Abstract
Helicobacter pylori are well acknowledged as a major cause of gastrointestinal ailments and gastric cancers. Therefore, the present study aimed to investigate the potential in vitro activity of Desmostachya bipinnata against H. pylori, focusing on the determination of the most active extract responsible for the anti-helicobacter activity to produce new active drug from natural source.
Desmostachya bipinnata total alcohol and successive extracts were in vitro tested against H. pylori. All extracts showed promising anti Helicobacter pylori activities. The most effective extract was diethyl ether extract, it showed 75% growth inhibition of the clinical Isolates bacterial Helicobacter pylori, in addition it showed high count reduction on the selected organisms in the different concentrations used (2xMIC, MIC & ½ MIC) compared with the untreated controls as well as the other extracts (chloroform, ethyl acetate and n-butanol). The oral median lethal dose (LD50) of the alcohol extract of the plant by doses up to 5000 mg/kg didn’t showed any mortality or morbidity, in addition no side effects were recorded on both liver and kidney functions this means that the extract was safe for use.
Keywords: Desmostachya bipinnata, Kidney function, Liver function, Antimicrobial activity, Non-polar extracts, Phytochemical contents
1. Introduction
Helicobacter pylori have been designated as critical organisms in the etiology of chronic gastritis; peptic ulcers (Megraud, 1993) and gastric cancers (NIH Consensus Development Panel, 1994, Mbulaiteye et al., 2009) and their suppression and eradication have been considered the gold standard therapy for infectious gastric diseases (Ibrahim et al., 2012). Many therapeutic agents are used for extermination of this bacterium, but the widespread use of these agents has increased resistance, failure and relapses among the isolated strains of H. pylori. Therefore, the world is tending to go back to the use of natural products for the treatment of various diseases. Studies have suggested many natural plant extracts with anti-H. pylori activity, including chamomile flowers, coneflower herbs, peppermint leaves, thyme herbs and grapes as well as “Halfa grass” (Malm et al., 2015, Brown et al., 2009, James, 2011).
“Halfa grass” or Desmostachya bipinnata is included in the family Gramineae. This family comprises more than 660 genera and 10,000 species. It is well known for its great economic and medicinal importance because it includes all cereals, bamboos and sugar cane. There are many medicinal activities of Gramineae species such as astringent and in treatment of wound, anti-emetic, diuretic (Shrestha, 2011), and in treatment of eye problems (Bolus, 2000). Desmostachya bipinnata is a native plant in northeast and west tropical, and northern Africa and countries in the Middle East, and temperate and tropical Asia. Commonly known in English by the names ‘Halfa grass’, Big cord grass, and Salt reed-grass (James, 2011).
In folk medicine, it has been used to treat many diseases. Researchers proved that this plant has some activities as a diuretic, antidiarrheal (Medha et al., 2010), analgesic, antipyretic and has an anti-inflammatory effect (Panda et al., 2009). It was also suggested to have hepatoprotective ability (Rahate et al., 2015), help against dysentery, menorrhagia, jaundice, (Joshi, 2003) and has antioxidant effect (Golla & Bhimathati, 2014) as well as anti-ulcerogenic properties (Awad et al., 2008).
Few reports have recognized this plant as a candidate that showed promise as a novel antimicrobial agent (Hashmi and Rashid, 2001, James, 2011, Joshi et al., 2017). These reports and others have encouraged the current investigation. Therefore, the present study aimed to investigate the potential in vitro activity of Desmostachya bipinnata against H. pylori, focusing on the determination of the most active extract responsible for the anti-helicobacter activity to produce new active drug from natural source.
2. Material and methods
2.1. Plant material
Arial parts of Desmostachya bipinnata (L.) Stapf were collected during flowering stage in March 2013 from Gazan territory, South west Saudi Arabia, the sample was identified by Dr. Jacob Thomas; Assistant Professor of taxonomy, Botany and Microbiology Department, Faculty of Science, King Saud University, Specimen was kept in the herbarium of botany Department, KS, KSA and were compared with published (Migahid,1996). Samples of the aerial parts were air dried in shade, reduced to fine powder and kept for phytochemical and biological investigation.
2.2. Phytochemical screening, extraction and isolation
For determination of the phytochemical active constituents, the powder of Desmostachya bipinnata was subjected to screening according to the published methods (Ayoola et al., 2008).
Dried aerial parts of Desmostachia bipennata (500 g) were extracted by percolation in ethanol (95%) at room temperature for two days. The ethanol extract was filtered and the residues were re-percolated as before (3 × 3 times). The combined filtrates were concentrated under reduced pressure to yield 80 g. The residue obtained was suspended in water (200 ml), filtered over a piece of cotton. The material obtained on top of the cotton piece (20 g) was symbolized as D-1(The non-polar components) and kept for further investigation. The aqueous layer which filtered off (polar components) were successively fractionated using: ether, chloroform, ethyl acetate and n-butanol respectively. Each extract was dried over anhydrous sodium sulphate and concentrated to yield 10, 8, 13 and 25 g. respectively. The dry extracts were kept for further investigation.
2.3. Anti – H. Pylori activity
2.3.1. Preparation of the extracts
The total methanol extract and the successive extracts (ether, chloroform, ethyl acetate and n-butanol) of Desmostachya bipinnata were suspended in DEMSO (dimetyl sulfoxide) to be used on the selected strains. The antibiotic amoxicillin (Sigma Chemical Co., St. Louis, Mo.) was used as the standard control in every run.
2.3.2. Bacterial isolates
Twelve clinical yields of Helicobacter pylori were isolated from 19 biopsies received from patients diagnosed with gastritis or peptic ulcer at King Khalid University hospital, Riyadh, Saudi Arabia. Identification was carried out using Gram staining, urease, oxidase, and catalase tests. They were then stored in aliquots of 1 ml glycerol-containing skimmed milk, following Han et al. method, at −70 °C (Han et al., 1995) till used.
On use, the thawed bacterial growths were sub-cultured on blood agar supplemented with 5% defibrinated sheep blood (Biolife) and Helicobacter pylori Selective Supplement (DENT, Oxoid, United Kingdom), incubated at 37 °C in a microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2; Campy Gen; Unipath) for 3–5 days. The reference strains H. pylori ATCC 43504 and Escherichia coli ATCC 25922 were involved as controls with each run.
2.3.3. Antimicrobial screening
H. pylori inhibitory zone testing for the plant extracts selected were performed according to the modified method of Johnson and Christine (1995). Fresh microbial inocula were prepared from the selected strains and suspended in tubes containing sterile saline adjusting their turbidity to 2 McFarland (12 × 108 CFU/ml). The bacteria were then cultivated on the surface of Mueller-Hinton agar (Unipath S.p.A., Garbagnate Milanese, Milan, Italy) supplemented with 5% defibrinated sheep blood (Biolife) and Helicobacter pylori Selective Supplement (DENT, Oxoid, United Kingdom). Wells sized 7-mm in diameter were punched on the plates with 30 μL of the prepared dissolved extracts incorporated in each well. DMSO was used as control in separate wells. The plates were permitted to diffused at 4 °C for 2 h, then, incubated in microaerophilic conditions as mentioned before. The inhibition zones around each well were observed and their diameters were recorded.
2.3.4. Determination of the minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) was carried out by the broth microdilution assay (Hachem et al., 1996).
A total of 100 µL of Brain Heart Infusion broth supplemented with 10% defibrinated sheep blood inoculated with (12 × 108CFU/ml) Helicobacter pylori (equal to 2 McFarland turbidity) and 100 µL of serial dilutions to reach final concentrations of 50, 25 > 50, 12.5 > 50, 6.25 > 50, 3.125 > 50, 1.5625 > 50, 0.78125 > 50 mg/mL. The standard drug, Amoxycillin, was diluted to the same concentrations. The microplate was incubated at 37 °C under microaerophilic conditions in an atmosphere of 5–15% O2 and 5–10% CO2, for 48–72 h. The MICs of each isolate were assessed visually as the lowest concentration of the extracts showing complete growth inhibition of the isolate as well as the reference strain. A positive control (containing inoculum without the tested extracts) and a negative control (containing the tested extracts without inoculum) were included on each microplate.
2.3.5. Time Kill-curve
The three most sensitive strains were selected and prepared in 100 µL of Brain Heart Infusion broth supplemented with 10% de-fibrinated sheep blood inoculated with (12 × 108 CFU/ml) Helicobacter pylori (equal to 2 McFarland turbidity). The ability of the most effective three extracts, in concentrations of MIC, 2 × MIC and ½MIC, to inhibit the three growths was evaluated based on the plotting of time-death curves. Each concentration of the 3 tested extracts was tested against the selected yields and incubated for 1, 4 and 24 h at 37 °C under microaerophilic conditions. Aliquots of the bacterial cultures were serially diluted and then plated on to MHA for colony counts. Parallel controls were carried out.
The effects of the extracts on the 3 H. pylori cell wall were also observed by the Scanning Electron Microscope following treatment by the extracts after 1, 4, and 24 h with MIC of each isolate.
2.4. Pharmacological study
2.4.1. Preparation of the plant extract
Dried aerial parts of Desmostachya bipinnata (L.) Stapf, were extracted as mentioned before in Section 2.2. The dried plant extract was freshly suspended in distilled water just before administration.
2.4.2. Determination of median lethal dose (LD50)
LD50 of the ethanol extract was determined as described before (El-Meligy et al., 2017). For this purpose, 5 groups of 5 mature mail Swiss albino mice (23–25 g body weight) each were used. The tested extract was administered orally in doses of 200–400 mg/kg b.wt in addition to a group used as a control (given the solvent). Mice were kept under observation for 24 h during which the number of dead animals in each group was recorded.
2.5. Statistical analysis
Data were collected and analyzed statistically using Statistical Package for Social Sciences program (SPSS v21). The following tests were used in this study: mean, standard deviation, T test for independent samples, ANOV A test (analysis of variance). Significance levels: P > 0.05 insignificant, P < 0.05 significant and P < 0.001 highly significant.
3. Results and discussions
3.1. Phytochemical screening
Phytochemical screening of Desmostachya bipinnata (L.) showed the presence of the following groups: carbohydrates and/or glycosides, flavonoids, sterols and/or triterpenes, protein and/or amino acids, phenolic compounds and tannins while saponin, Anthraquinones, alkaloids, and cardinolides were absence in this plant.
3.2. Anti-H. Pylori activity
Twelve clinical yields of Helicobacter pylori were identified from 19 biopsies received from patients diagnosed with gastritis or peptic ulcer at King Khalid University hospital, Riyadh, Saudi Arabia.
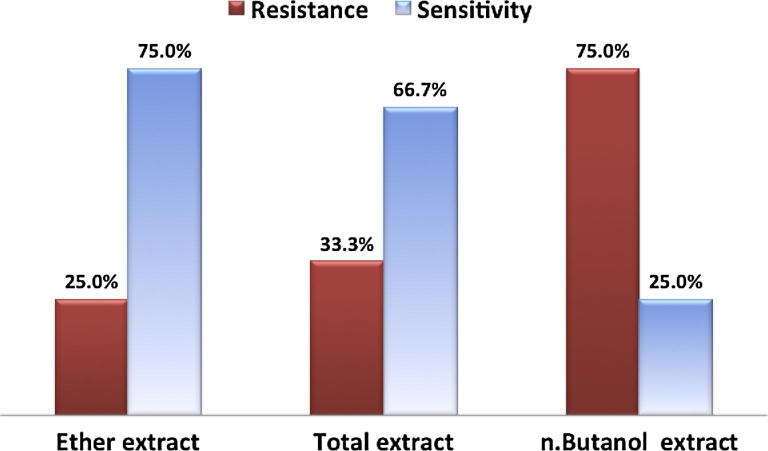
On screening of the 5 prepared extracts by the cup diffusion method, the results showed susceptibility and inhibition zones for the different extracts. The most effective were ether extract (EE), total extract (TE) and n.butanol extract (BE) which showed inhibition zones in 9 (75%), 9 (75%) and 8 (66.7%) isolates respectively. These results were statistical significant when compared with the control wells containing the solvent alone (p value of 0.0003) (table 1).
Table 1.
Analysis of anti-Helicobacter pylori activity of plant extracts by cup diffusion method.
| Extracts |
|||||
|---|---|---|---|---|---|
| Isolates | Inhibition zone in (mm) |
||||
| Total alcohol | Non polar | Ether | Ethyl acetate | n.butanol | |
| 1 | 13 | 9 | 14 | 10 | 17 |
| 2 | 18 | 10 | 16 | ≥7 | 13 |
| 3 | 17 | 15 | 20 | 13 | 18 |
| 4 | 15 | ≥7 | 16 | 14 | 15 |
| 5 | 12 | 13 | 10 | ≥7 | 12 |
| 6 | 13 | ≥7 | 11 | ≥7 | 15 |
| 7 | 18 | 13 | 14 | 13 | 13 |
| 8 | 13 | ≥7 | 12 | ≥7 | 11 |
| 9 | ≥7 | ≥7 | 11 | ≥7 | ≥7 |
| 10 | 10 | ≥7 | ≥ 7 | ≥7 | ≥7 |
| 11 | ≥7 | ≥7 | ≥ 7 | ≥7 | ≥7 |
| 12 | ≥7 | ≥7 | ≥ 7 | ≥7 | ≥7 |
MICs of the 3 most active extracts were assessed against the 12 selected isolates by broth microdilution method. The findings revealed that Total extract was the most effective extract against the growths, though with no statistical significance, moreover, isolates 2, 3 and 7 were the most sensitive yields, which made them candidates for further evaluation (Table 2 & Fig. 1).
Table 2.
MICs of the 3 selected extracts by broth micro-dilution assay.
| Extracts |
|||
|---|---|---|---|
| Isolates | Minimum inhibitory concentration MIC (mg/ml) |
||
| Ether extract | Total extract | n.Butanol | |
| 1 | 25 | 50 | 12.5 |
| 2 | 12.5 | 12.5 | >50 |
| 3 | 25 | 6.25 | 6.25 |
| 4 | 25 | 12.5 | >50 |
| 5 | 50 | 50 | 12.5 |
| 6 | 12.5 | 25 | >50 |
| 7 | 12.5 | 6.25 | >50 |
| 8 | 25 | 25 | >50 |
| 9 | >50 | >50 | >50 |
| 10 | 25 | >50 | >50 |
| 11 | >50 | >50 | >50 |
| 12 | >50 | >50 | >50 |
Fig. 1.
Comparative in vitro MIC activities of different extracts against 12 clinical H. pylori isolates.
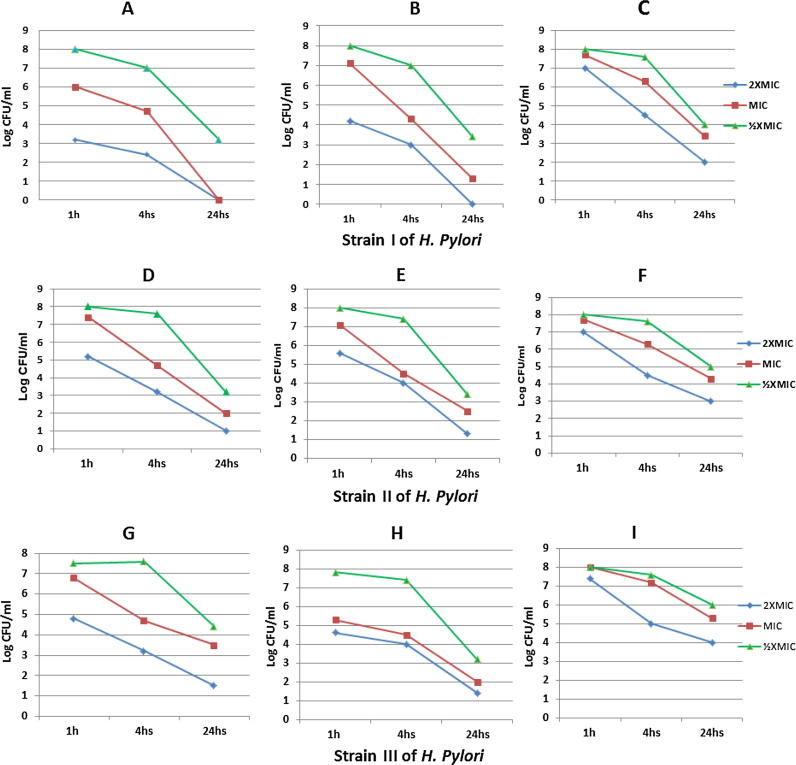
The 3 most sensitive isolates (2, 3 &7 renamed as I, II & III) were selected and subjected to the effect of the most effective three extracts, in concentrations of MIC, 2 × MIC and ½MIC, to inhibit the three growths. They were evaluated based on the plotting of time-death curves; each concentration of the 3 tested extracts was tested against the selected yields and incubated for 1, 4 and 24 h at 37 °C under microaerophilic conditions. There were statistical significant difference regarding time and concentrations when presented in means, ½MIC concentration when compared with MIC and 2 × MIC of the selected strains p value were 0.04 and 0.000 respectively. Different time periods used showed also statistical significance; 1 h when compared with 4 h and 24 h, p value were 0.01 and 0.000 respectively. However, there was no statistical significant difference in-between the different extracts, except for, between total and n.butanol extract in their MIC for 24 h; the p value was 0.01 (Fig. 2).
Fig. 2.
A, D & G: the time dependent effect of (2 × MIC, MIC & 1/2 MIC) of diethyl ether extract on the three selected isolates at 1, 4, 24 h exposure time. B, E & H: the time dependent effect of (2XMIC, MIC & 1/2 MIC) of the total extract on the three selected isolates at 1, 4, 24 h exposure time. C, F &I: the time dependent effect of (2XMIC, MIC & 1/2 MIC) of n.butanol extract on the three selected isolates at 1, 4, 24 h exposure time.
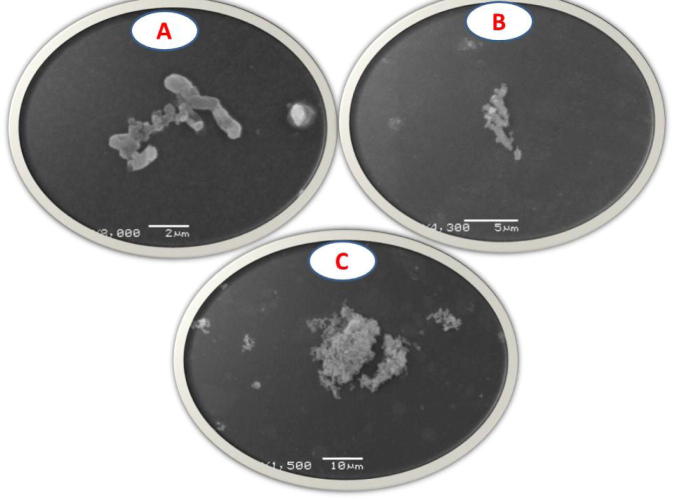
The in vitro effectiveness of the extracts on the 3H. pylori’s cell wall were also assessed using the Scanning Electron Microscope following treatment by the extracts after 1, 4, and 24 h with MIC of each isolate. The cell wall was indented and showed many convolutions after only one hour, after 4 h, it became more damaged to be more and more destructed at the 24 h time period (Fig. 3).
Fig. 3.
Scanning electron microscopy images of H. pylori following treatment by diethyl ether extract after 1, 4, and 24 h with MIC of each isolate. A: the isolate after 1 h of treatment, B: the organism after 4 h, C: the organism after 24 h.
3.3. Determination of median lethal dose (LD50)
The oral median lethal dose (LD50) of the alcohol extract of Desmostachya bipinnata (L.) Stapf by doses up to 5000 mg/kg didn’t showed any mortality or morbidity. This means that, the extract was safe for use (Lorke, 1983, Awaad Amani et al., 2015).
The non-toxic nature of Desmostachya bipinnata (L) Stapf ethanol extract in acute toxicity study is well supported by the biochemical data following 35 consecutive days of oral administration of the total alcohol extract to rats in a dose of 500 mg/kg which neither show any significant effect on the activity of ALT and AST, nor any significant changes in the mean values of urea and creatinine in rat’s sera compared to controls (Table 3). The alcohol extract fractions of Desmostachia bipennata (L.) Stapf are therefore, neither hepatotoxic nor nephrotoxic in rats.
Table 3.
Effect of Desmostachya bipinnata (L)Stapf extract on liver and kidney functions of rats.
| Groups | ALT(U/l) | AST(U/l) | Blood urea (mg/dl) | Serum creatinine (mg/dl) |
|---|---|---|---|---|
| Control | 4.70 ± 0.47 | 4.90 ± 0.27 | 44.60 ± 1.26 | 0.79 ± 0.03 |
| Desmostachia bipennata (1000 mg/kg) | 4.80 ± 0.02 | 4.80 ± 0.50 | 45.00 ± 0.05 | 0.80 ± 0.02 |
3.4. Antihelicopacter
The relationship between Helicobacter pylori and acquired resistance to various drugs from conventional therapy is of worldwide concern. Several global agreement assemblies have been conducted to ensure that therapeutic guidelines are constantly revised on various issues involving the controlling of infections (Malfertheiner et al., 2012). With antibiotic resistance reaching a predicament in many hospitals around the world and the growing resistance rate disturbing the communities, there is a crucial need to reestablish the armory of new antimicrobial agents (Tim Cushnie and Lamb, 2011). Furthermore, the prospects for incompliance to treatment are large, mainly, due to side effects or recurrence of infection. Therefore, it was imperative to search for new remedies with anti-Helicobacter pylori action. Currently, plants are viewed as the main source for the discovery of new compounds (Cogo et al., 2010, Peek and Crabtree, 2006).
For centuries, many medicinal plants were employed to treat various gastrointestinal tract illnesses. They contain a lot of biologically active compounds that may display potential antimicrobial properties, including anti-H. pylori activity. Plants with potent anti-H. pylori effects were found to belong to several families (Lang and Buchbauer, 2012, Safavi et al., 2014)
The anti-H. pylori activity of plant extracts is studied in vitro usually by way of the disc or cup diffusion method or the microdilution method. The latter is recommended by the Clinical and Laboratory Standards Institute (CLSI, 2006, Ramadan and Safwat, 2009) in and the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2003) for determining MIC of antimicrobial substances (Wiegand et al., 2008).
In the current study, dried aerial parts of Desmostachya bipinnata (L.) Stapf were extracted by percolation in ethanol (95%). Total methanol extract and the successive extracts (ether, chloroform, ethyl acetate and n-butanol) were prepared and their anti-helicobacter effects were evaluated. The most effective extracts were ether extract (EE) and total extract (TE) followed by n.butanol extract (BE), which showed evident inhibition zones in 9 (75%), 9 (75%) and 8 (66.7%) isolates respectively (p value of 0.0003) (Table 1). MICs of the 3 most active extracts were assessed against the 12 selected isolates by broth microdilution method. The findings revealed that TE was the most effective extract against the growths. These results were comparable to the findings of Hashmi & Rashid (2001); James (2011) and Joshi et al. (2017), who found anti- bacterial activity of the extracts of the investigated plant, nevertheless, the tested parts in these reports were the roots.
The 3 most sensitive isolates were selected and subjected to the effect of the most effective three extracts, in concentrations of MIC, 2 × MIC and ½MIC, to inhibit the selected 3 yields. They were evaluated based on the plotting of time-death curves; each concentration of the 3 tested extracts was tested and incubated for 1, 4 and 24 h. There were statistical significant difference regarding time and concentrations when presented in means, ½MIC concentration when compared with MIC and 2 × MIC of the selected strains p value were 0.04 and 0.000 respectively. Different time periods used showed also statistical significance; 1 h when compared with 4 h and 24 h, p value were 0.01 and 0.000 respectively (Fig. 2). The in vitro effectiveness of the extracts on the 3H. pylori’s cell wall were also assessed using the Scanning Electron Microscope following treatment by the extracts after 1, 4, and 24 h with MIC of each isolate. The cell wall was indented and showed many convolutions after only one hour, after 4 h, it became more damaged to be more and more destructed at the 24 h time period (Fig. 3). Further studies should be performed to confirm the activity of these extracts against more clinical H. pylori isolates, especially those showing drug resistance, and to study possible other mechanisms of their anti-H. pylori activity.
4. Conclusion
The present findings suggest that Desmostachya bipinnata (L.) Stapf aerial parts can be considered as a potential natural medicine. It has promising bioactivity against H. pylori, and has the ability for being used safely in prophylaxis or as an enhancing agent in H. pylori infection treatment.
Footnotes
Peer review under responsibility of King Saud University.
References
- Awaad Amani S, Asmaa A. Al-Rifai, Reham M. El-Meligy, Ahmed M. Alafeefy and Mohamed E. Zain. (2015). New activities for isolated compounds from convolvulus austro-aegyptiacus as anti-ulcerogenic, anti- helicobacter pylori and their mimic synthesis using bio-guided fractionation. Phytother. Res. 1311–1316. [DOI] [PubMed]
- Awaad Amani S., Nawal H.M., Derek J.M., Solimon G.A. Antiulcerogenic activity of extract and some isolated flavonoids from Desmostachya bipinnata. Rec. Nat. Prod. 2008;2:76–82. [Google Scholar]
- Ayoola G.A., Coker H.A.B., Adesegun S.A., Adepoju-Bello A.A., Obaweya K., Ezennia EC., Atangbayila TO. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008;7(3):1019–1024. [Google Scholar]
- Bolus L (2000): Flora of Egypt. Vol. II, al Hadara Publishing, Cairo, Egypt. pp. 449.
- Brown JH, Huang G, Haley-Zitlin V, Jiang X. Antibacterial effects of grape extracts on helicobacter pylori. Appl. Environ. Microbiol. 2009, p. 848–852. [DOI] [PMC free article] [PubMed]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; sixteenth informational supplement. CLSI document M100-S16CLSI, Wayne, PA (2006).
- Cogo L.L., Monteiro C.L.B., Miguel M.D., Miguel O.G., Cunico M.M., Ribeiro M.L., Camargo E.R., Kussen G.M.B., Nogueira K.S., Costa L.M.D. Anti- Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Braz. J. Microbiol. 2010;41:304–309. doi: 10.1590/S1517-83822010000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Meligy Reham M., Amani S.Awaad, Gamal A. Soliman, Abir B. Bacha, Ahmed M. Alafeefy and Sanaa A. Kenawy (2017). Prophylactic and Curative Anti-ulcerative colitis activity and the possible mechanisms of action of some desert plants. J. Enzyme Inhib. Med. Chem. 30(2):250–8. [DOI] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2003. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9, ix–xv.
- Golla U., Bhimathati S.S. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci. World J. 2014;19(2014):215084. doi: 10.1155/2014/215084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem J.P., Fowler A., Behne M., Fluhr J., Feingold K.R., Elias P.M. Increased stratum orneum pH promotes activation and release of primary cytokines from the stratum corneum attributable to activation of serine proteases. J Invest Dermatol. 1996;119:207–350. abstr 306. [Google Scholar]
- Han S.W., Flamm R., Hachem C.Y., Kim H.Y., Clarridge J.E., Evans D.G., Beyer J., Drnec J., Graham D. Transport and storage of Helicobacter pylori from gastric mucosal biopsies and clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 1995;14:349–352. doi: 10.1007/BF02116531. [DOI] [PubMed] [Google Scholar]
- Hashmi H., Rashid A. Isolation of fungi from roots of Parthenium hysterophorus and Desmostachya bipinnata and antibacterial activity of their root extracts. J. Biol. Sci. 2001;1:350–351. [Google Scholar]
- Ibrahim NH, Gomaa AA, Abu-Sief MA, Hifnawy TM, Tohamy MA., 2012. The use of different laboratory methods in diagnosis of Helicobacter pylori infection; a comparative study. Life Sci. J. 9 (4).
- James A. Duke.,2011. Desmostachya bipinnata (POACEAE). Green Farmacy Garden, Fulton, Maryland: Dr. Duke's Phytochemical and Ethnobotanical Databases. (Retrieved June 15, 2011).
- Johnson T.R., Christine L.C. The Benjamin/Cummings Pub. Co., Inc.; New York: 1995. Laboratory Experi- ments in Microbiology; pp. 177–179. [Google Scholar]
- Joshi SG.,2003. Medicinal Plants. New Delhi, India: Oxford and IBH Co. Pvt. Ltd., p. 318.
- Joshi K.B., Mandavia M.K., Golakiya B.A. Comparative study of phytochemical analysis, antimicrobial and antioxidant activity of different root extracts of Desmostachya bipinnata Stapf (Kush) Int. J. Curr. Microbiol. App. Sci. 2017;6(5):129–137. [Google Scholar]
- Lang G., Buchbauer G. A review on recent research results (2008–2010) on essential oils as antimicrobial and antifungals. A Rev. Flavour Fragr. J. 2012;27:13. [Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:251–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Malm A., Glowniak-Lipa A., Korona-Glowniak I., Baj T. Anti-Helicobacter pylori activity in vitro of chamomile flowers, coneflower herbs, peppermint leaves and thyme herbs – a preliminary report. Curr. Issues Pharm. Med. Sci. 2015;28(1):30–32. [Google Scholar]
- Malfertheiner P., Megraud F., O’Morain C.A., Atherton J., Axon A.T.R., Bazzoli F., Gensini G.F., Gisbert J.P., Graham D.Y., Rokka T., El-Omar E.M., Kuipers E.J. Management of Helicobacter pylori infection-the maastricht IV/ florence consensus report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- Mbulaiteye S.M., Hisada M., El-Omar E.M. Helicobacter pylori associated global gastric cancer burden. Front Biosci. 2009;14:1490–1504. doi: 10.2741/3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medha MH, Lakshman K, Girija K, Ashok Kumar BS, Lakshmiprasanna V.,2010. Assessment of antidiarrhoeal activity of Desmostachya bipinnata L. (Poaceae) root extracts. Bol Latinoam Caribe Plant Med Aromat, 9, 312–18.
- Megraud F. Epidemiology of Helicobacter pylori infection. Gastroenterol Clin North Am. 1993;22:73–88. [PubMed] [Google Scholar]
- Migahid A.M. 1996. Flora of Saudi Arabia. fourth ed. King Saud University Press. vol. 1, 127.
- NIH Consensus Development Panel Helicobacter pylori in peptic ulcer disease. J. Am. Med. Assoc. 1994;272:65–69. [PubMed] [Google Scholar]
- Panda S., Chaudhary N.S., Patro V.J., Pradhan D.K., Jana G.K. Analgesic, antipyretic and anti-inflammatory effect of the whole plant extract of Desmostachya bipinnata Stapf (Poaceae) in albino rats. Drug Invention Today. 2009;1:150–153. [Google Scholar]
- Peek R.M., Crabtree J.E. Helicobacter infection and gastric neoplasia. J. Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- Rahate KP, Rajasekaran A., 2015. Hepatoprotection by active fractions from Desmostachya bipinnata stapf (L.) against tamoxifen-induced hepatotoxicity. Indian J. Pharmacol. 47(3), 311–5. [DOI] [PMC free article] [PubMed]
- Ramadan M.A., Safwat N.A. Antihelicobacter activity of a flavonoid compound isolated from Desmostachya bipinnata. Aust. J. Basic Appl. Sci. 2009;3:2270–2277. [Google Scholar]
- Safavi M., Shams-Ardakani M., Foroumadi A. Medicinal plants in treatment of Helicobacter pylori infections. Pharm. Biol. 2014;28:1. doi: 10.3109/13880209.2014.952837. [DOI] [PubMed] [Google Scholar]
- Shrestha S., Park J.H., Lee D.Y. A new xanthene from Desmostachya bipinnata (L.) stapf inhibits signal transducer and activator of transcription 3 (STAT3) and low-density lipoprotein-oxidation. J. Appl. Biol. Chem. 2011;54(2):308–311. [Google Scholar]
- Tim Cushnie T.P., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Ag. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]