (total T number: R median 713 vs NR median 1,268; p=0.019). In contrast, there was no difference between NR and R patients in leukocyte (not shown), neutrophil, and monocyte absolute counts or proportion (Figure 1).
Figure 1.
Total lymphocytes and T lymphocytes differences in R and NR patients.
Notes: Bars show median PB cells absolute number (upper panel) and percentage (lower panel), from R (gray) and NR (white) patients. Wilcoxon rank sum test was performed. Significant p-values are shown in the graph.
Abbreviations: NR, nonrelapsed; PB, peripheral blood; R, relapsed.
Decreased NLR is associated with prolonged DFS
Pretreatment NLR association with clinical and pathological variables was evaluated first. No relation was found with age (p=0.84), tumor size (p=0.06), stage (p=0.11), lymph node status (p=0.51), tumor grade (p=0.56), Ki-67 (p=0.24), or LVI (p=0.97). We performed ROC analysis (100% sensibility and 45% specificity, AUC 0.71), and classified patients’ NLR as high (≥2) or low (<2). Patients with a NLR ≥2 presented a significantly lower DFS (5-year DFS: 100% vs 69.55%, p=0.048; Figure 2) and a tendency toward higher recurrence risk (p=0.058; data not shown).
Figure 2.
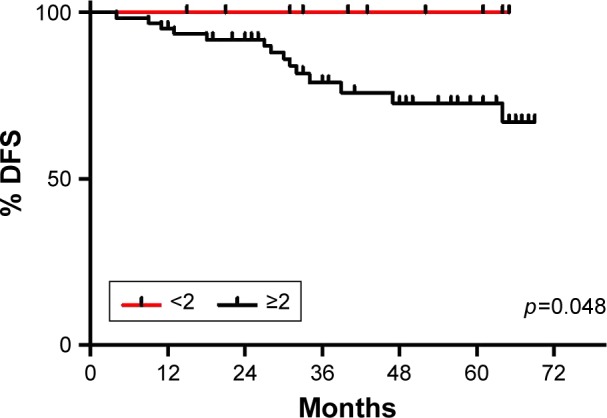
NLR association with DFS.
Notes: NLR variable was dichotomized as high (≥2) or low (<2). Patients with a NLR ≥2 presented a significantly lower DFS (5-year DFS: 100% vs 69.55%, p=0.048) and a tendency toward higher recurrence risk (p=0.058). Significant p-value is shown in the graph. Low NLR is depicted with a red line and high NLR with a black line.
Abbreviations: DFS, disease-free survival; NLR, neutrophil to lymphocyte ratio.
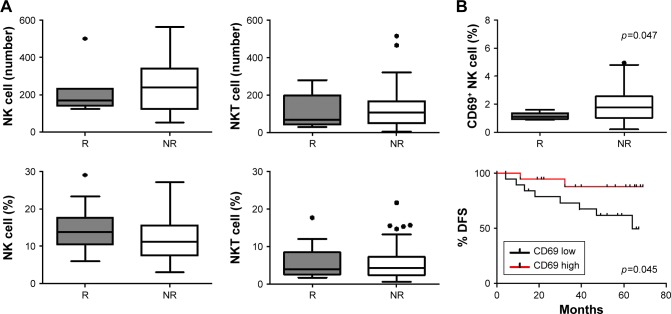
NK cell expression of activation receptor CD69 is associated with recurrence and longer DFS
NK cell population was studied specifically because, previously, in the same study population we observed that there was a significantly higher proportion of NK cells in BC PBMC compared to HD.27 Absolute count and relative frequency of NK and NKT cells did not differ between NR and R patients (Figure 3A).
Figure 3.
NK cells analysis in R and NR patients.
Notes: (A) Bars show median NK cells and NKT cells absolute number (up) and percentage (down) from R (gray) and NR (white) patients. There is no difference between R and NR patients. (B) Patients were classified as CD69 “high” or “low” expression in relation to population median (1.7%). NK cells from R patients expressed lower CD69 than NR (p=0.047). This association was also reflected in DFS (5-year DFS high 87.70% vs low 63.75%, p=0.045). χ2 and log rank test were performed, respectively. Significant p-value is shown in the graph. In Kaplan–Meier curve, CD69 high was depicted with a red line and CD69 low with a black one.
Abbreviations: DFS, disease-free survival; NK, natural killer; NR, nonrelapsed; R, relapsed.
To evaluate the NK cell phenotype, we analyzed the expression of both activating and inhibitory receptors, including NKG2D, DNAM-1, NKp30, NKp44, NKp46, and CD69, and CD161, CD94, NKG2A, and CD85J, respectively. Patients were classified as “high” or “low” if the NK cell receptor expression analyzed was above or below the median value of the study population. The only receptor that associated with prognostic variables was activation marker CD69. We found that PB NK cells from R patients expressed lower levels of CD69 than NR patients (p=0.047; Figures 3B and S2). This association was also reflected in a prolonged DFS in patients with high expression of the CD69 receptor (5-year DFS high 87.70% vs low 63.75%, p=0.045; Figure 3B).
Discussion
Individual risk of tumor recurrence differs considerably between patients with early-stage BC. Several intrinsic patho-logic and molecular tumor factors have been described as being prognostic and/or predictive.28 Despite the importance of the immune system in tumor surveillance, immunological parameters are not routinely used during the therapeutic decision-making process. Here, we described immunological characteristics associated with higher recurrence risk in early-stage BC patients. The importance of determining immune markers relies on the fact that in early-stage BC, administration of adjuvant treatment depends on the predicted recurrence risk. Characteristic pathologic parameters used in the decision-making process are lymph node involvement, histologic grade, tumor size, LVI, estrogen and progesterone receptor expression, Her2 overexpression, and Ki-67 index.2 Molecular platforms such as Oncotype® (Genomic Health, Inc., Redwood City, CA, USA) or Mammaprint® (Agendia, Amsterdam, the Netherlands) are used in selected cases to evaluate the likelihood of recurrence more accurately.29 In our study population, we found axillary lymph node involvement, TNM stages, and LVI to be associated with tumor recurrence.
The importance of immune surveillance in determining the prognosis of various types of cancers is increasingly recognized, but the mechanisms underlying the immune defects noticed in cancer patients have not been fully elucidated. The innate and adaptive immune responses modulate tumor growth and progression.4 To assess the prognostic impact of PB cells, we performed CBC. In this study, we determined that NR patients showed a higher absolute count of total lymphocytes, augmented total T lymphocytes number, and a lower NLR than NR patients. These observations could be evidence of adaptive immune surveillance in BC patients’ PB. On the other hand, we did not find differences in the total numbers of leukocytes, neutrophils, or monocytes. The prognostic impact of NLR in neoplastic disease has been shown previously in several tumor models.8–14 Consistent with prior evidence, we found that high NLR was associated with higher recurrence and a lower DFS.
The association between high NLR and poor prognosis is probably complex and largely unclear, but, as a multifactorial process, there are several possible explanations. The mechanism that links tumor and host biology remains uncertain. However, recent studies have proposed potential mediators linking the tumor and host interaction (deeply reviewed by Guthrie et al8). In this sense, a high NLR may reflect the key role of systemic inflammation in enhancing angiogenesis, tumor growth, and development of metastasis. Important associations between the NLR and other markers of the systemic inflammatory response were described in patients with operable cancer, in particular with elevated C-reactive protein and hypoalbuminemia. Also, several studies have undertaken measurements of circulating cytokines together with the NLR.30,31
Neutrophils may inhibit the immune system, by suppressing the cytolytic activity of lymphocytes, NK cells, and activated T-cells.32 On the other hand, a low lymphocyte count has been associated with poor outcomes in patients with advanced cancer attributed immunity, with the destruction of host cancer cells.33 It is plausible that host cell-mediated immunity continues to exert important effects on the destruction of any residual tumor cells and micrometastases.34 Tumor infiltration by lymphocytes has been reported to indicate the generation of an effective antitumor cellular immune response and increased lymphocyte infiltration correlated with a better prognosis.32
Particularly in BC, Noh et al16 found that patients with an elevated pretreatment NLR showed poorer disease-specific survival than patients without elevated NLR, most evident in the luminal A subtype. More recently, a significant correlation between high NLR and worse prognosis in Caucasian patients with early BC was characterized by Orditura et al.35 Moreover, low NLR may indicate high efficacy and favorable outcome after neoadjuvant chemotherapy in patients with triple-negative BC.36
Our NLR cutoff value (≥2) was calculated using a ROC analysis. We consider that ROC analysis is the most appropriate approach, and it is in agreement with the value of 1.97 used by Orditura et al.35 Two variables could influence the relative low NLR cutoff value compared with Asano et al36 and Noh et al.16 First, the low proportion of triple-negative BC patients included in our study population, and second, the Latin American ethnic composition that has not been previously evaluated.
Human NK cells exert effector functions such as cytotoxic activity and cytokine production in antiviral and antitumor responses.17 A pioneering study in human populations reported that medium and high cytotoxic activity of PB lymphocytes is associated with reduced cancer risk, whereas low activity is linked to increased cancer risk.37 Since then, the prognostic value of NK cells has been explored in solid tumors.23 A high count of CD56+NK cells in prostate cancer after androgen deprivation therapy was associated with a good prognosis,38 and there was an inverse correlation between the density of CD56+NK cells and seminal vesicle invasion.23 Even though we previously observed that there were a significantly higher proportion of NK cells in BC PBMC as compared to HD,27 we did not find any association between the relative number of NK cells and patients’ prognosis. Moreover, we did not find differences in absolute numbers in NKT population between R and NR patients. We also analyzed a panel of NK cell receptors finding no trait associations with recurrence, except for CD69. These were striking results because our and other researchers’ data did find an association between NK cell receptor expression and patients’ outcome. NKp30 status was described as a simple and early prognostic biomarker that identifies intermediate-risk patients with poor prognosis in patients with intermediate-risk acute myeloid leukemia.39 Recently, we also described that NKp46 receptor expression correlated with relapse-free survival of CRC patients upon a maximum follow-up of 71 months.40 CD69 is not usually expressed on resting cells, but it is one of the earliest activation markers expressed on the cell surface of T lymphocytes.41 CD69 is rapidly induced on NK and other hemopoietic cells in response to cytokines or other activating stimuli42,43 and has been shown to induce cytotoxic activity and costimulate cytokine production of activated NK cells and T-cell clones.41,44,45 It thus represents one of the receptors that endow activated NK cells with new recognition capability in the context of natural killing activity.41,44,46–48
It has been described that CD69-mediated NK cytotoxicity can be abrogated by the CD94 inhibitory receptor.49 In our data set, there is no correlation between the expression of CD69 and CD94 (Pearson -0.19). There is also no difference between the CD94 mean expression between NK cells with CD69 high or low. Patients with high expression of CD69 did not present more recurrence if CD94 expression was high (data not shown).
An increase in CD69 may represent an immunoreactive phenotype. In fact, the expression is accompanied by an enhanced cytotoxicity against various target cells.50 Moreover, it was reported in patients with BC and ovarian cancer that the increase of CD69 after immunotherapy was associated with better survival.51 Thus, our results revealed that patients with higher CD69 expression had longer DFS compared to patients with lower receptor expression. However, further validation work and feasibility study are required before the results of this study can be considered for clinical use.
It has been broadly described how tumors act systemically to sustain cancer progression, affecting physiological processes in the host and triggering responses in the PB cells.52 The PB cells monitor the body’s physiological status and modify their immune phenotype in response to pathological changes.53,54 In particular, immune cell dysfunctions have been found in BC patients.55 This present study showed that high NLR is a prognostic factor for DFS in early-stage BC patients using easily accessible PB samples. This finding is explained, at least partially, by a higher number and proportion of total and T lymphocytes. Furthermore, an activated NK cell phenotype was correlated with a longer DFS interval. Further prospective studies are therefore warranted to confirm these preliminary results and to investigate the correlations between BC characteristics and NLR and NK cell phenotype.
Supplementary materials
Gating strategy.
Notes: The percentage of T lymphocyte, NKT cell, and NK cell population were selected based on the CD3 and CD56 expression of gated lymphocytes, according to forward-side scatter (A) and CD45 expression (B). The NK cell phenotype was analyzed on CD3−CD56+ population (C). As an example, this graph shows the NK-CD16+ cells (D).
Abbreviations: FSC, forward-scatter; NK, natural killer; SSC, side-scatter.
CD69 density plots.
Notes: Representative CD69 density plots of patients with CD69low (C) and CD69high (D), evaluated on CD3−CD56+ (NK cells) population. Isotype controls are depicted in (A) and (B).
Abbreviation: NK, natural killer.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Peto R, Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017;7:156. doi: 10.3389/fonc.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil–lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Gomez D, Farid S, Malik H, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32(8):1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 10.Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13(3):170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 11.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31(9):2995–2998. [PubMed] [Google Scholar]
- 12.Lee YY, Choi CH, Kim HJ, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32(4):1555–1561. [PubMed] [Google Scholar]
- 13.Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187(2):411–417. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16(3):614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 15.Pistelli M, De Lisa M, Ballatore Z, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15(1):195. doi: 10.1186/s12885-015-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16(1):55–59. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Zamai L, Ponti C, Mirandola P, et al. NK cells and cancer. J Immunol. 2007;178(7):4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 19.Vivier E, Ugolini S. Natural killer cells: from basic research to treatments. Front Immunol. 2011;2:18. doi: 10.3389/fimmu.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narni-Mancinelli E, Ugolini S, Vivier E. Tuning the threshold of natural killer cell responses. Curr Opin Immunol. 2013;25(1):53–58. doi: 10.1016/j.coi.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214(1):73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochoa MC, Minute L, Rodriguez I, et al. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95(4):347–355. doi: 10.1038/icb.2017.6. [DOI] [PubMed] [Google Scholar]
- 23.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348(1–2):9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Roberti MP, Rocca YS, Amat M, et al. IL-2-or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res Treat. 2012;136(3):659–671. doi: 10.1007/s10549-012-2287-y. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Xu L, Lin Q, et al. Levels of lymphocyte subsets in peripheral blood prior treatment are associated with aggressive breast cancer phenotypes or subtypes. Med Oncol. 2014;31(6):981. doi: 10.1007/s12032-014-0981-9. [DOI] [PubMed] [Google Scholar]
- 26.de Kruijf EM, Sajet A, van Nes JG, et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer. 2012;12(1):24. doi: 10.1186/1471-2407-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberti MP, Juliá EP, Rocca YS, et al. Overexpression of CD85j in TNBC patients inhibits cetuximab-mediated NK-cell ADCC but can be restored with CD85j functional blockade. Eur J Immunol. 2015;45(5):1560–1569. doi: 10.1002/eji.201445353. [DOI] [PubMed] [Google Scholar]
- 28.Colzani E, Liljegren A, Johansson AL, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29(30):4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 29.Krop I, Ismaila N, Andre F, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical oncology Clinical Practice Guideline Focused Update. J Clin Oncol. 2017;35(24):2838–2847. doi: 10.1200/JCO.2017.74.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motomura T, Shirabe K, Mano Y, et al. Neutrophil–lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepat. 2013;58(1):58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Kantola T, Klintrup K, Väyrynen J, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73(3–4):215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 33.Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 34.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Orditura M, Galizia G, Diana A, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open. 2016;1(2):e000038. doi: 10.1136/esmoopen-2016-000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. 2016;23(4):1104–1110. doi: 10.1245/s10434-015-4934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 38.Pasero C, Gravis G, Granjeaud S, et al. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6(16):14360–14373. doi: 10.18632/oncotarget.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chretien A-S, Fauriat C, Orlanducci F, et al. NKp30 expression is a prognostic immune biomarker for stratification of patients with intermediate-risk acute myeloid leukemia. Oncotarget. 2017;8(30):49548–49563. doi: 10.18632/oncotarget.17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocca YS, Roberti MP, Juliá EP, et al. Phenotypic and functional dysregulated blood NK cells in colorectal cancer patients can be activated by cetuximab plus IL-2 or IL-15. Front Immunol. 2016;7:413. doi: 10.3389/fimmu.2016.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moretta A, Poggi A, Pende D, et al. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor α/β. J Exp Med. 1991;174(6):1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15(10):479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 43.Lanier L, Buck DW, Rhodes L, et al. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988;167(5):1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zingoni A, Palmieri G, Morrone S, et al. CD69-triggered ERK activation and functions are negatively regulated by CD94/NKG2-A inhibitory receptor. Eur J Immunol. 2000;30(2):644–651. doi: 10.1002/1521-4141(200002)30:2<644::AID-IMMU644>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Santis AG, Campanero MR, Alonso JL, et al. Tumor necrosis factor-α production induced in T lymphocytes through the AIM/CD69 activation pathway. Eur J Immunol. 1992;22(5):1253–1259. doi: 10.1002/eji.1830220521. [DOI] [PubMed] [Google Scholar]
- 46.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19(1):197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 47.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40–CD40 ligand interaction. J Exp Med. 1997;185(12):2053–2060. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson JL, Charo J, Martín-Fontecha A, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immun. 1999;163(8):4207–4212. [PubMed] [Google Scholar]
- 49.Borrego F, Robertson MJ, Ritz J, Pena J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97(1):159–165. doi: 10.1046/j.1365-2567.1999.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207(2):85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 51.Yacyshyn MBB, Poppema S, Berg A, et al. CD69+ and HLA-DR+ activation antigens on peripheral blood lymphocyte populations in metastatic breast and ovarian cancer patients: correlations with survival following active specific immunotherapy. Int J Cancer. 1995;61(4):470–474. doi: 10.1002/ijc.2910610407. [DOI] [PubMed] [Google Scholar]
- 52.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Ann Rev Immunol. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 53.Verma C, Eremin JM, Robins A, et al. Abnormal T regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived suppressor cells (MDSCs: monocytic, granulocytic) and polarised T helper cell profiles (Th1, Th2, Th17) in women with large and locally advanced breast cancers undergoing neoadjuvant chemotherapy (NAC) and surgery: failure of abolition of abnormal treg profile with treatment and correlation of treg levels with pathological response to NAC. J Transl Med. 2013;11(1):16. doi: 10.1186/1479-5876-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91(2):163–171. doi: 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
- 55.Verronèse E, Delgado A, Valladeau-Guilemond J, et al. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. Oncoimmunology. 2016;5(3):e1100791. doi: 10.1080/2162402X.2015.1100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy.
Notes: The percentage of T lymphocyte, NKT cell, and NK cell population were selected based on the CD3 and CD56 expression of gated lymphocytes, according to forward-side scatter (A) and CD45 expression (B). The NK cell phenotype was analyzed on CD3−CD56+ population (C). As an example, this graph shows the NK-CD16+ cells (D).
Abbreviations: FSC, forward-scatter; NK, natural killer; SSC, side-scatter.
CD69 density plots.
Notes: Representative CD69 density plots of patients with CD69low (C) and CD69high (D), evaluated on CD3−CD56+ (NK cells) population. Isotype controls are depicted in (A) and (B).
Abbreviation: NK, natural killer.