Abstract
Protein misfolding is implicated in many diseases, including serpinopathies. For the canonical inhibitory serpin α1-antitrypsin, mutations can result in protein deficiencies leading to lung disease, and misfolded mutants can accumulate in hepatocytes, leading to liver disease. Using all-atom simulations based on the recently developed bias functional algorithm, we elucidate how wild-type α1-antitrypsin folds and how the disease-associated S (Glu264Val) and Z (Glu342Lys) mutations lead to misfolding. The deleterious Z mutation disrupts folding at an early stage, whereas the relatively benign S mutant shows late-stage minor misfolding. A number of suppressor mutations ameliorate the effects of the Z mutation, and simulations on these mutants help to elucidate the relative roles of steric clashes and electrostatic interactions in Z misfolding. These results demonstrate a striking correlation between atomistic events and disease severity and shine light on the mechanisms driving chains away from their correct folding routes.
Introduction
Understanding how mutations alter protein misfolding propensities and the physicochemical mechanisms underlying this shift is key to clarifying the molecular basis of many diseases. One set of relatively common protein-misfolding diseases known as serpinopathies arises when mutations in inhibitory serpins lead to misfolding, thus reducing the secreted levels of these important protease inhibitors (1). Mutations in the canonical secretory serpin α1-antitrypsin (A1AT) result in the most common serpinopathies: the A1AT deficiencies. In these deficiencies, low circulating A1AT levels dysregulate leukocyte serine proteases, resulting in lung disease, which can be slowed but not halted by A1AT augmentation therapy (2). Extremely pathogenic A1AT mutations such as Z (Glu342Lys) can lead to both lung disease, because of loss of function, and liver disease, because of A1AT accumulation in the endoplasmic reticulum of hepatocytes, which generate most of the circulating A1AT. With the exception of liver transplants, there are no effective treatments for A1AT-associated liver disease (3).
In vitro, the pathogenic A1AT Z mutant folds very slowly, spending hours in at least one partially folded intermediate state (4). Similarly, Z secretion from cells is slow, and although some Z species are targeted for degradation (5, 6, 7), misfolded Z accumulates in the endoplasmic reticulum, where it can polymerize (8). Despite numerous experimental studies (9, 10, 11, 12, 13), little is known about the structure of misfolded species for any A1AT disease-associated mutant, hindering efforts to either rescue the folding of these species or to target them for degradation.
Molecular dynamics (MD) simulations offer an attractive approach to studying protein folding and misfolding, as they can in principle reveal folding pathways and intermediates in atomistic detail. To date, the application of all-atom MD simulations to investigate protein folding and misfolding has been limited to small, single-domain proteins with relatively short folding times. In particular, recent developments, such as the Anton special-purpose supercomputer (14) and the massively distributed folding@home project (15), have made it possible to generate in silico several reversible folding/unfolding events for a number of small globular proteins (<100 amino acids) with folding times up to the millisecond range. These studies have demonstrated that current all-atom force fields in explicit solvent can lead to the correct native states of proteins and predict with good accuracy their folding kinetics. Unfortunately, most biologically relevant proteins are much larger than 100 amino acids and have folding times as long as seconds and beyond. In particular, A1AT and other serpins contain ∼400 amino acids and fold over tens of minutes (9, 12, 13). Because of their large size and slow folding kinetics, simulating serpin folding with conventional MD simulations is not feasible, even using the most powerful available supercomputers.
Although the folding of a single large protein is generally much too slow to simulate using standard MD, this is chiefly due to the exponentially long time spent in local free-energy minima. However, single-molecule studies have shown that even for slow-folding proteins, actual transitions between unfolded and folded conformations are rapid (typically on the microsecond timescale) and similar to those of small fast-folding proteins (16). Therefore, if we can preferentially sample only the “reactive” parts of folding trajectories, it becomes feasible to accurately compute the folding pathways of even large proteins (17, 18, 19). In this work, we rely on a recently developed variational method called the bias functional (BF) approach (see Methods) to achieve this goal and sample transition paths. We use this scheme to characterize the folding and misfolding of wild-type (WT) A1AT, the pathological Z mutant, and the relatively benign S (Glu264Val) mutant (1), starting from several fully denatured conformations. The results of these all-atom simulations provide testable, atomistic models for how WT A1AT folds, how disease-associated mutants misfold, and how suppressor mutations can rescue misfolding. These results also shine light on the connections between single-point mutations and the pathogenicity of misfolding-prone proteins and provide physical mechanisms responsible for misfolding phenotypes.
Methods
The computational limitations of conventional MD have motivated the development of more sophisticated algorithms and approximations to study rare biomolecular transitions. Among these, well-tempered metadynamics has proved to be a powerful tool to profile the potential of the mean force of the slowest collective variables in a system (20). Conversely, transition-path sampling (17) offers a framework to directly sample reaction pathways, whereas transition-interface sampling (21) and milestoning (22) enable the investigation of reaction kinetics. Unfortunately, to our knowledge, none of these powerful equilibrium and nonequilibrium methods have been successfully applied to reactions as complex and slow as serpin folding and misfolding.
The BF approach
In the BF approach (23), a set of variationally optimized trajectories connecting an ensemble of fully denatured serpin conformations to folded and misfolded conformations is obtained through the following three-step procedure, which is schematically represented in Fig. 1.
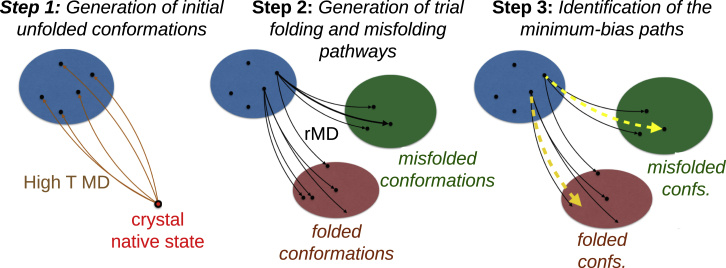
Figure 1.
Schematic representation of the BF algorithm. Black solid lines are trial ratchet-and-pawl (rMD) trajectories obtained starting from conformations in the unfolded set U (blue) generated in step 1. The dashed yellow line is the minimal bias path, for which the value of the bias functional T given in Eq. 2 is least. To see this figure in color, go online.
Step 1: Generation of initial denatured conformations
We sampled an ensemble of fully unfolded conformations (with a fraction of native contacts, Q < 0.1) by performing thermal unfolding MD simulations at 1600 K for 10 ns with an integration time of 1 fs starting from the WT native structure. Thermal unfolding was followed by a 10 ns relaxation at room temperature, T = 300 K.
Step 2: Generation of trial folding and misfolding pathways
From each unfolded conformation Xi obtained in step 1, we initiated 12 ratchet-and-pawl MD (rMD) simulations. In rMD, the standard MD at room temperature T is modified by adding an unphysical, history-dependent biasing force FrMDi, which is defined as follows (24, 25):
| (1) |
where, for a given BF folding trajectory, X(t) is the A1AT conformation at time t. In this equation, z(X) is a collective variable that measures the distance between the protein’s instantaneous contact map Cij(X(t)) and a reference contact map, calculated from the native-state crystal structure Xnative, i.e., z(X) = Σi<j [ Cij(X) − Cij(Xnative)]2. Cij is a function of the distance between atoms i and j, which interpolates between 0 and 1 and provides a continuum representation of the contact map Cij(X) = [1 − (rij/r0)6]/[1 − (rij/r0)10], where r0 = 0.75 nm. The variable zm(t) in Eq. 1 denotes the minimal value assumed by the collective coordinate z up to time t along the given rMD trajectory. Thus, the biasing force (Eq. 1) remains latent (equals 0) anytime an rMD step leads to no change or to an increase of the overlap between the instantaneous and the native contact maps, i.e., if z(X(t + Δt)) ≤ zm(t). On the other hand, the bias switches on whenever an elementary integration step leads to a decrease of the overlap with the native state, i.e., for z(X(t + Δt)) > zm(t).
Step 3: Identification of the least-biased transition pathways
The rMD paths generated in step 2 are all affected by the presence of the unphysical biasing force FirMD. However, it can be rigorously shown (23) that the trajectories in this trial path ensemble with the largest probability to occur in an unbiased Langevin simulation are those with the smallest value of the so-called bias functional:
| (2) |
Here, γi and mi denote the viscosity and mass of the ith atom, respectively.
Thus, for each denatured conformation Xi, we retain only one minimal bias trajectory and discard all other trial rMD paths. This procedure keeps to a minimum the systematic errors introduced by the biasing force. It is important to recall that, in rMD, any time the chain progresses toward conformations with increased numbers of native contacts, the biasing force is not active. Furthermore, the strength of the biasing force is chosen to ensure that the modulus of the total biasing force is always at least two orders of magnitude smaller than that of the physical force. Consequently, the partially folded and misfolded structures obtained in our simulations are sensitive to the details of the underlying force field. The parameter kR in Eq. 1, setting the strength of the biasing force, was 0.02 kJ/mol.
On the other hand, the presence of a nonphysical biasing force could in principle promote the early formation of some local native contacts. Although the BF variational principle is supposed to minimize these artifacts, some residual systematic errors may persist.
Simulations details
The x-ray crystal structure of active, native WT A1AT from Elliott and co-workers (26) (Protein Data Bank (PDB): 1QLP) was used as the target structure for WT BF folding simulations. To our knowledge, the first 22 amino acids are disordered in all published A1AT structures. Thus, the sequences for the folding simulations start at Phe23. We note that, from a kinetic point of view, this active native state is only metastable. Indeed, A1AT spontaneously performs an irreversible conformational reaction into a fully stable yet biologically latent structure with a mean first-passage time on the order of days to weeks. The latency transition was previously characterized in atomistic detail using a variant of the BF algorithm (27). Further details on the simulations can be found in Supporting Materials and Methods.
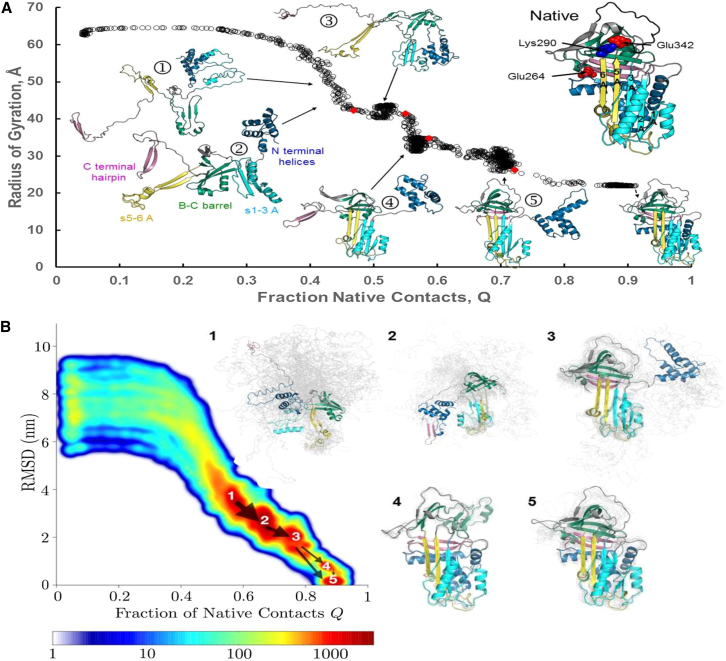
Even under the effect of the rMD biasing force, a residual degree of frustration persists. As a result, only 2% of our short WT A1AT least-biased trajectories (LBTs) reach the fully folded structure within the simulated time window. The majority of LBTs reach a fraction of native contacts of at least 0.4, corresponding to the formation of the local foldons (Fig. 2, stage 2A). 45% of all WT LBTs reach stage 4 or 5, as shown in Fig. 2 A, during which the central β-sheet A is fully formed and all that remains is the docking of the N- and C-termini. We observe that in BF trajectories, the final state may display single-strand crossovers that do not appreciably change the native contact map (Fig. S1). In particular, in the active, metastable A1AT structure, strands 3 and 4C cross over each other, and the direction of this crossover is reversed in the BF trajectories; see Fig. S1. However, this single-strand crossover does not alter the native contact map.
Figure 2.
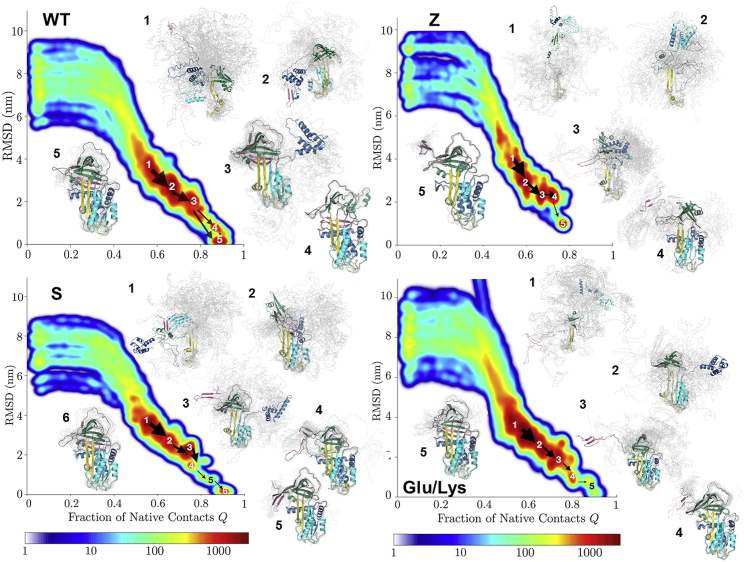
WT A1AT folding. (A) Changes in the WT radius of gyration, Rg, as a function of native contacts formed along a typical successful WT folding trajectory. Change points (34) are indicated by red diamonds. The A1AT structure colored by foldon and indicating the locations of the Z and S mutations and Lys290 is shown on the upper right. (B) A kinetic free-energy landscape generated from all frames in all WT LBTs that reach the native state is shown and colored according to population density from blue (low populations) to red (high populations). The arrows indicate fluxes between the labeled local minima, and arrow widths are proportional to the flux. Representative conformational ensembles from each of the labeled minima, harvested as described in the Supporting Materials and Methods, are shown to the right in gray with a single representative conformation colored as in (A).
Results and Discussion
The BF approach
Using the BF algorithm (23), it is feasible to use standard computer clusters to generate many folding and misfolding events for proteins as large as serpins using state-of-the-art all-atom force fields. This algorithm identifies the most realistic reaction pathways within an ensemble of uncorrelated trial trajectories, generated by the so-called rMD (24, 25). In rMD, a biasing force is introduced only when the chain tends to backtrack toward the unfolded state, and it is not applied when the chain spontaneously progresses toward the native state. In the BF approach, a rigorously derived variational condition is applied to identify the trial rMD pathways that have the largest probability to occur in the absence of any bias. In the following discussion, we shall refer to these paths as LBTs.
The accuracy of all variational approaches critically depends on the quality of the model subspace, i.e., the set of possible solutions, within which the optimal approximate solution is identified through the variational condition. In the BF approach, the model subspace is defined by the trial-folding trajectories generated using the rMD protocol. The BF method thus provides the relative statistical weights of the trial trajectories in our variational space rather than the absolute statistical weights for individual trajectories. Because a suboptimal choice of the model subspace may introduce systematic errors, variational approaches must be carefully benchmarked against exact methods and extensively validated against experimental data.
The BF method was benchmarked against the results of MD folding simulations performed using an Anton supercomputer (23, 28), with both methods using the same all-atom force field. In particular, the folding mechanism and the precise order of native contact formation during the folding of an all-beta protein (WW domain Fip35) and of an all-alpha protein (villin headpiece subdomain) predicted by the BF method were found to be statistically indistinguishable from those obtained using conventional MD methods. The predictions of the BF approach have also been validated against experimental data. For example, the BF method was used to study the folding of two alpha proteins consisting of nearly 100 amino acids (IM7 and IM9). These two proteins have highly homologous native structures but very different folding mechanisms: Im7 folds via a three-state mechanism, whereas the folding of Im9 is in two states (29). The BF method correctly reproduced these experimental observations (30). Furthermore, in a very recent investigation (31), the BF approach has been interfaced with quantum electronic structure calculations to yield a direct prediction of the expected time-resolved near and far ultraviolet circular dichroism spectra in the folding of the proteins canine lysozyme and IM7. The BF results quantitatively agree with the experimental data, correctly predicting the existence of a folding intermediate and reproducing the difference between the intermediate and native state near ultraviolet circular dichroism spectra, which reports on the local environment of aromatic residues.
An early version of the BF algorithm (called the dominant reaction pathway) was also successfully applied to sample the conformational transition leading to serpin latency (27). These BF simulations predicted the atomistic structure of an intermediate in the active-to-latent transition for the serpin plasminogen activator inhibitor (PAI-1) and provided an atomistically detailed picture that explained why binding a specific small molecule accelerates the PAI-1 latency transition. For both PAI-1 and A1AT, the BF simulations reproduced the experimentally observed propensity of specific point mutations to accelerate or retard the latency transition. This study also delineated differences between the PAI-1 latency transition and that of A1AT to help explain why PAI-1 easily accesses the latent state whereas A1AT does not.
To further explore serpin conformational changes and to validate the BF simulation results, we begin this study by simulating and analyzing the folding of the WT A1AT. We compare the results of the simulations to available experimental results and provide testable, atomistically resolved data, which to our knowledge are new, on how WT A1AT folds. These WT simulations also provide the reference for comparing folding and misfolding pathways. We therefore proceed to simulate and analyze the folding of disease-associated A1AT variants and suppressor mutants.
WT A1AT folding pathways
For all of the A1AT variants, folding starts with the independent formation of local structures (Fig. 2 A, stage 1) that we refer to as foldons, based on the usage of Wolynes, Englander, and co-workers (32, 33). In the majority of successful WT trajectories, foldons dock in a well-defined order, as determined from visual inspection, from the plot of the radius of gyration versus fraction of native contacts and from an automated statistical analysis that identifies the most relevant change points (34) (Fig. 2 A).
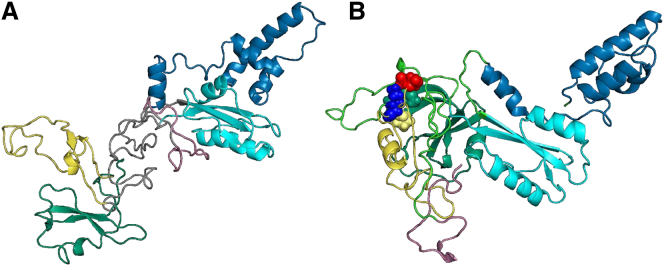
In WT LBTs, initial foldon formation is followed by the formation of native interactions between residues at the top of strands 5 and 6A (s5/6A) in the nascent sheet A and the nascent B-C barrel formed by parts of strands 3C, 4C, and 1 to 3B (Fig. 2 A, stage 2). In particular, a hydrogen bond is formed between Glu342 and Thr203, and van der Waals interactions are established between Pro289 and Met residues 220 and 221. To assess the role of rMD forces in maintaining these interactions, the structures corresponding to stages 1 (foldon formation) and 2 (early interfoldon interactions) in Fig. 2 A were subjected to 200 ns of conventional MD simulations in explicit solvent. In these MD simulations, the C-terminal β-hairpin formed by strands 4/5B is not stable, which is consistent with the experimental finding that the isolated peptide composed of the 36 C-terminal A1AT residues lacks stable structure (35). In contrast, as shown in Fig. 3, the three N-terminal foldons and the network of interactions between the nascent B-C barrel and s5/6A are stable on the timescale of these conventional MD simulations even in the absence of any additional stabilizing forces.
Figure 3.
(A) Stability of A1AT foldons. The structure from stage 1 in Fig. 2A after 200 ns of conventional MD simulations in explicit solvent is shown. The three foldons in the N-terminal two-thirds of A1AT are stable on this timescale. (B) Stability of the contacts between s5/6A and the B-C barrel is shown. The structure from stage 2 in Fig. 2A after 200 ns of conventional MD simulations in explicit solvent is shown. Glu342, red; Lys290, blue; Pro289, yellow; Met220 and Met221, green; Thr 203, gray. The ribbon structure is colored by foldon, as described in Fig. 2A. To see this figure in color, go online.
In most of the WT trajectories that reach the native conformation (see Video S1), nonnative interactions between the loop at the top of strand 3A and β-strands 2 and 3B prevent correct positioning of the B-C barrel leading to the first barrier (Fig. 2 A, stage 3). Disruption of this steric hindrance allows for the correct positioning of the B-C barrel and docking of s5/6A to s1-3A, thus completing β-sheet A formation (Fig. 2 A, stage 4). At this stage, the C-terminal β-hairpin (s4/5B) and the N-terminal helices are solvent exposed and free to move on flexible linkers. Folding completes when the C-terminal hairpin docks to strands 1 to 3B (Fig. 2 A, stage 5) followed by packing of the N-terminal helices and s6B on the back of the β-sheets.
Although the results from one successful WT folding trajectory are informative, further insight into A1AT folding can be gained by studying patterns that emerge from all of the successful LBTs. To this end, a density plot was generated by projecting all frames from all successful WT simulations onto the plane defined by the root mean-square distance from the native x-ray crystal structure (PDB: 1QLP (26)) and fraction native contacts, Q (Fig. 2 B) (see Supporting Materials and Methods). In this projection, metastable states (local energy minima) are identified as densely populated regions (red) separated by sparsely populated regions corresponding to barriers. Due to the action of the rMD force, the barriers in this representation can only provide lower bounds on the true free-energy barriers. This representation can therefore be thought of as a kinetic free-energy landscape. Also shown are representative conformational ensembles (gray) harvested from each basin, as described in Supporting Materials and Methods, with a representative structure shown in color.
As expected, the metastable states in the WT density plot correlate with the most highly populated intermediates along the single LBT (Fig. 2 A), with the addition of a new near-native metastable state (basin 4) associated with a minor folding pathway in which consolidation of the B-C barrel is the last folding step. Local minima corresponding to the progressive docking of the initially formed foldons are clearly evident, identifying the major successful folding pathway. Closer inspection of the major basins and the fluxes between them reveals that for the major pathway, the order of folding events is as follows: initial nonnative interactions between the foldons, the completion of sheet A while both N- and C-termini remain exposed, docking of the C-terminal β-hairpin, and finally, docking of the N-terminal helices, in agreement with the single representative trajectory in Fig. 2 A.
Our finding that completion of sheet A precedes the incorporation of C-terminal hairpin into sheet B agrees with fragment complementation studies in which docking of a fragment containing the C-terminal hairpin with a larger N-terminal A1AT fragment requires the presence of s5A (11) and thus, presumably, completion of sheet A. Similarly, in kinetic refolding experiments monitored by oxidative labeling of side chains or hydrogen/deuterium exchange of backbone amides detected by mass spectrometry (MS), s5A is one of the last regions to acquire native-like protection (12, 13). These same MS-based studies found that s4B remains solvent-exposed until late in the folding process, which is consistent with the late packing of the C-terminal hairpin (s4/5B) seen in our simulations.
Although A1AT has only a single Cys residue and no disulfide bonds, folding studies of two serpins containing disulfide bonds, ovalbumin and antithrombin III, show that N-terminal disulfide bond formation and rearrangements occur after the C-terminus packs (36, 37). These experimental results imply that the N-terminus is involved in the final stage of serpin folding. The large conformational transition performed by the N-terminal foldon in the last stage of WT A1AT folding suggests that the last steps in A1AT, ovalbumin, and antithrombin III folding are similar and provides a testable, atomistically detailed explanation for the experimental results. Recently, the folding of A1AT was investigated using a coarse-grained, one-bead-per-residue Go model (38). Detailed comparisons with this study are difficult because of the different representations of the polypeptide chain, the differences in the force fields used, and the high temperature used for the Go model equilibrium simulations. Nonetheless, although the details differ, we do note broad agreement between the two studies in that formation of the B-C barrel region precedes full formation of the domain containing sheet A and the N-terminal helices.
The BF WT A1AT folding simulations are in good agreement with existing serpin folding data and set the stage for generating detailed models of mutation-induced serpin misfolding.
Misfolding of A1AT disease-associated variants
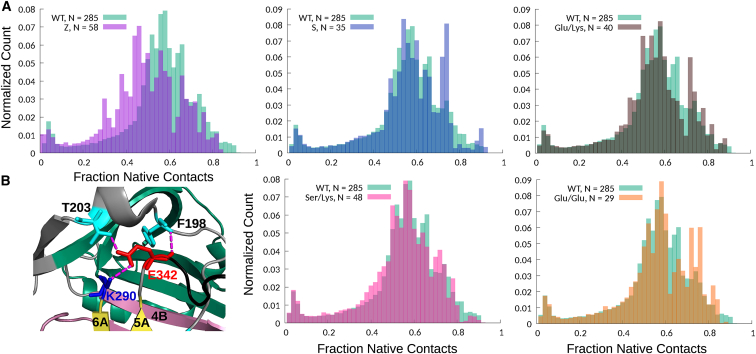
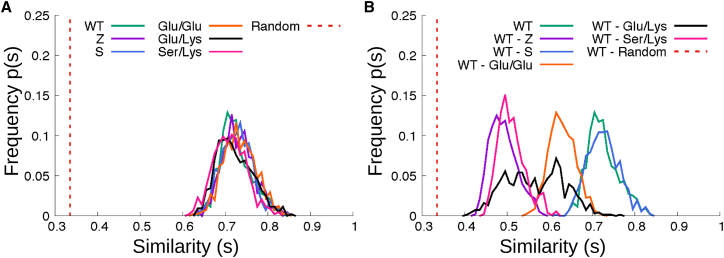
BF folding simulations for the S (Glu264Val) and Z (Glu342Lys) mutants suggest that these two A1AT variants misfold differently. To effectively analyze the differences among WT, S, and Z folding and misfolding for these nonequilibrium BF folding simulations, we focused on the observation that mutants with higher misfolding propensity are less likely to reach conformations in which almost all of the native contacts have been formed. To quantify this measure for each A1AT variant, we used all of the frames from all of the LBTs generated for each A1AT variant to construct a histogram describing how often each variant visits conformations with a given fraction of native contacts, Q (Fig. 4 A). Comparisons between such histograms for WT and Z simulations correctly predict that the pathological Z mutant is significantly less able to populate structures close to the native state, and the statistical significance of this difference is confirmed by a Kolmogorov-Smirnov test, as described in Supporting Materials and Methods. In fact, none of the Z mutant LBTs successfully reached the fully folded state. In contrast, the Q histograms for S and WT trajectories are similar, which is consistent with the observation that the S mutation has only mild effects on A1AT folding (1) (Fig. 4 A).
Figure 4.
(A) Comparisons of the fraction of native contacts, Q, formed during folding and misfolding LBTs. For the indicated variants with N total LBTs, histograms were calculated using all frames from all N trajectories. The data for Z (E342K, N = 58) and S (E264V, N = 35) are in purple and blue, respectively. Suppressor variants with mutations at residues 290 and/or 342 are Glu/Glu (K290E/E342, N = 25), Glu/Lys (K290E/E342K, N = 40), and Ser/Lys (K290S/E342K, N = 48) in orange, gray, and pink, respectively. In all of the histograms, the WT (N = 285) data are in green, and darker-colored regions represent the overlap between WT and mutant distributions. (B) A close-up view shows the network of electrostatic and hydrogen-bonding interactions formed by Glu342 in WT A1AT.
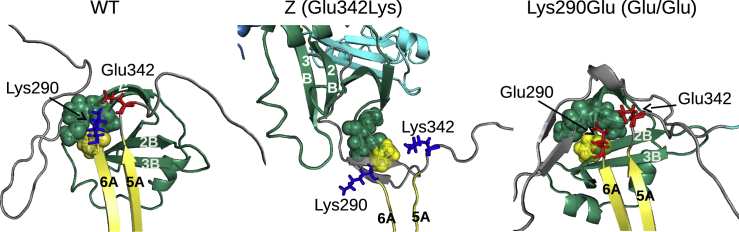
The Z mutation disrupts a network of electrostatic and hydrogen-bonding interactions at the top of β-sheet A (Fig. 4 B). Experimentally, Z misfolding can be rescued by mutating Lys290 to Glu (39), leading to a reversed version of the original salt bridge (Lys290Glu/Glu342Lys or Glu/Lys) and reducing the probability of steric clashes. Consistent with the experiment, our Glu/Lys simulations show a WT-like distribution of native contacts (Fig. 4 A).
One way to test the relative roles of electrostatics and sterics in Z misfolding is to place Glu at both 342 and 290 (Lys290Glu/Glu342 or Glu/Glu), thus preserving the electrostatic repulsion present in Z but reducing the side chain length. Consistent with cellular studies in which Z and Glu/Glu show ∼20 and 75% secretion efficiency, respectively, relative to WT A1AT (40), the distribution of native contacts in the Glu/Glu simulations resembles that of WT (Fig. 4 A; Video S2). The fact that both experiments and computations indicate that Glu/Glu can fold with reasonable efficiency suggests that the 290/342 salt bridge may not be essential for A1AT folding. To further test this hypothesis, we determined whether replacing Lys290 with Ser could rescue Z (K290S/E342K or Ser/Lys) simply by alleviating steric clashes. The resulting LBTs are more WT-like than Z-like (Fig. 4 A).
Further insight into the effects of mutations on intermediates populated during A1AT folding/misfolding can be gained by comparing the kinetic free-energy landscapes of WT and the various mutants (Figs. 5, S2, and S3). The Z mutant energy landscape is markedly different from that of WT. In addition to the fact that none of the Z trajectories achieve more than 80% of the total native contacts, all of the major free-energy basins in the Z landscape are populated by structures that are less folded than the (roughly) corresponding WT intermediates. This result suggests that the folding of Z diverges from that of WT early in the folding pathway. Even in the most native-like structures attained by Z, sheet B fails to fully form, leaving strands 4 and 5B solvent-exposed (basin 5 in Fig. 5). However, few trajectories reach this state, and most are trapped in more unfolded conformations in which both sheets A and B fail to fully form. It is known that Z polymerizes from a partially folded conformation (4, 41), although the nature of this species, like the structure of the resulting polymers, is a matter of debate. The heterogenous mixture of partially folded Z conformations generated by the BF folding simulations makes it difficult to identify a single candidate polymerization-prone species.
Figure 5.
Kinetic free-energy landscapes for WT A1AT and the Z, S, and Glu/Lys variants. Also shown are ensembles of representative structures from the identified basins generated, as described in Supporting Materials and Methods and Fig. 2B. Landscapes and ensembles for the Ser/Lys and Glu/Glu mutants are shown in Figs. S2 and S3.
Because the majority of the trajectories result in Z conformations with incomplete β-sheets, we selected one such representative structure and subjected it to conventional MD simulations in explicit solvent to allow secondary structure to relax in the absence of rMD forces. After 100 ns, sheet A and half of sheet B as well as regions of sheet C are substantially disordered (Fig. S4). Nevertheless, enough secondary structure is retained to give a calculated circular dichroism spectrum (42) that is more characteristic of a folded protein than an unstructured coil. The structure is also relatively compact, with a radius of gyration of 24.9 Å2, which is only 12% larger than that of native A1AT (21.9 Å2). Ekeowa et al. used ion mobility MS to measure the collisional cross-section (CCS) of a polymerigenic species of WT A1AT that is populated upon mild heating and has been proposed as a model for the polymerization-prone form of Z (43). They found that the CCS of the polymerigenic species is 118 ± 17% that of the folded monomer. The CCS of our misfolded Z structure after 100 ns of MD is 123% that of folded WT A1AT (CCS calculated from structures using the program IMPACT (44)). All of these results suggest that Z misfolds to a state that, in terms of compactness and secondary structure, has substantial native-like character, which is consistent with arguments that Z polymerizes from a state that is at least somewhat native-like rather than globally unfolded (41).
In contrast to Z, the energy landscape of the mildly deficient S mutant resembles that of WT (Fig. 5), and the major intermediates populated during folding are more structured than those of Z. The same is true for the Glu/Lys rescue mutant. Interestingly, in both of these variants, the order of the C- and N-termini docking is the opposite of that in WT. In fact, in S and Glu/Lys, Ser/Lys, and Glu/Glu, the N-terminal helices dock first, followed by packing of the C-terminal hairpin (Figs. 5, S2, and S3). In the case of S, this delayed C-terminal packing is likely explained by the fact that the Glu264Val mutation eliminates a salt bridge between Glu264 in helix G and Lys387 in the C-terminal hairpin (45). This electrostatic interaction would favor docking of the C-terminus. The reason why C-terminal packing is delayed is less clear for Glu/Lys and the other rescue mutants, but the relative orientations of the B-C barrel and sheet A are subtly different from that observed in WT (Fig. 5).
In S and WT trajectories, misfolding occurs late, resulting from premature docking of the N- terminal helices, which leaves the C-terminus solvent exposed (Fig. 5; Video S3). Premature docking of the N-terminal helices to the rest of A1AT is essentially irreversible in the BF simulations because of the number of native contacts gained in these interactions. However, in experiments, this misfolding may be reversible, and in cells, interactions between the lectin chaperones and the N-terminal glycans at Asn residues 46 and 83 could help protect against this late misfolding. 100 ns of standard MD simulations of misfolded S with the C-terminus exposed show that it remains structured on this timescale (Fig. S5). In contrast, misfolded Z loses ordered structure during simulations on this same timescale (Fig. S4). This difference in stability mirrors the relative pathogenicity of these two mutations (1, 41).
In striking contrast, Z and WT folding diverge much earlier (Video S4). As discussed above, the Z mutation replaces a conserved salt bridge between Lys290 and Glu342 (Fig. 6) with a charge-charge repulsion. This mutation appears to disrupt early native and nonnative interactions between β-strands 5/6A and the B-C barrel. Electrostatic repulsion and steric hindrance, because of the length of the two Lys side chains (Lys290/Glu342Lys), increase the probability that these residues assume a nonnative spatial orientation. In contrast to Z, Glu/Glu achieves WT-like docking between s5/6A and the B-C barrel despite the electrostatic repulsion between the two Glu residues. This supports the hypothesis that sterics in addition to electrostatics plays a key role in Z misfolding.
Figure 6.
The divergence of WT and Z folding with close-up views of the region containing residues 290 and 342 (E: red and K: blue) and P289 (yellow) with T203, M220, and M221 in green. Glu/Glu is also shown. Structures are representative snapshots taken from basin 2 (Figs. 2 and 5) of the BF simulations. To see this figure in color, go online.
Investigating the order of contact formation
Although the various rescue mutants restore the correct docking of s5/6A to the B-C barrel, it can be seen from the ensembles in Figs. 5, S2, and S3 that they do not necessarily restore the full WT folding pathway. To investigate this more quantitatively, we performed a statistical analysis based on the distribution of path similarity s introduced in (46) and defined in Supporting Materials and Methods. This parameter is equal to 1 when native contacts in two reactive trajectories are formed in exactly the same order, whereas s = 0 when the order is entirely different. For completely random sequences of native contact formation, the similarity distribution is sharply peaked at around 0.3. Path similarities calculated between LBTs for the same variant (self-similarity) or between trajectories of different variants (cross-similarity) are shown in Fig. 7. For any given A1AT variant, the self-similarity distribution obtained from all of the LBTs peaks around 0.6 (Fig. 7 A). In contrast, comparisons between different variants show that Z LBTs differ significantly from WTs (peak s ∼ 0.4), whereas S and WT pathways are similar (peak s ∼ 0.6) (Fig. 7 B).
Figure 7.
Distributions of path similarities for WT and mutants (see Supporting Materials and Methods). The vertical dotted line indicates the average similarity score for two random sequences of native contact formation. Self-similarities are shown in (A). Differences between the WT paths and the various mutant paths are shown in (B). The coloring is the same as in Fig. 4A. To see this figure in color, go online.
Path similarity analyses also indicate that although mutations at Lys290 may suppress Z misfolding, the paths are very different for different combinations of residues at positions 290 and 342. Folding of the Glu/Glu variant, where Lys290 is mutated to Glu in the WT background providing an anionic charge repulsion between residues 290 and 342 compared to the cationic repulsion in the Z mutant, is most WT-like. The folding of the Ser/Lys variant diverges the most from WT, whereas the order in which contacts are formed in Glu/Lys—the charge-reversed salt bridge in the Z background—spans a large range from WT-like to quite different (Fig. 7 B).
We note that the native-state contact map, which defines the collective coordinate along which the biasing force acts, is not significantly affected by the mutations, as the structures of WT and the mutants are nearly indistinguishable apart from the mutated residue itself. This is unlikely to be due to the fact that we introduced the mutations computationally, as the crystal structure of the Z mutant was recently published, and its structure is essentially superimposable on that of WT (34). Thus, the collective coordinate along which the biasing force acts was essentially identical for all variants studied. This strongly suggests that the differences observed are due to the sterics and energetics of the mutations themselves rather than to the biasing force.
These results, along with the finding that in the BF folding simulations all of the salt-bridge variants are more likely to fold than Z, suggest that A1AT can fold using a number of alternative folding pathways. Moreover, they show that the Lys290-Glu342 salt bridge, although not required for folding, is important for increasing the probability of the major folding mechanism observed in the WT A1AT BF folding simulations.
Experimental observables
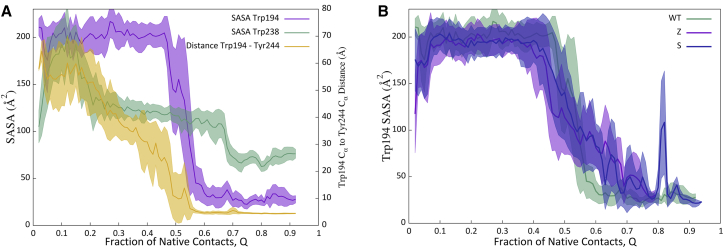
WT A1AT contains two Trp residues: 238 in strand 2B, and 194, C-terminal to strand 3A in the “breach” region at the top of sheet A. Studies of A1AT single Trp mutants in the WT background show that during equilibrium unfolding, Trp194 shows two distinct transitions corresponding to a native-to-intermediate transition followed by an intermediate-to-unfolded transition. In contrast, Trp238 shows a single broad transition (47). In addition, stopped-flow studies of WT A1AT refolding monitored by Trp fluorescence emission reported at least three kinetic phases for A1AT WT refolding: a very fast ∼50 ms phase, a slower ∼500 ms phase, and a very slow hundreds-of-seconds-long phase (9). Trp194 fluorescence may be altered both by changes in solvent accessibility and by quenching by Tyr244. Consistent with experimental observations, the Trp194-Tyr244 distance changes in two stages: one corresponding roughly to the formation of the foldons and the other occurring with the docking of strands 5 and 6A to the B-C barrel (Fig. 8 A). For WT A1AT Trp194, burial occurs approximately in a single step during the completion of β-sheet A (Fig. 8 A). Trp238 burial does not occur in a single step. Rather, it shows an initial large decrease in accessibility followed by a gradual decrease and then a later sharp drop. This is qualitatively consistent with the observation that Trp238 shows a single broad transition during equilibrium unfolding. The multiple transitions in the local Trp environments in our simulations are consistent with the multiple kinetic phases seen when refolding is monitored by Trp emission (9). Comparison of WT with S and Z (Fig. 8 B) and the rescue mutants (Fig. S6) shows that Trp194 is buried earlier in WT than in any of the mutants. This computational observation could potentially be tested using kinetic folding experiments monitored by Trp lifetimes or fast oxidative footprinting coupled to MS.
Figure 8.
(A) Evolution of solvent-accessible surface areas for Trp194 and Trp238 and the Trp194-Tyr244 distance during WT A1AT folding. Solid lines show the averages over all successful simulations, whereas shaded areas show the SDs. (B) The evolution of Tryp194 solvent-accessible surface area during folding of WT and the S and Z mutants is shown. To see this figure in color, go online.
The A1AT BF folding simulations suggest that the biggest folding differences between WT and other variants involve the relative timing for packing the N-terminal helices and the C-terminal β-hairpin. Perhaps the best way to monitor this difference is by looking at how the N-terminal helices/β-sheet A and the β-sheet A/C-terminal hairpin distances change as A1AT variants fold. Kinetic refolding experiments monitored by Förster resonance energy transfer have the potential to reveal such differences. A1AT contains a single Cys, Cys232 in s1B, and a number of amino acids in A1AT have been mutated to Cys without significantly impairing function or stability (48, 49, 50, 51). In particular, both Ser47 in the N-terminal helical region and Ser313 in the loop from helix I to strand 5A have been mutated to Cys. Calculation of the 47–232 and 47–313 distances suggest that Förster resonance energy transfer pairs at these locations are likely to be sensitive to docking of the N-terminal helices (Fig. S7). Similarly, comparative kinetic refolding experiments using oxidative labeling of side chains monitored by MS would provide a rigorous test of the BF-predicted folding mechanisms because foldon docking should significantly alter side chain accessibility.
Conclusions
In conclusion, our calculations provide a coherent atomistic and physics-based picture of serpin folding and misfolding. For WT A1AT, we find that there is a major folding pathway that begins with the initial assembly of local structural units, followed by higher-order associations, some of which involve nonnative contacts. The pathway ends with the incorporation of the C-terminal β-hairpin followed by docking of the N-terminal helices. These findings are supported by existing experimental data (9, 10, 11, 12, 13, 36, 37, 39, 40, 47), and the detailed molecular mechanisms provided here are experimentally testable. The multidimensional free-energy landscape for folding is complex, and as exemplified by the Z suppressor mutants, there are alternative ways to successfully fold to the native state. Our simulations of pathological and suppressor mutants also elucidate the mechanism of Z misfolding and make the prediction that misfolding occurs early in the folding process, which is a prediction that should be amenable to experimental testing.
The scheme presented in this work opens a way to investigate many important disease-associated processes that occur over minutes or hours using only ordinary, medium-sized computer clusters of the type available to most computational laboratories.
Author Contributions
F.W. designed, performed, and analyzed the simulations and wrote the article. S.O. analyzed the simulations, developed new analysis methods as necessary, and wrote the article. A.I. and G.S. analyzed the simulations and developed new analysis methods as necessary. S.a.B. designed and wrote the software to perform BF simulations. P.F. designed and analyzed simulations, developed new analysis methods as necessary, and wrote the article. A.G. and P.L.W. designed and analyzed simulations and wrote the article.
Acknowledgments
We thank David E. Shaw Research for providing the code for performing change-point analysis. We also thank several anonymous referees for valuable suggestions. S.O., G.S., and P.F. are cofounders of Sibylla Biotech (https://www.sibyllabiotech.it), a startup company focused on using advanced molecular simulation methods to develop new therapeutics.
This work was funded by the Alpha-1 Foundation (P.L.W. and P.F.) and National Institutes of Health grant GM094848 (A.G.).
Editor: Rohit Pappu.
Footnotes
Fang Wang and Simone Orioli contributed equally to this work.
Supporting Materials and Methods, seven figures, and four videos are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30397-7.
Contributor Information
Anne Gershenson, Email: gershenson@biochem.umass.edu.
Pietro Faccioli, Email: pietro.faccioli@unitn.it.
Patrick L. Wintrode, Email: pwintrod@rx.umaryland.edu.
Supporting Citations
References (52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62) appear in the Supporting Material.
Supporting Material
References
- 1.Gooptu B., Lomas D.A. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu. Rev. Biochem. 2009;78:147–176. doi: 10.1146/annurev.biochem.78.082107.133320. [DOI] [PubMed] [Google Scholar]
- 2.Chapman K.R., Burdon J.G., McElvaney N.G., RAPID Trial Study Group Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 3.Teckman J.H., Jain A. Advances in alpha-1-antitrypsin deficiency liver disease. Curr. Gastroenterol. Rep. 2014;16:367. doi: 10.1007/s11894-013-0367-8. [DOI] [PubMed] [Google Scholar]
- 4.Yu M.H., Lee K.N., Kim J. The Z type variation of human α1-antitrypsin causes a protein folding defect. Nat. Struct. Biol. 1995;2:363–367. doi: 10.1038/nsb0595-363. [DOI] [PubMed] [Google Scholar]
- 5.Novoradovskaya N., Lee J., Brantly M. Inhibition of intracellular degradation increases secretion of a mutant form of α1-antitrypsin associated with profound deficiency. J. Clin. Invest. 1998;101:2693–2701. doi: 10.1172/JCI549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teckman J.H., Perlmutter D.H. Retention of mutant α(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G961–G974. doi: 10.1152/ajpgi.2000.279.5.G961. [DOI] [PubMed] [Google Scholar]
- 7.Kamimoto T., Shoji S., Yoshimori T. Intracellular inclusions containing mutant α1-antitrypsin Z are propagated in the absence of autophagic activity. J. Biol. Chem. 2006;281:4467–4476. doi: 10.1074/jbc.M509409200. [DOI] [PubMed] [Google Scholar]
- 8.Lomas D.A., Evans D.L., Carrell R.W. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 9.Kim D., Yu M.H. Folding pathway of human α1-antitrypsin: characterization of an intermediate that is active but prone to aggregation. Biochem. Biophys. Res. Commun. 1996;226:378–384. doi: 10.1006/bbrc.1996.1364. [DOI] [PubMed] [Google Scholar]
- 10.James E.L., Whisstock J.C., Bottomley S.P. Probing the unfolding pathway of α1-antitrypsin. J. Biol. Chem. 1999;274:9482–9488. doi: 10.1074/jbc.274.14.9482. [DOI] [PubMed] [Google Scholar]
- 11.Dolmer K., Gettins P.G. How the serpin α1-proteinase inhibitor folds. J. Biol. Chem. 2012;287:12425–12432. doi: 10.1074/jbc.M111.315465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsui Y., Dela Cruz R., Wintrode P.L. Folding mechanism of the metastable serpin α1-antitrypsin. Proc. Natl. Acad. Sci. USA. 2012;109:4467–4472. doi: 10.1073/pnas.1109125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocks B.B., Sarkar A., Konermann L. Early hydrophobic collapse of α1-antitrypsin facilitates formation of a metastable state: insights from oxidative labeling and mass spectrometry. J. Mol. Biol. 2012;423:789–799. doi: 10.1016/j.jmb.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Lindorff-Larsen K., Piana S., Shaw D.E. How fast-folding proteins fold. Science. 2011;334:517–520. doi: 10.1126/science.1208351. [DOI] [PubMed] [Google Scholar]
- 15.Bowman, G. R., V. S. Pande, and F. Noé, eds. 2013. An introduction to Markov state models and their application to long timescale molecular simulation. Advances in Experimental Medicine and Biology v. 797 (Springer).
- 16.Chung H.S., McHale K., Eaton W.A. Single-molecule fluorescence experiments determine protein folding transition path times. Science. 2012;335:981–984. doi: 10.1126/science.1215768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolhuis P.G., Chandler D., Geissler P.L. Transition path sampling: throwing ropes over rough mountain passes, in the dark. Annu. Rev. Phys. Chem. 2002;53:291–318. doi: 10.1146/annurev.physchem.53.082301.113146. [DOI] [PubMed] [Google Scholar]
- 18.Faccioli P., a Beccara S. Computing reaction pathways of rare biomolecular transitions using atomistic force-fields. Biophys. Chem. 2016;208:62–67. doi: 10.1016/j.bpc.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Rohrdanz M.A., Zheng W., Clementi C. Discovering mountain passes via torchlight: methods for the definition of reaction coordinates and pathways in complex macromolecular reactions. Annu. Rev. Phys. Chem. 2013;64:295–316. doi: 10.1146/annurev-physchem-040412-110006. [DOI] [PubMed] [Google Scholar]
- 20.Barducci A., Bussi G., Parrinello M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. Lett. 2008;100:020603. doi: 10.1103/PhysRevLett.100.020603. [DOI] [PubMed] [Google Scholar]
- 21.Escobedo F.A., Borrero E.E., Araque J.C. Transition path sampling and forward flux sampling. Applications to biological systems. J. Phys. Condens. Matter. 2009;21:333101. doi: 10.1088/0953-8984/21/33/333101. [DOI] [PubMed] [Google Scholar]
- 22.West A.M., Elber R., Shalloway D. Extending molecular dynamics time scales with milestoning: example of complex kinetics in a solvated peptide. J. Chem. Phys. 2007;126:145104. doi: 10.1063/1.2716389. [DOI] [PubMed] [Google Scholar]
- 23.a Beccara S., Fant L., Faccioli P. Variational scheme to compute protein reaction pathways using atomistic force fields with explicit solvent. Phys. Rev. Lett. 2015;114:098103. doi: 10.1103/PhysRevLett.114.098103. [DOI] [PubMed] [Google Scholar]
- 24.Paci E., Karplus M. Forced unfolding of fibronectin type 3 modules: an analysis by biased molecular dynamics simulations. J. Mol. Biol. 1999;288:441–459. doi: 10.1006/jmbi.1999.2670. [DOI] [PubMed] [Google Scholar]
- 25.Tiana G., Camilloni C. Ratcheted molecular-dynamics simulations identify efficiently the transition state of protein folding. J. Chem. Phys. 2012;137:235101. doi: 10.1063/1.4769085. [DOI] [PubMed] [Google Scholar]
- 26.Elliott P.R., Pei X.Y., Lomas D.A. Topography of a 2.0 A structure of alpha1-antitrypsin reveals targets for rational drug design to prevent conformational disease. Protein Sci. 2000;9:1274–1281. doi: 10.1110/ps.9.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cazzolli G., Wang F., Wintrode P.L. Serpin latency transition at atomic resolution. Proc. Natl. Acad. Sci. USA. 2014;111:15414–15419. doi: 10.1073/pnas.1407528111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.a Beccara S., Škrbić T., Faccioli P. Dominant folding pathways of a WW domain. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2330–2335. doi: 10.1073/pnas.1111796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friel C.T., Capaldi A.P., Radford S.E. Structural analysis of the rate-limiting transition states in the folding of Im7 and Im9: similarities and differences in the folding of homologous proteins. J. Mol. Biol. 2003;326:293–305. doi: 10.1016/s0022-2836(02)01249-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang F., Cazzolli G., Faccioli P. Folding mechanism of proteins Im7 and Im9: insight from all-atom simulations in implicit and explicit solvent. J. Phys. Chem. B. 2016;120:9297–9307. doi: 10.1021/acs.jpcb.6b05819. [DOI] [PubMed] [Google Scholar]
- 31.Ianeselli A., Orioli S., Mennucci B. Atomic detail of protein folding revealed by an ab initio reappraisal of circular dichroism. J. Am. Chem. Soc. 2018;140:3674–3682. doi: 10.1021/jacs.7b12399. [DOI] [PubMed] [Google Scholar]
- 32.Panchenko A.R., Luthey-Schulten Z., Wolynes P.G. Foldons, protein structural modules, and exons. Proc. Natl. Acad. Sci. USA. 1996;93:2008–2013. doi: 10.1073/pnas.93.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Englander S.W., Mayne L. The nature of protein folding pathways. Proc. Natl. Acad. Sci. USA. 2014;111:15873–15880. doi: 10.1073/pnas.1411798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Z., Dror R.O., Shaw D.E. Identifying localized changes in large systems: change-point detection for biomolecular simulations. Proc. Natl. Acad. Sci. USA. 2015;112:7454–7459. doi: 10.1073/pnas.1415846112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oktaviani N.A., Risør M.W., Mulder F.A. Optimized co-solute paramagnetic relaxation enhancement for the rapid NMR analysis of a highly fibrillogenic peptide. J. Biomol. NMR. 2015;62:129–142. doi: 10.1007/s10858-015-9925-8. [DOI] [PubMed] [Google Scholar]
- 36.Onda M., Nakatani K., Hirose M. Cleaved serpin refolds into the relaxed state via a stressed conformer. J. Biol. Chem. 2008;283:17568–17578. doi: 10.1074/jbc.M709262200. [DOI] [PubMed] [Google Scholar]
- 37.Chandrasekhar K., Ke H., Hebert D.N. Cellular folding pathway of a metastable serpin. Proc. Natl. Acad. Sci. USA. 2016;113:6484–6489. doi: 10.1073/pnas.1603386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giri Rao V.V.H., Gosavi S. On the folding of a structurally complex protein to its metastable active state. Proc. Natl. Acad. Sci. USA. 2018;115:1998–2003. doi: 10.1073/pnas.1708173115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brantly M., Courtney M., Crystal R.G. Repair of the secretion defect in the Z form of α 1-antitrypsin by addition of a second mutation. Science. 1988;242:1700–1702. doi: 10.1126/science.2904702. [DOI] [PubMed] [Google Scholar]
- 40.Sifers R.N., Hardick C.P., Woo S.L. Disruption of the 290-342 salt bridge is not responsible for the secretory defect of the PiZ α 1-antitrypsin variant. J. Biol. Chem. 1989;264:2997–3001. [PubMed] [Google Scholar]
- 41.Lomas D.A., Hurst J.R., Gooptu B. Update on α-1 antitrypsin deficiency: new therapies. J. Hepatol. 2016;65:413–424. doi: 10.1016/j.jhep.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Mavridis L., Janes R.W. PDB2CD: a web-based application for the generation of circular dichroism spectra from protein atomic coordinates. Bioinformatics. 2017;33:56–63. doi: 10.1093/bioinformatics/btw554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekeowa U.I., Freeke J., Lomas D.A. Defining the mechanism of polymerization in the serpinopathies. Proc. Natl. Acad. Sci. USA. 2010;107:17146–17151. doi: 10.1073/pnas.1004785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marklund E.G., Degiacomi M.T., Benesch J.L. Collision cross sections for structural proteomics. Structure. 2015;23:791–799. doi: 10.1016/j.str.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Loebermann H., Tokuoka R., Huber R. Human α 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J. Mol. Biol. 1984;177:531–557. [PubMed] [Google Scholar]
- 46.Camilloni C., Broglia R.A., Tiana G. Hierarchy of folding and unfolding events of protein G, CI2, and ACBP from explicit-solvent simulations. J. Chem. Phys. 2011;134:045105. doi: 10.1063/1.3523345. [DOI] [PubMed] [Google Scholar]
- 47.Tew D.J., Bottomley S.P. Probing the equilibrium denaturation of the serpin α(1)-antitrypsin with single tryptophan mutants; evidence for structure in the urea unfolded state. J. Mol. Biol. 2001;313:1161–1169. doi: 10.1006/jmbi.2001.5104. [DOI] [PubMed] [Google Scholar]
- 48.Sivasothy P., Dafforn T.R., Lomas D.A. Pathogenic α1-antitrypsin polymers are formed by reactive loop-beta-sheet A linkage. J. Biol. Chem. 2000;275:33663–33668. doi: 10.1074/jbc.M004054200. [DOI] [PubMed] [Google Scholar]
- 49.Liu L., Mushero N., Gershenson A. Conformational distributions of protease-serpin complexes: a partially translocated complex. Biochemistry. 2006;45:10865–10872. doi: 10.1021/bi0609568. [DOI] [PubMed] [Google Scholar]
- 50.Liu L., Werner M., Gershenson A. Collapse of a long axis: single-molecule Förster resonance energy transfer and serpin equilibrium unfolding. Biochemistry. 2014;53:2903–2914. doi: 10.1021/bi401622n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maddur A.A., Swanson R., Olson S.T. Kinetic intermediates en route to the final serpin-protease complex: studies of complexes of α1-protease inhibitor with trypsin. J. Biol. Chem. 2013;288:32020–32035. doi: 10.1074/jbc.M113.510990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindorff-Larsen K., Piana S., Palmo K., Maragakis P., Klepeis J.L., Dror R.O., Shaw D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinforma. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hess B., Kutzner C., Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 54.Orioli S., a Beccara S., Faccioli P. Self-consistent calculation of protein folding pathways. J. Chem. Phys. 2017;147:064108. doi: 10.1063/1.4997197. [DOI] [PubMed] [Google Scholar]
- 55.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38, 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 56.Huang X., Zheng Y., Zhou A. Molecular mechanism of Z α1-antitrypsin deficiency. J. Biol. Chem. 2016;291:15674–15686. doi: 10.1074/jbc.M116.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jorgensen W.L., Chandrasekhar J., Klein M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- 58.Best R.B., Zhu X., Mackerell A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 60.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 61.Oliphant T.E. Python for scientific computing. Comput. Sci. Eng. 2007;9:10–20. [Google Scholar]
- 62.McGibbon R.T., Beauchamp K.A., Pande V.S. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 2015;109:1528–1532. doi: 10.1016/j.bpj.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.