Abstract
Objective
To examine the relationship between lower-extremity muscle cross-sectional area, muscle strength, specific torque, and age in ambulatory boys with Duchenne muscular dystrophy (DMD) compared with controls.
Design
Observational cross-sectional study.
Setting
University research setting.
Participants
Volunteer sample of boys with DMD (n = 22) and healthy control boys (n = 10), ages 5 through 14 years.
Interventions
Not applicable.
Main Outcome Measures
Maximal muscle cross-sectional area (CSAmax) assessed by magnetic resonance imaging of quadriceps, plantarflexors (PFs) and dorsiflexors (DFs), peak isometric torque from dynamometry, and timed functional tests.
Results
The average CSAmax of the triceps surae muscle group was approximately 60% higher in boys with DMD compared with controls (39.1 ± 13.6 cm2 vs 24.5±9.3 cm2; P = .002), while the tibialis anterior muscle showed age-appropriate increases in CSAmax. The increase in quadriceps CSAmax was also distinctly different in boys with DMD compared with controls. Specific torque (ie, peak torque/CSAmax) was impaired in all 3 muscles groups, with the knee extensor (KE) and PF muscles showing 4-fold, and the DF muscles 2-fold, higher values in controls compared with boys with DMD. Large age-related gains in specific torque were observed in all 3 muscle groups of control subjects, which were absent in ambulatory boys with DMD. Correlations were observed between performance on functional tasks and quadriceps and PF torque production (r= −.45 to − 57, P < .05), but not with DF strength.
Conclusions
Age-related changes in muscle cross-sectional area and specific torque production in lower-extremity muscles showed distinctly different patterns in the KE, PF, and DF muscles of boys with DMD compared with controls.
Keywords: Magnetic resonance imaging, Muscular dystrophy, Duchenne, Muscle strength, Rehabilitation
Duchenne muscular dystrophy is an X-linked recessive disease affecting approximately 1 in 3500 newborn boys.1 This condition occurs from a mutation in the gene coding for the sarcolemmal protein dystrophin, which connects the myofilaments to the muscle cell membrane.2 In DMD, the lack of functional dystrophin results in an increased susceptibility to contraction-induced muscle damage.3 The muscles of boys with DMD undergo repeated cycles of damage, inflammation, and repair, which ultimately leads to a loss of contractile tissue and progressive muscle weakness.2
Boys with DMD experience profound muscle weakness and functional limitations as early as ages 3 to 5 years.4 The progressive decline in muscle function is first observed in the proximal muscle groups (eg, shoulder girdle, hips), with distal muscle groups being affected at a later age.5–7 Previous studies examining age-related changes in function in boys with DMD have used the MMT,7 and this method has also been used in clinical trials.8–10 However, the MMT has been criticized for having limited reliability,11,12 accuracy,13 and sensitivity;13 therefore, other muscle testing methods such as dynamometry are preferred for assessing muscle strength.
A hallmark of muscle quality is the amount of force a muscle can produce per unit of muscle mass.14 A recent study on boys with DMD showed only low to moderate correlations (r = .164 –.514) between regional lean body mass measured using DXA and muscle strength, indicating poor muscle quality in this population.15 The authors noted that a limitation of their study was that DXA could not be used to quantify the size of specific muscle groups but only provided an estimate of regional lean body mass.15
The CSA of individual muscles or muscle groups can be specifically quantified using MRI, and this technique has been applied in previous studies examining muscle atrophy after disuse16 and spinal cord injury17,18 and in other chronic conditions.19–21 Unlike DXA and bioelectric impedance, which provide measurements of body composition (ie, lean body mass, fat mass), MRI can be used to quantify the CSA of individual muscles. Previous MRI studies in muscular dystrophy have focused on describing patterns of fatty-tissue infiltration;5,22 however, age-related changes in the CSA of specific muscles and their relationship to muscle force output have not been evaluated.
Therefore, the primary objectives of this study were to (1) quantify the CSA and torque production of primary lower-extremity muscles in ambulatory boys with DMD; (2) examine the relationship of age with muscle strength, CSA, and specific torque in boys with DMD and compare these relationships with controls; and (3) examine the relationship of lower-limb muscle strength with functional ability in boys with DMD. We hypothesized that all lower-limb muscles in boys with DMD would have a larger CSA but lower torque production compared with healthy controls. In addition, we hypothesized that the slope of increase in muscle CSA and torque production versus age would be lower in boys with DMD compared with controls. Last, we hypothesized that torque production of the lower-extremity muscles would be related to performance on functional tasks such as walking and transfers in boys with DMD.
METHODS
Participants
Twenty-two boys with DMD and 10 healthy boys from the general population (controls) aged 5 to 14 years old volunteered to participate in the study. A report confirming the diagnosis of DMD using molecular genetic testing (eg, polymerase chain reaction amplification) and/or immunohistochemical staining of muscle biopsy was obtained from the subject’s pediatric neurologist. All DMD participants were ambulatory and were being treated with corticosteroids (either prednisone or deflazacort). The study was approved by the institutional review board at the University of Florida. Written informed consent was obtained from the participants’ parents, and written assent was obtained from each participant.
Each subject’s height and weight were measured in light clothing with shoes off.
Isometric Muscle Strength Testing
Isometric peak torque of the KEs, PFs, and DFs of the right leg was measured using a Biodexa dynamometer. For KE testing, the knee and hip joints were placed at 90° of flexion. For PFs and DFs, the knee was placed between 0° to 10° of flexion and the ankle was placed in neutral (for PFs) or 30° of plantarflexion (for DFs). The subject was instructed to push or pull as hard as possible for 5 seconds, followed by a 2-minute rest. Five trials were performed for each muscle group, and the highest torque value was used for analysis (peak torque). One subject with DMD was unable to perform the dorsiflexion contraction. This protocol was tested for between-day reliability (2-month interval) by the same tester, in a subset of children with DMD (n = 6) and controls (n = 10). ICCs2,1 were calculated for both groups of subjects. High reliability was found for all 3 muscle groups (KEs: ICC = .89 in DMD and .99 in controls, PFs: ICC = .88 in DMD and .98 in controls, and DFs: ICC = .87 in DMD and .95 in controls [P < .001 for all ICCs]).
Because the body size of boys between the ages of 5 and 14 years varies considerably, peak torque was normalized to BSA. BSA is widely used as a biometric unit to normalize physiologic parameters. Hence for each of the functional muscle groups, torque normalized to BSA (ie, normalized torque; units = N*m/m2) was reported. BSA (m2) was calculated using the equation developed by Gehan and George23 and modified by Mosteller24:
Muscle CSA
MRI was performed on either a 1.5T (Signab) or 3.0T (Achievac) whole-body scanner. Subjects were placed in a supine position with their lower leg positioned in either a lower-extremity quadrature coil (1.5T) or an 8-channel sensitivity encoding, receive-only extremity coil (3.0T) for lower-leg imaging or a flexible surface coil for thigh imaging. Thigh imaging was not completed in 2 of the control subjects and 2 of the boys with DMD. Three-dimensional transaxial fat-suppressed gradient echo images were acquired with the following parameters: repetition time = 24ms, echo time = 1.8ms, flip angle = 20°, and optimized field of view (calf: 12–14cm2, thigh: 18cm2).
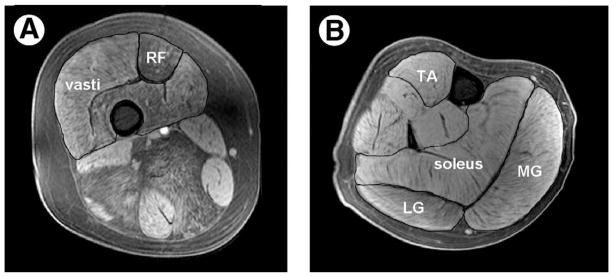
Individual muscles (quadriceps, soleus, medial, and lateral gastrocnemius and tibialis anterior) were manually outlined using OsiriXd, an open-source software (fig 1).25 To determine the CSAmax of a muscle, the axial image with the largest CSA in the series was identified and the mean of 3 consecutive images (ie, the slice with the greatest CSA and the immediate proximal and distal slices) was used to obtain the value for CSAmax. The CSAmax of the triceps surae muscle group was taken as the sum of the individual CSAmax of the soleus, medial, and lateral gastrocnemius muscles. The CSAmax of the quadriceps was taken as the sum of the CSAmax of the 3 vasti together and the rectus femoris.
Fig 1.
T1-weighted fat-suppressed transaxial images of the thigh (A) and lower leg (B) obtained from an 11-year-old boy with DMD. The rectus femoris and vasti muscles are outlined on the thigh, and the TA, soleus, MG, and LG are outlined on the lower leg. Note the presence of fatty infiltration to a greater extent in the thigh musculature than in the calf. Abbreviations: LG, lateral gastrocnemius; MG, medial gastrocnemius; RF, rectus femoris; TA, tibialis anterior.
Specific Torque
The force-generating capacity of the muscle per unit area (ie, specific torque; units = Nm/cm2) was calculated by dividing the isometric peak torque by the CSAmax, measured by MRI. KE peak torque was divided by quadriceps CSAmax, while the PF torque was divided by the triceps surae CSAmax and DF torque by the tibialis anterior CSAmax.
Functional Ability
Subjects performed 4 timed functional tasks, in the following order: time to rise from the floor, rise from a chair, walk 9m and ascend 4 stairs.6 The subject was given rest breaks between tasks to minimize the effects of fatigue. Subjects performed each task 3 times, and the best time was used for analysis. The functional ability of the DMD subjects was ranked using the Vignos Lower Extremity Functional Scale.26 This scale ranges from grade 1 (able to walk and climb stairs independently) to grade 10 (confined to bed).
Statistical Analysis
Data were analyzed in SPSSe (version 11.0), and are described using means and SDs. To examine differences in muscle strength, muscle CSAmax, and specific torque between boys with DMD and controls, independent sample t tests were used after testing for equality of variance (Levene’s test). The difference between groups in the slopes of the relationships between age and muscle strength, muscle CSAmax, and specific torque was analyzed using analysis of covariance. The level of significance for between-group comparisons was set at an alpha of .05 and adjusted for multiple comparisons using the modified Bonferroni correction.27 To examine the relationships between functional test times and muscle strength, bivariate linear correlations were made using Pearson product moment correlations (α = .05).
RESULTS
Boys with DMD were shorter in stature and had a higher body mass index than controls (table 1); however, BSA was similar between groups. No significant differences were noted for age between the 2 groups. The lower-extremity Vignos score ranged from grade 1 (independence in walking and climbing stairs) to grade 4 (walks unassisted and rises from chair but cannot climb stairs) in boys with DMD.
Table 1.
Demographics of the Participants
| Characteristic | DMD (n = 22) | Controls (n = 10) | P |
|---|---|---|---|
| Age (y) | 9.6 ± 2.7 | 9.7 ± 3.0 | .480 |
| Height (m) | 1.20 ± 0.13 | 1.40 ± 0.18 | .002* |
| Weight (kg) | 31.3 ± 11.2 | 33.1 ± 11.6 | .670 |
| BMI (kg/m2) | 20.7 ± 5.3 | 16.3 ± 1.7 | .002* |
| BSA (m2) | 1.02 ± 0.23 | 1.15 ± 0.25 | .160 |
| Vignos score (median) | 1.5 (1–4) | 1.0 | NA |
NOTE: Values are mean ± SD or as otherwise indicated.
Abbreviation: NA, not applicable.
Significantly different between groups.
Muscle Strength
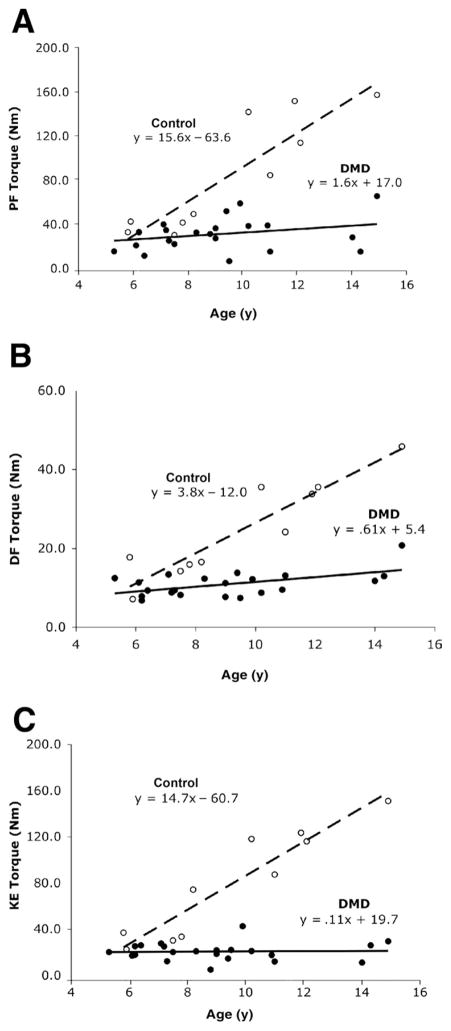
Peak torques of the PFs, DFs, and KEs were significantly lower in boys with DMD compared with controls (PFs: 31.3 ± 13.8Nm vs 84.9 ± 52.1Nm; DFs: 10.9 ± 3.2Nm vs 24.6 ± 12.3Nm; KEs: 20.5 ± 7.9Nm vs 79.5 ± 46.6Nm in boys with DMD and controls, respectively; P < .001). The slopes of peak torque versus age (fig 2) for all 3 muscle groups were also significantly different between boys with DMD and controls (P < .001). The difference in slopes between the DMD and control groups are the result of a considerable gain in muscle strength as a function of age in the control group, which was largely absent in boys with DMD. In the control group, peak torque was 3 to 4 times greater in all muscle groups across the age range. In boys with DMD, the PFs and DFs showed only a slight increase in peak torque with age, while KE peak torque did not vary with age.
Fig 2.
Scatter plots of age versus peak torque (Nm) in boys with DMD (filled circles, solid line) and controls (open circles, dashed line): (A) PFs (n = 22 DMD, n = 10 control), (B) DFs (n = 21 DMD, n = 10 control), and (C) KEs (n = 22 DMD, n = 10 control). Please note that in A, 2 subjects with DMD had overlapping values for plantarflexion peak torque (age 6.2y, 33.2Nm and 33.4Nm); in B, 1 subject with DMD was unable to perform the dorsiflexion torque measurement.
The difference in peak torque between groups was also examined after normalization to body size, using BSA which incorporates the age, height, and weight of the child.23,24 Normalized peak torque of the KE and PF was 3 times higher in controls than boys with DMD (KE: 64.2 ± 27.3Nm/m2 vs 20.9 ± 7.8Nm/m2, P < .0021; PFs: 68.6 ± 30.2Nm/m2 vs 20.9 ± 7.8Nm/m2, P< .001). Normalized peak torque of the DFs was twice as high in controls as DMD subjects (DFs: 20.4 ± 6.4Nm/m2 vs 10.8 ± 2.6Nm/m2, respectively; P < .001). As expected, normalized torque in controls showed an increase with age (slopes = .08 – 8.6), while a negative slope was found in the boys with DMD in all 3 muscle groups (slopes = −.22 to −1.4).
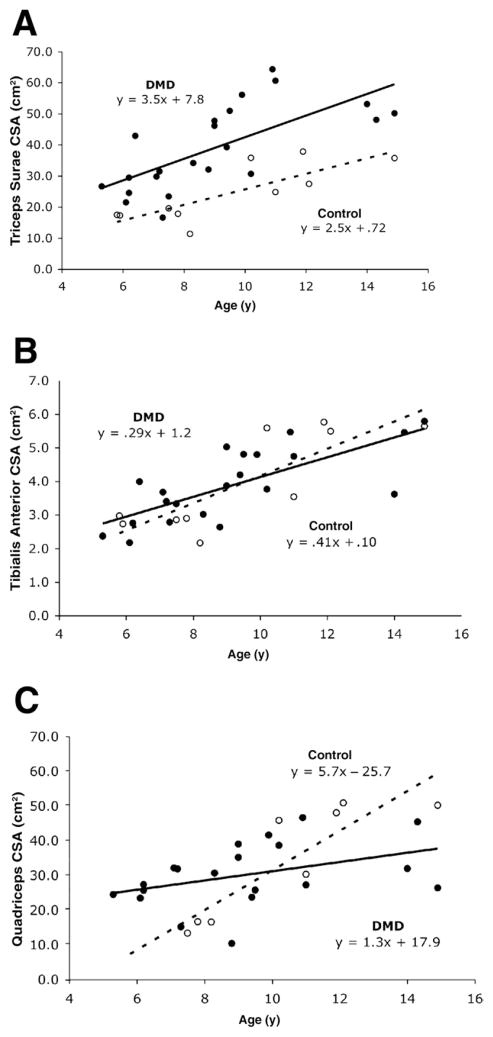
Maximal CSA
The average CSAmax of the triceps surae muscle group was about 60% higher in boys with DMD compared with controls (39.1 ± 13.6cm2 vs 24.5 ± 9.3cm2; P < .002). In contrast, no significant difference was found between groups in the CSAmax of the tibialis anterior muscle (3.8 ± 1.1cm2 vs 4.0 ± 1.5cm2, P < .78). Furthermore, the slopes of lines expressing muscle CSAmax versus age for the triceps surae and tibialis anterior were not significantly different between boys with DMD and controls (P< .44 and .31, respectively; fig 3A and B). However, there was a significant difference in slope for the CSAmax of the quadriceps muscle group between boys with DMD and controls (P< .006; fig 3C). The CSAmax of the quadriceps appeared larger in the boys with DMD under the age of 10 years compared with controls. However, in subjects aged 11 years and older, boys with DMD tended to have a smaller CSAmax than controls (see fig 3C).
Fig 3.
Scatter plots of CSAmax (cm2) versus age in boys with DMD (filled circles, solid line) and controls (open circles, dashed line): (A) triceps surae (n = 22 DMD, n = 10 control), (B) tibialis anterior (n = 22 DMD, n = 10 control), and (C) quadriceps (n = 20 DMD, n = 8 control). Please note that in B, 2 subjects with DMD had overlapping values for tibialis anterior CSAmax (age 6.2y, 2.7cm 2 and 2.8cm2).
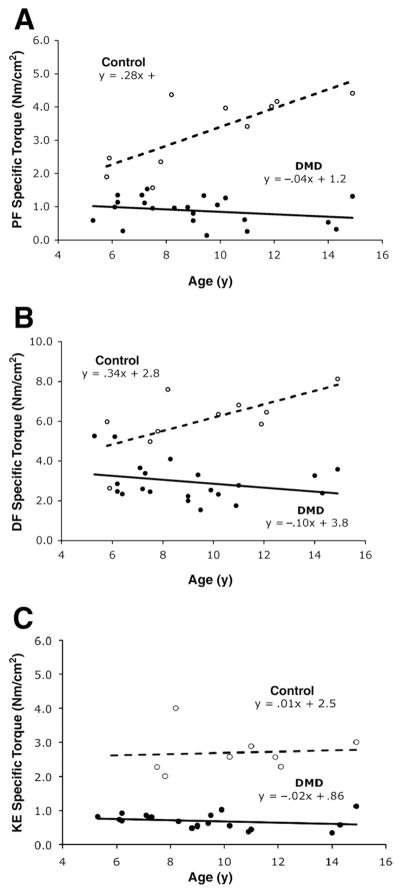
Specific Torque
Specific torque (ie, peak torque normalized to muscle CSAmax) was significantly different between groups for the PFs (.88 ± .41Nm/cm2 in DMD vs 3.26 ± 1.08Nm/cm2 in controls; P< .001), DFs (2.95 ±.99Nm/cm2 in DMD vs 6.03 ± 1.52Nm/cm2 in controls, P< .001), and KEs (.69 ±.21Nm/cm2 in DMD vs 2.76 ±.77Nm/cm2, P< .001). The specific torque of the KEs and PFs was approximately 4 times as high in the controls compared with boys with DMD. In contrast, a 2-fold difference was found in the DFs. The change in specific torque with age is shown in figure 4. A significant difference in slope was observed between the boys with DMD and controls for specific torque of the PFs (P< .001) and DFs (P = .006). For both muscle groups, the boys with DMD showed a decline in specific torque with age, whereas controls showed an increase with age. No difference was found between groups for the slope of KE specific torque versus age (P = .78).
Fig 4.
Scatter plots of age versus specific torque (torque normalized to CSAmax of muscle [Nm/cm2]) in boys with DMD (filled circles, solid line) and controls (open circles, dashed line). (A) PFs (n = 22 DMD, n = 10 control), (B) DFs (n = 21 DMD, n = 10 control), and (C) KEs (n = 20 DMD, n = 8 control). Please note in C, there are 2 overlapping points in the DMD group (age 9y, KE specific torques of .56 and .53Nm/cm2).
Functional Ability
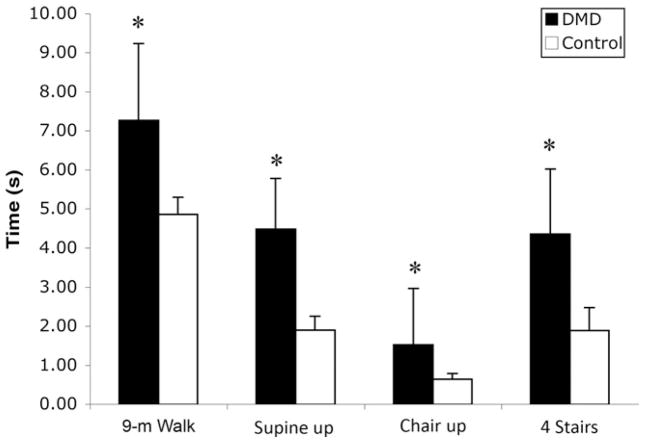
Boys with DMD required significantly more time to complete all functional tasks compared with controls (fig 5) (P<.001). There was a progressive increase in the time required to complete functional tasks with age in the boys with DMD, whereas the controls were relatively consistent in their times to complete all 4 tasks. Out of the sample of 22 boys with DMD, 5 were not able to rise from supine (ages 8.8 –14y) and 3 were unable to climb 4 stairs (ages 8.8 –14y).
Fig 5.
Group means (± SD) of 4 timed functional tasks in boys with DMD and controls. *P<.001.
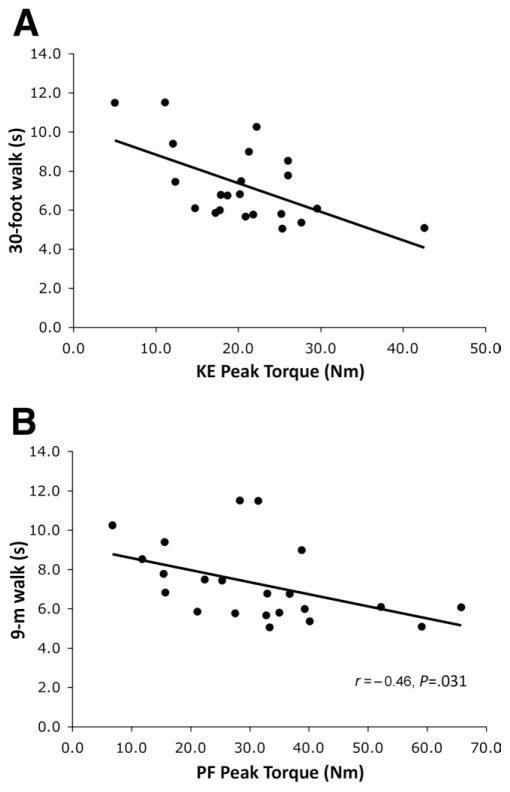
In boys with DMD, significant relationships were observed between time to walk 9m and KE peak torque (r= −.57, P < .005) (fig 6A), as well as PF peak torque, (r= −.46) (P< .031) (fig 6B). Furthermore, torque normalized to CSAmax of the KEs was correlated to both the time to walk 9m and time to rise from a chair (r= −.51 and r= −.56, respectively; P< .05, not shown). Similar correlations were seen for PF normalized torque with these 2 functional tasks (r= −.45 and r= −.48, respectively; P< .05, not shown). Interestingly, no significant relationships were observed between the functional tasks with DF strength.
Fig 6.
Relationships between peak torque of the KEs (A) and PFs (B) and time to walk 9m.
DISCUSSION
The findings of this study provide new insights into the age-related changes in CSAmax and specific torque production of important lower-extremity muscle groups in ambulatory boys with DMD. We found approximately 60% greater CSAmax of the triceps surae muscles (soleus, gastrocnemius) of ambulatory boys with DMD compared with controls. The increase in triceps surae CSAmax was observed across all ages, and in fact the slope between muscle CSAmax and age was similar in boys with DMD and controls. In contrast, the age-related increase in the tibialis anterior CSAmax appeared to be unaffected by dystrophy. The quadriceps muscle showed a distinct pattern with larger muscles in young boys with DMD, whereas boys older than 11 years demonstrated relatively smaller CSAs than controls. Torque production was impaired in all 3 muscle groups, including the DFs in boys with DMD, both when expressed as absolute values as well as after normalization to BSA or to muscle CSAmax (ie, specific torque). Specific torque showed a modest decline with age in boys with DMD, while healthy boys showed large gains in specific torque between the ages of 5 and 14 years. The torque production of the KEs and PFs, but not the DFs, was correlated to performance on functional tasks such as walking and rising from a chair.
Muscle weakness was observed in all 3 muscle groups in boys with DMD, although KE strength deficits were observed at an earlier age. KE torque in DMD was on average 71% of controls at 5 to 7 years old and declined to 15% of controls in subjects over 11 years old. This difference was primarily the result of a 4-fold increase in KE torque of the controls with age. McDonald et al6 reported a sharp decline in KE strength with age in boys with DMD, both by isometric testing and the MMT. In their study, boys aged 4 to 6 years had an MMT grade of 4, which corresponded to an isometric strength of 40% to 50% of control values.6 The MMT grade continued to decline with age, and isometric strength was reported to be less than 5% of controls by the age of 8 years.6 In comparison, we found that although the relative difference between controls and boys with DMD increased with age, there was little change in absolute torque production of the KE in DMD.
Our findings showed that peak torque of the PF and DF were about two thirds of controls at ages 5 to 9 years, whereas McDonald et al6 reported an earlier loss of DF strength compared with PFs using the MMT. This may be attributed to differences in the position of the ankle joint in testing the DFs between studies. In the MMT, the ankle is placed at or near full DF,28 whereas in our study, the DFs were tested near their optimal angle for force generation (30° plantarflexion).29,30 Between the ages of 5 and 14, we observed a 3-fold increase in PF and DF muscle strength in controls. Only a small increase in absolute torque was observed among boys with DMD over the same age. Similarly, Beenakker et al7 reported that the greater disparity in lower-extremity muscle strength in older boys with DMD was primarily the result of an increase in strength among the controls.
Another important factor to consider in muscle strength changes with age in our subjects is that all subjects in our study were taking corticosteroids, whereas the previous study by McDonald et al6 did not indicate the use of corticosteroids. However, at the time at which they collected longitudinal data (1982–1992), corticosteroids were not part of the standard clinical care for patients with DMD. Because corticosteroids have been shown to stabilize muscle force,31 this intervention may have contributed to an improved preservation of muscle torque in our subjects compared with the previous study. This theory is substantiated by a recent study by Parreira et al32 in which the progressive loss in muscle strength in boys with DMD who had initiated corticosteroids was found to be less than that reported in an older study by Scott et al,33 in which the cohort of boys with DMD were not taking corticosteroids.
Previous MRI studies have focused on examining fatty-tissue infiltration, and in particular the distribution of muscle involvement in a variety of muscular dystrophies and inherited myopathies.5,34–36 We used MRI to measure the total muscle CSAmax including both muscle and fat tissue, although we also observed differential fatty-tissue infiltration across the lower-extremity muscles (see fig 1), intramuscular fat infiltration was not quantified in the current study. Although advanced MR techniques such as proton spectroscopy can be used to quantify lipid content of muscle,37 this study was conducted to specifically measure differences in CSAmax with age and to examine the relationship between muscle size and muscle strength in boys with DMD. We found that PF CSAmax was larger in the boys with DMD relative to controls across all ages. In contrast, DF CSAmax was not different between groups and showed a similar increase in size with age in both controls and DMD. For the quadriceps, however, the slope of line relating CSAmax to age was lower in boys with DMD compared with controls. From the scatter plot it appears that young boys with DMD (under 10y) had a higher quadriceps CSAmax than controls, whereas older boys with DMD (over age 10) had a lower CSAmax than controls. This difference between DMD and controls must be considered in light of our limited sample of young controls for quadriceps CSAmax, because the 2 control subjects who were under 7 years old did not participate in this measurement.
Peak torque normalized to muscle CSA (ie, specific torque) provides an index of muscle quality. Our findings clearly showed that increases in muscle size were not reflected by proportional increases in muscle strength in boys with DMD, consistent with a decline in muscle quality. This is similar to recent findings using DXA, which show low correlations between muscle strength and regional lean body mass in boys with DMD between 5 and 13 years of age.15 In our study, specific torque was impaired in all 3 muscle groups, with the average KE and PF muscles showing 4 times higher values in controls compared with boys with DMD. However, absolute specific torque only showed a modest decline with age in boys with DMD, while in controls, large age-related gains in specific torque were noted. Interestingly, even though age-related increases in the DF CSAmax mirrored those of controls, the average specific torque in controls was 2-fold higher than that of boys with DMD. In addition, the relationship between DF specific torque and age was significantly different in ambulatory boys with DMD compared with controls. These data demonstrate that while muscle growth (increase in CSAmax) may appear normal in the tibialis anterior muscle, there is a loss of muscle quality.
In healthy boys, there is a sharp rise in specific torque around the onset of puberty (age 13–15y).38 This rapid increase in force-generating capacity may be related to increases in anabolic hormone levels39 and stature.40 Oral corticosteroid use has been shown to decelerate growth rate in boys and suppress adrenal function.41 The subjects with DMD in this study were of shorter stature than controls, which may also have contributed to a smaller gain in specific torque with age. Interestingly, we noted that there were differences in the specific torques of the different muscle groups in healthy subjects. There was a 2-fold difference between PFs and DFs in normalized peak torque: approximately 3Nm/cm2 in PF and approximately 6Nm/cm2 in DF. Specific tension of the DFs has been reported to be higher than PFs in healthy adults and may be partially attributed to differences in muscle architecture.42
We found that the ability to rise from the floor and climb stairs was lost in 50% of the boys with DMD over age 10, although their absolute lower-limb muscle strength was similar to younger boys who were still able to complete these tasks. This is most likely because of an increase in body stature with little increase in absolute muscle strength to compensate, as indicated by the lower muscle torques normalized to BSA in boys with DMD. Furthermore, we did not evaluate the strength of the upper limbs and trunk and their contribution to the ability of the boys with DMD to perform functional tasks. These muscle groups also play an important role in functional ability in boys with DMD, especially as leg muscle strength declines and compensatory movements are required to maintain independent mobility. Relationships between muscle strength and ability to walk and perform sit-to-stand transfers were observed in the KEs and PFs. Previous studies have also found relationships between lower-limb muscle strength and ambulation and transfers.7,43 Furthermore, PF strength has been implicated as a key muscle in maintaining gait in boys with DMD.44 Interestingly, we did not observe any relationships between DF muscle strength and functional ability, although a previous study45 found that ankle DF muscle strength of less than grade 4 was a key prognostic factor in determining time to wheelchair dependence in boys with DMD. Because all subjects in our study were ambulatory, DF torque may not have played a role in their current walking ability.
Although we observed differential fatty-tissue infiltration across the lower-extremity muscles (see fig 1) in boys with DMD, fibrosis and intramuscular fat infiltration was not quantified in the current study. All magnetic resonance images in this study were acquired using a standard T1-weighted imaging sequence, with fat suppression, to facilitate segmentation of the muscle boundaries and quantification of the muscle CSA. Advanced MR techniques such as volume-localized spectroscopy and 3-point Dixon can be used to measure the intramuscular lipid composition37; however, the quantification of fibrosis in skeletal muscle presents significant challenges because of extremely short T2 of collagen. MRI of human skeletal muscle also suffers from partial volume filling because of limited spatial resolution, adding to the complexity of quantifying noncontractile tissue in muscular dystrophies.
CONCLUSIONS
Using MRI in combination with dynamometry allowed us to measure the muscle CSAmax and specific torque production of primary lower-extremity muscles in children with DMD. Age-related changes in muscle CSA and specific torque production in lower-extremity muscles showed distinctly different patterns in the KE, PF, and DF muscles of boys with DMD. The distal triceps surae muscles showed a larger CSAmax by approximately 60% in ambulatory boys with DMD compared with controls across ages, while the tibialis anterior muscle showed age-appropriate increases in CSAmax. In the quadriceps muscle, CSAmax tended to be higher than controls in younger boys with DMD but lower in older boys with DMD over 10 to 11 years of age. However, all muscles showed significant deficits in specific torque, with a 4-fold difference in the PF and KE, and 2-fold difference in the DFs. Specific torque showed a modest decline with age in boys with DMD, while in controls, large age-related gains in specific torque were noted.
Future clinical trials for therapeutic strategies in DMD should consider examining multiple muscles to evaluate the efficacy of treatment, as well as the inclusion of physiologic measures such as muscle CSA and specific torque. This study also underlines the importance of including age-matched healthy controls in evaluating boys with DMD.
Acknowledgments
MRI was conducted at the Advanced Magnetic Resonance Imaging and Spectroscopy facility at the University of Florida.
Supported by the Muscular Dystrophy Association (grant no. MDA4170) and Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center (grant no. U54AR052646).
List of Abbreviations
- BSA
body surface area
- CSA
cross-sectional area
- CSAmax
maximal cross-sectional area
- DF
dorsiflexor
- DMD
Duchenne muscular dystrophy
- DXA
dual energy x-ray absorptiometry
- ICC
intraclass correlation coefficient
- KE
knee extensor
- MMT
manual muscle test
- MRI
magnetic resonance imaging
- PF
plantarflexor
Footnotes
Biodex Medical Systems, 20 Ramsay Rd, Shirley, NY 11967-4704.
Signa; GE Medical Systems, 3000 N Grandview Blvd, Waukesha, WI 53188.
Achieva; Philips Medical Systems, 3000 Minuteman Rd, Andover, MA 01810-1099.
OsiriX Imaging Software, available from: http://www.osirix-viewer.com/.
SPSS Inc, 233 S Wacker Dr, 11th Fl, Chicago, IL 60606.
Presented in part to the American College of Sports Medicine, May 31, 2008, Indianapolis, IN.
Reprints are not available from the author.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy. Pediatric Neurology. 2007;36:1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Petrof B, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–4. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sussman M. Duchenne muscular dystrophy. J Amer Acad Orthop Surg. 2002;10:138–51. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–8. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- 6.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuro-muscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74(5 Suppl):S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Beenakker EA, Maurits NM, Fock JM, Brouwer OF, van der Hoeven JH. Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2005;9:387–93. doi: 10.1016/j.ejpn.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–8. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 9.Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–7. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MM, Hannan MA, Mondol BA, Bhoumick NB, Haque A. Prednisolone in Duchenne muscular dystrophy. Bangladesh Med Res Counc Bull. 2001;27:38–42. [PubMed] [Google Scholar]
- 11.Escolar DM, Henricson EK, Mayhew J, et al. Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. Muscle Nerve. 2001;24:787–93. doi: 10.1002/mus.1070. [DOI] [PubMed] [Google Scholar]
- 12.Mayhew JE, Florence JM, Mayhew TP, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 13.Bohannon RW. Manual muscle testing: does it meet the standards of an adequate screening test? Clin Rehabil. 2005;19:662–7. doi: 10.1191/0269215505cr873oa. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 15.Skalsky AJ, Han JJ, Abresch RT, Shin CS, McDonald CM. Assessment of regional body composition with dual-energy X-ray absorptiometry in Duchenne muscular dystrophy: correlation of regional lean mass and quantitative strength. Muscle Nerve. 2009;39:647–51. doi: 10.1002/mus.21212. [DOI] [PubMed] [Google Scholar]
- 16.Stevens JE, Walter GA, Okereke E, et al. Muscle adaptations with immobilization and rehabilitation after ankle fracture. Med Sci Sports Exerc. 2004;36:1695–701. doi: 10.1249/01.mss.0000142407.25188.05. [DOI] [PubMed] [Google Scholar]
- 17.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–9. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 18.Shah PK, Stevens JE, Gregory CM, et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil. 2006;87:772–8. doi: 10.1016/j.apmr.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Mathur S, Takai KP, Macintyre DL, Reid D. Estimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary disease. Phys Ther. 2008;88:219–30. doi: 10.2522/ptj.20070052. [DOI] [PubMed] [Google Scholar]
- 20.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol. 2001;90:2070–4. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 21.Weber MA, Krakowski-Roosen H, Schroder L, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009;48:116–24. doi: 10.1080/02841860802130001. [DOI] [PubMed] [Google Scholar]
- 22.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]
- 23.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–35. [PubMed] [Google Scholar]
- 24.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 25.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–16. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vignos PJ, Jr, Spencer GE, Jr, Archibald KC. Management of progressive muscular dystrophy in childhood. JAMA. 1963;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 27.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 28.Kendall FP, Kendall EM, Provance PC, Rodger MM. Muscles: testing and function, with posture and pain. 5. Baltimore: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 29.Maganaris CN. Force-length characteristics of in vivo human skeletal muscle. Acta Physiol Scand. 2001;172:279–85. doi: 10.1046/j.1365-201x.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 30.Manal K, Roberts DP, Buchanan TS. Optimal pennation angle of the primary ankle plantar and dorsiflexors: variations with sex, contraction intensity, and limb. J Appl Biomech. 2006;22:255–63. doi: 10.1123/jab.22.4.255. [DOI] [PubMed] [Google Scholar]
- 31.Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle Nerve. 2007;36:424–35. doi: 10.1002/mus.20812. [DOI] [PubMed] [Google Scholar]
- 32.Parreira SL, Resende MB, Della Corte Peduto M, Marie SK, Carvalho MS, Reed UC. Quantification of muscle strength and motor ability in patients with Duchenne muscular dystrophy on steroid therapy. Arq Neuropsiquiatr. 2007;65:245–50. doi: 10.1590/s0004-282x2007000200011. [DOI] [PubMed] [Google Scholar]
- 33.Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle Nerve. 1982;5:291–301. doi: 10.1002/mus.880050405. [DOI] [PubMed] [Google Scholar]
- 34.Jungbluth H, Davis MR, Muller C, et al. Magnetic resonance imaging of muscle in congenital myopathies associated with RYR1 mutations. Neuromuscul Disord. 2004;14:785–90. doi: 10.1016/j.nmd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Kornblum C, Lutterbey G, Bogdanow M, et al. Distinct neuro-muscular phenotypes in myotonic dystrophy types 1 and 2: a whole body highfield MRI study. J Neurol. 2006;253:753–61. doi: 10.1007/s00415-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 36.Mercuri E, Bushby K, Ricci E, et al. Muscle MRI findings in patients with limb girdle muscular dystrophy with calpain 3 deficiency (LGMD2A) and early contractures. Neuromuscul Disord. 2005;15:164–71. doi: 10.1016/j.nmd.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Boesch C. Musculoskeletal spectroscopy. J Magn Reson Imaging. 2007;25:321–38. doi: 10.1002/jmri.20806. [DOI] [PubMed] [Google Scholar]
- 38.Kanehisa H, Yata H, Ikegawa S, Fukunaga T. A cross-sectional study of the size and strength of the lower leg muscles during growth. Eur J Appl Physiol Occup Physiol. 1995;72:150–6. doi: 10.1007/BF00964130. [DOI] [PubMed] [Google Scholar]
- 39.Ramos E, Frontera WR, Llopart A, Feliciano D. Muscle strength and hormonal levels in adolescents: gender related differences. Int J Sports Med. 1998;19:526–31. doi: 10.1055/s-2007-971955. [DOI] [PubMed] [Google Scholar]
- 40.Wood LE, Dixon S, Grant C, Armstrong N. Elbow flexion and extension strength relative to body or muscle size in children. Med Sci Sports Exerc. 2004;36:1977–84. doi: 10.1249/01.mss.0000145453.02598.7e. [DOI] [PubMed] [Google Scholar]
- 41.Lai HC, FitzSimmons SC, Allen DB, et al. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med. 2000;342:851–9. doi: 10.1056/NEJM200003233421204. [DOI] [PubMed] [Google Scholar]
- 42.Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Edgerton VR. Specific tension of human plantar flexors and dorsiflexors. J Appl Physiol. 1996;80:158–65. doi: 10.1152/jappl.1996.80.1.158. [DOI] [PubMed] [Google Scholar]
- 43.Uchikawa K, Liu M, Hanayama K, Tsuji T, Fujiwara T, Chino N. Functional status and muscle strength in people with Duchenne muscular dystrophy living in the community. J Rehabil Med. 2004;36:124–9. doi: 10.1080/16501970410023461. [DOI] [PubMed] [Google Scholar]
- 44.Armand S, Mercier M, Watelain E, Patte K, Pelissier J, Rivier F. A comparison of gait in spinal muscular atrophy, type II and Duchenne muscular dystrophy. Gait Posture. 2005;21:369–78. doi: 10.1016/j.gaitpost.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Bakker J, De Groot IJ, Beelen A, Lankhorst GJ. Predictive factors of cessation of ambulation in patients with Duchenne muscular dystrophy. American Journal of Physical Medicine and Rehabilitation. 2002;81:906–12. doi: 10.1097/00002060-200212000-00004. [DOI] [PubMed] [Google Scholar]