Abstract
Aquatic animals are known for their myriad of beneficial bacteria with diverse biologically active compounds. The current study was aimed to isolate and characterize potentially beneficial lactic acid bacteria from Nile Tilapia and evaluate their pharmaceutical applications. The fish samples were dissected and stomach, intestine, and gills were collected and serially diluted for the isolation of lactic acid bacteria (LAB) on BCP agar media. Identification of isolate was carried by biochemical and molecular characterization using API kit and 16S rRNA gene sequencing analysis, respectively. Further, KS-TN11 was assessed for α-glucosidase inhibitory potential using the chromogenic method. A lactic acid bacterium KS-TN11 was isolated from the stomach of Nile Tilapia and identified as Leuconostoc mesenteroides. Effect of KS-TN11 on lipid accumulation in adipocytes was done by using Oil Red O staining. The isolate showed strong antibacterial activity against a number of pathogenic bacteria in vitro. In addition, L. mesenteroides KS-TN11 KS-TN11 (50 mg/ml and 100 mg/ml) tends to inhibit adipogenesis in 3T3-L1 adipocytes and thus may have possible anti-obesity effects. Moreover, L. mesenteroides KS-TN11 exhibited substantial α–glucosidase inhibitory activities by 41.33% at 50 mg/ml and 64% at 100 mg/ml, respectively. The bacterium showed potent antibacterial activity against a number of pathogenic bacteria; in addition to alpha-glucosidase activity, and inhibition of lipid accumulation in 3T3-L1 cell line. These results reinforce KS-TN11 as a novel bacterium with an impending pharmaceutical application.
Keywords: Lactic acid bacteria, Antimicrobial, Fish microflora, Anti-diabetic
1. Introduction
There are different types of indigenous lactic acid bacteria (LAB) present in nature, and aquatic animals hold a plethora of potential LAB with probiotic properties (Balcazar et al., 2008). Currently, LAB are used as probiotics adhering number of beneficial properties such as antibacterial activity (Rather et al., 2013), antifungal activity (Rather et al., 2014), immune modulation (Kamiya et al., 2017, Kim et al., 2012, Kober and Bowe, 2015, Tsai et al., 2010), anti-obesity (Tsai et al., 2014), and anti-diabetic properties (Bajpai et al., 2016) and so on. Due to the production of metabolic characteristics, LAB are considered as novel bacteria for their applications in food and pharmaceutical industry.
Further, obesity has become a very common and serious lifestyle disease over past few decades and continues to increase in developing countries. However, obesity is preventable by choosing a healthier diet and lifestyle. Lactic acid bacteria have been used since ages in fermented food products and have a number of beneficial properties. There are vast number of reports suggesting an antimicrobial effect of Lactobacillus spp. against intestinal pathogens particularly against Escherichia coli O157:H7 and Salmonella spp. (Tsai et al., 2014, Bajpai et al., 2016). In addition, LAB also showed anti-obesity effect (Choi et al., 2007, Kim et al., 2008).
Diabetes is one of the loathsome chronic diseases among humans, especially in elderly people. Its dramatic increase worldwide has led to the increasing appearance of diabetes-related comorbidities. The disease has affected around 100 million elderly people aged 60–78 years old in 2010 and is expected to double in next 20 years (Shaw et al., 2010). There are a number of risk factors associated with diabetes such as food, genetics, or environment. Since diabetes is directly associated with the increase in blood glucose level, a number of reports suggesting that intake of LAB decreases the blood glucose level (Honda et al., 2012a, Honda et al., 2012b, Yun et al., 2009, Matsumoto et al., 2016). Several LAB are reported to decrease postprandial blood glucose level by suppressing the glucose absorption and decreasing the glucose available from digestion of foodstuff (Tabuchi et al., 2004, Honda et al., 2011, Honda et al., 2012a, Honda et al., 2012b). Nevertheless, insufficient studies have been done with other LAB species. In addition, in most of the reports, the effect of live bacteria has been studied (Honda et al., 2012a, Honda et al., 2012b).
In this study, the antibacterial, anti-obesity, and alpha-glucosidase potential of a fish isolate L. mesenteroides KS-TN1 were evaluated to confirm its pharmacological significance.
2. Materials and methods
2.1. Media and reagents
The de Man, Rogosa and Sharpe (MRS) agar medium were purchased from Difco (USA). The agar medium, Bromocresol Purple (BCP), p-nitrophenyl-α-d-glucopyranoside, yeast α-glucosidase, and 3,4-dihydroxy-l-phenylalanine (DOPA) were purchased from Sigma-Aldrich (Sigma, MO, USA). All other chemicals or reagents used were of analytical grade.
2.2. Target pathogenic strains
The highly pathogenic bacteria such as Escherichia coli O157:H7, Salmonella enterica ATCC 4731, Listeria monocytogenes KCTC 3569, Staphylococcus aureus KCTC 1621 and Bacillus subtilis KCTC 1021 were used as target bacteria. The strains were collected from American Type Culture Collection and Korean Type Culture Collection and were cultured on nutrient broth and agar at 37 °C. The stock culture samples were stored at −80 °C in cryopreservative vials.
2.3. Fish collection and isolation of LAB
Nile Tilapia, Oreochromis niloticus samples were captured using the net (weighed between 150 and 300 g) from the Wadi Namar in the western area of Riyadh, Saudi Arabia. The isolation of lactic acid bacteria was carried by sacrificing fish and dissect its gills, stomach, and intestine. The collected samples were homogenized for a short period of time and serially diluted using phosphate buffer saline (Cho et al., 2013). The dilutions were made from 10−1 to 10−7 and 100 µl from each dilution was plated on BCP agar plates. The inoculated plates were incubated for 24 h at 37 °C. The clean zone around each colony was taken as lactic acid bacteria (Zapata, 2013). Further, the identified colonies were selected using inoculation loop and inoculated in the de Man, Rogosa and Sharpe (MRS) broth and incubated at 37 °C for 24 h. The samples were further spread on MRS plates and re-cultured for long-term preservation at −80 °C.
2.4. Antibacterial spectrum
The initial screening of fish isolates was carried out against pathogenic bacteria using agar well diffusion assay (Murray et al., 1995, Rather et al., 2013). Briefly, the plates of nutrient agar were prepared and allowed to solidify. The plates were spread with 200 μl of target bacteria (107 cfu/ml) and allowed to dry for 10 min. The 24 h culture broths of 32 LAB isolates grown in MRS media at 37 °C were centrifuged at 10,000g for 10 min. The supernatant was collected and filter-sterilized through a 0·45-μm-pore-size filter (Sartorius Stedim Biotech, Goettingen, Germany). An autoclaved borer was used to make uniform wells poured with 100 μl filter sterilized cell-free-supernatant of the isolated bacterium. The plates were incubated for 24 h at 37 °C. After incubation, the antibacterial activity was determined by measuring the zones of inhibition against tested bacteria. The assay was done in triplicates.
2.5. Biochemical and morphological identification of fish isolate
Based on the initial screening of highly antibacterial activity against pathogenic strains, KS-TN11 was selected as a potential strain. The isolate was identified by observing the colony shape on BCP agar plates, and by Gram-staining and cell morphology under a microscope. Further, for biochemical characterization, API 50CH strips with API 50CHL medium was used as per manufacturer instructions (Shin et al., 2007, Rather et al., 2013). The strips were processed for analyzing the API profiles using computer APILAB Plus Version.
The isolate was further characterized by 16S rRNA gene sequencing. The sequences were compared on National Center for Biotechnology Information (NCBI) for homology and submitted to Genebank.
2.6. Extraction and sample preparation
For extraction of biologically active metabolites, SK-TN11 was cultured in MRS broth for 24 h at 37 °C. The culture was mixed with ethanol (1:2 ratio v/v) and incubated on a rotatory shaker for 4 h at room temperature. Further, the mixture was centrifuged at 10,000 rpm for 15 min. The ethanol extract in the upper phase was collected and vacuum evaporated under reduced pressure at 40 °C. The sample was freeze-dried for three days and stored at 4 °C for further use.
2.7. Cell culture
The cell line 3T3-L1 (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) in presence of 10% fetal bovine serum in a humidified environment supplied with 5% CO2 at 37 °C. The differentiation of 3T3-L1 preadipocytes to mature adipocytes were evaluated as reported previously (Tominaga et al., 2009). During treatments, unless otherwise stated, cells were maintained in complete medium containing KS-TN11 for 6–8 days before further analysis, and maturation medium was changed every 2 days. KS-TN11 was supplemented with both induction mediums. Oil Red O staining
On day 7, accumulation of lipid in adipocytes was evaluated by oil Red O staining. Briefly, after cell maturation, the control and KS-TN11 (50 mg and 100 mg) treated cells were washed with sterile 1X phosphate buffered saline (PBS), followed by one-hour fixation in 10% formalin at room temperature. Further, the cells were washed three times with sterile deionized water followed by coating with 0.6% Oil Red O dye in isopropanol and water (6:4 ratio v/v) for 20 min. Finally, the cells were washed 3–4 times with sterile deionized water before microscopic observation.
2.8. α-glucosidase inhibitory activity
LAB are known for its beneficial properties and have been used since ages for food fermentation. A large number of the food-based LAB are now potential probiotics. In this study, two concentrations of KS-TN11 were evaluated for its α-glucosidase inhibitory potential to confirm its anti-diabetic property using chromogenic method (Yuan et al., 2012, Bajpai et al., 2016). A 50 μl of various concentrations (2.5 mg and 5 mg) of SK-TN11 and 100 μl of α-glucosidase was dissolved in 0.1 M PBS, the solutions were gently mixed in a 96-well microplate and incubated for 10 min at 25 °C. In addition, a 50 μl of p-nitrophenyl-α-d-glucopyranoside (5 mM) using PBS as a substrate solution was added after 10 min incubation to wells and the plate was again incubated for 5 min at 25 °C. However, the reading for absorbance was taken before and after incubation at 405 nm using microplate reader. Simultaneously, the absorbance of 50 μl PBS buffer was taken separately for comparison with test groups. The experiments were replicated three times and α-glucosidase inhibitory activity was calculated using the formula:
2.9. Statistical analysis
All data are expressed as the mean ± SD., and comparison was made by using Student's t-test.
3. Results and discussion
3.1. Isolation and screening of LAB
The small colonies appeared yellow color on BCP agar confirms the presence LAB. In this study, 32 fish LAB were isolated from stomach, intestine, and gills of Nile Tilapia, O. niloticus as shown in Table 1. Further, each yellow colony was carefully picked and grown in MRS broth and checked for its antibacterial activity against various pathogenic bacteria.
Table 1.
List of isolated lactic acid bacteria from Nile Tilapia (Oreochromis niloticus).
| Lab name | Source | Media | Identification |
|---|---|---|---|
| KS-TN1 | Stomach | BCP | Lactic acid bacteria |
| KS-TN2 | Stomach | BCP | Lactic acid bacteria |
| KS-TN3 | Stomach | BCP | Lactic acid bacteria |
| KS-TN4 | Stomach | BCP | Lactic acid bacteria |
| KS-TN5 | Stomach | BCP | Lactic acid bacteria |
| KS-TN6 | Stomach | BCP | Lactic acid bacteria |
| KS-TN7 | Stomach | BCP | Lactic acid bacteria |
| KS-TN8 | Stomach | BCP | Lactic acid bacteria |
| KS-TN9 | Stomach | BCP | Lactic acid bacteria |
| KS-TN10 | Stomach | BCP | Lactic acid bacteria |
| KS-TN11 | Stomach | BCP | Lactic acid bacteria |
| KS-TN12 | Stomach | BCP | Lactic acid bacteria |
| KS-TN13 | Stomach | BCP | Lactic acid bacteria |
| KS-TN14 | Stomach | BCP | Lactic acid bacteria |
| KS-TN15 | Stomach | BCP | Lactic acid bacteria |
| KS-TN16 | Stomach | BCP | Lactic acid bacteria |
| KS-TN17 | Stomach | BCP | Lactic acid bacteria |
| KI-TN18 | Intestine | BCP | Lactic acid bacteria |
| KI-TN19 | Intestine | BCP | Lactic acid bacteria |
| KI-TN20 | Intestine | BCP | Lactic acid bacteria |
| KI-TN21 | Intestine | BCP | Lactic acid bacteria |
| KI-TN22 | Intestine | BCP | Lactic acid bacteria |
| KI-TN23 | Intestine | BCP | Lactic acid bacteria |
| KI-TN24 | Intestine | BCP | Lactic acid bacteria |
| KI-TN25 | Intestine | BCP | Lactic acid bacteria |
| KI-TN26 | Intestine | BCP | Lactic acid bacteria |
| KI-TN27 | Intestine | BCP | Lactic acid bacteria |
| KG-TN28 | Gills | BCP | Lactic acid bacteria |
| KG-TN29 | Gills | BCP | Lactic acid bacteria |
| KG-TN30 | Gills | BCP | Lactic acid bacteria |
| KG-TN31 | Gills | BCP | Lactic acid bacteria |
| KG-TN32 | Gills | BCP | Lactic acid bacteria |
3.2. Anti-bacterial activity and selection of LAB isolates from Nile Tilapia
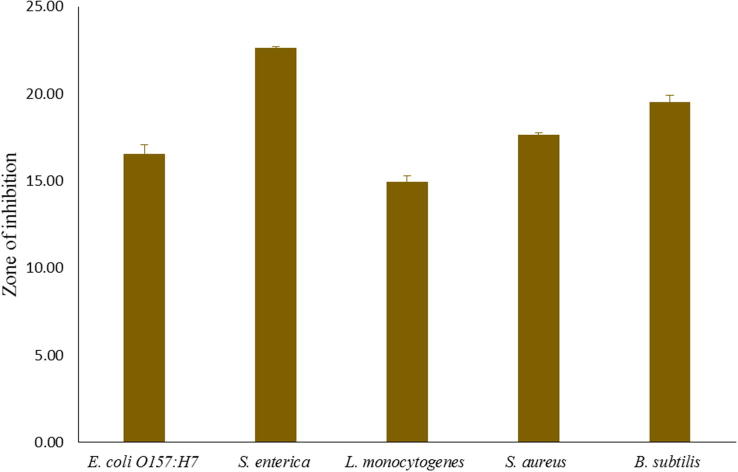
The cell-free-supernatant of each isolate was checked for antibacterial activity against Escherichia coli O157:H7 and Salmonella enterica ATCC 4731, Listeria monocytogenes KCTC 3569, Staphylococcus aureus KCTC 1621 and Bacillus subtilis KCTC1021. The isolate KS-TN11 showed highest antibacterial activity (Fig. 1), and was chosen for further study.
Fig. 1.
Antibacterial activity of cell-free-supernatant of Leuconostoc mesenteroides KS-TN11 against pathogenic strains.
3.3. Biochemical and molecular characterization of KS-TN11
The morphological identification of KS-TN11 was carried out according to Bergey’s Manual of Systematic Bacteriology (Holt et al., 1994). The small colonies on BCP agar appearing in yellow color confirm the presence of LAB strain (Ashraf and Shah, 2011). The BCP is a specific media for the LAB as it inhibits the formation of colonies by concomitant bacteria and hence recommended for the selective enumeration of the LAB (Bielecka et al., 2000).
In addition, KS-TN11 was further identified by biochemical characterization using API50 kit (Table 2). The strain was identified as a rod-shaped Gram-positive bacterium and showed a close association with Leuconostoc mesenteroides. The change in color from violet to yellow in the strip capsule indicated the complete fermentation and KS-TN11 showed utilization of 24 type carbohydrates such as l-arabinose, Ribose-d-ribose, d-xylose, d-galactose, d-glucose, d-fructose, d-mannose, Dulcitol, d-sorbitol, N-acetylglucosamine, Amygdalin, Arbutin, Esculin, d-cellobiose, d-maltose, d-lactose, d-melibiose, d-saccharose, d-trehalose, d-raffinose, Gentiobiose, d-turanose, Potassium Gluconate.
Table 2.
Biochemical characterization of Leuconostoc mesenteroides KS-TN11 using API 50 CHL.
| Active ingredients | Results | Active ingredients | Results |
|---|---|---|---|
| Glycerol | −1 | Salicin | − |
| Erythritol | − | d-Cellobiose | + |
| d-Arabinose | − | d-Maltose | + |
| l-Arabinose | +2 | d-Lactose (bovine origin) | + |
| d-Ribose | + | d-Melibiose | + |
| d-Xylose | + | d-Saccharose | + |
| l-Xylose | − | d-Trehalose | + |
| d-Adonitol | − | Inulin | − |
| Methyl-β-d-Xylopyranoside | − | d-Melezitose | − |
| d-Galactose | + | d-Raffinose | + |
| d-Glucose | + | Amidon (starch) | − |
| d-Fructose | + | Glycogen | − |
| d-Mannose | + | Xylitol | − |
| l-Sorbose | − | Gentiobiose | + |
| l-Rhamnose | − | d-Turanose | + |
| Dulcitol | + | d-Lyxose | − |
| Inositol | − | d-Tagatose | − |
| d-Mannitol | − | d-Fuccose | − |
| d-Sorbitol | + | l-Fuccose | − |
| Methyl-α-d-Mannopyranoside | − | d-Arabitol | − |
| Methyl-α-noside | − | l-Arabitol | − |
| N-acetylglucosamine | + | Potassium Gluconate | + |
| Amygdalin | + | Potassium 2-Ketogluconate | − |
| Arbutin | + | Potassium 5-Ketogluconate | − |
| Esculin | + |
Note: 24 type carbohydrates: l-arabinose, Ribose-d-ribose, d-xylose, d-galactose, d-glucose, d-fructose, d-mannose, Dulcitol, d-sorbitol, N-acetylglucosamine, Amygdalin, Arbutin, Esculin, d-cellobiose, d-maltose, d-lactose, d-melibiose, d-saccharose, d-trehalose, d-raffinose, Gentiobiose, d-turanose, Potassium Gluconate.
The bacterium does not use this carbohydrate.
The bacterium uses this carbohydrate.
Based on the molecular analysis with 16S rRNA gene sequencing, the stain KS-TN11 showed 96% similarity with Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293. The sequence was submitted to GenBank with nucleotide accession number (KY933715). Hence, KS-TN11 was finally confirmed as Leuconostoc mesenteroides KS-TN11.
3.4. KS-TN11 inhibits adipocyte differentiation
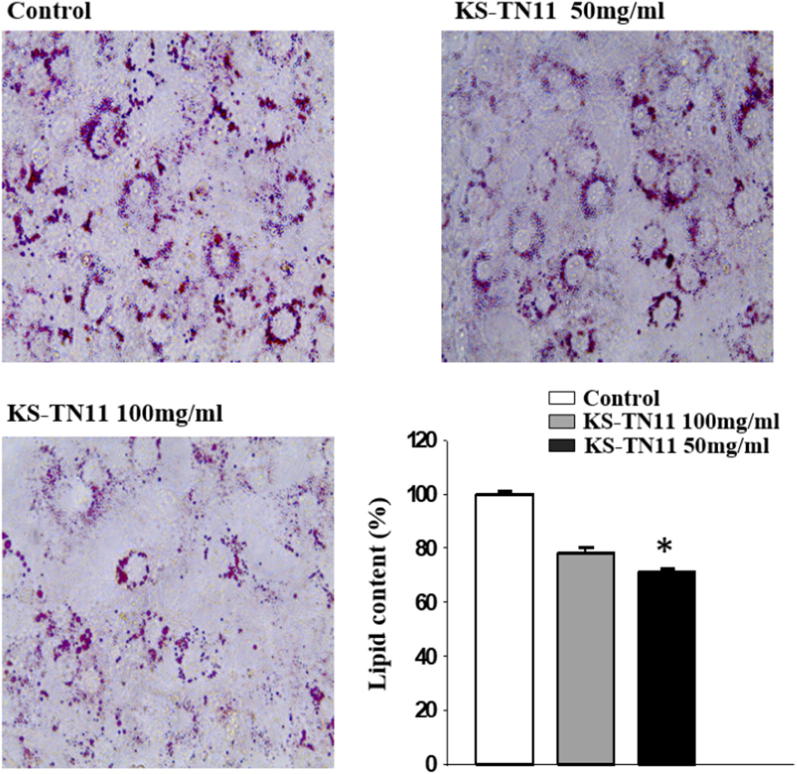
To evaluate the effect of KS-TN11 on adipogenesis of adipocytes, the 3T3-L1 adipocytes were treated with different concentrations of KS-TN11 (50 mg and 100 mg). Adipogenesis is the diagnostic feature of obese subjects. To, this end it was observed that KS-TN11 decreased the lipid content and prevented the accumulation of lipid droplets as depicted by reduced intensity of Oil Red O staining. The effect was not much different at higher and lower dosage of ethanol extract of KS-TN11. However, when compared with control cells the effect on accumulation of lipid was significantly reduced with KS-TN11 at 100 mg/ml (P < 0.05), as shown in Fig. 2.
Fig. 2.
Effect of Leuconostoc mesenteroides KS-TN11 on lipid accumulation in 3T3-L1 adipocytes. *P < 0.05 is significant. Analysed by using Student's t-test.
3.5. α-glucosidase inhibitory activity
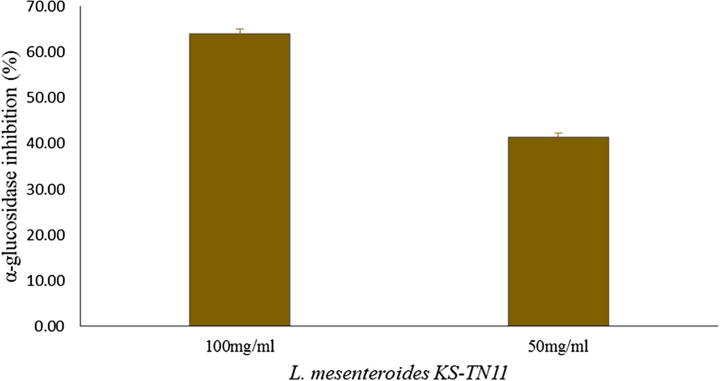
The antidiabetic effect of 50 mg and 100 mg of KS-TN11 was evaluated by α-glucosidase assay using p-nitrophenyl-α-d-glucopyranoside (pNPG) as a substrate. The highest inhibition of α-glucosidase was seen with 100 mg/ml sample and minimum inhibition was seen by 50 mg/ml ethanol extract of KS-TN11 as 64% and 41.33%, respectively, as shown in Fig. 3. These results suggest that KS-TN11 as a novel strain with antidiabetic effect.
Fig. 3.
α-Glucosidase inhibitory activity of L. mesenteroides KS-TN11.
Aquatic animals have been a great source of lactic acid bacteria (Nair and Surendran, 2005). Interestingly, the majority of the microorganisms isolated from aquatic animals such as fish bear same Lactobacillus species as found in animals, including human gastrointestinal tract (Kandler and Weiss, 1986). It has been reported that LAB isolated from fish samples showed potential antibacterial activity against human pathogenic bacteria (Jini et al., 2011). In addition, these fish isolated LAB have gained tremendous attention due to their probiotics functionality. A new probiotic LAB was isolated from O. nilotocus and the bacterium could be used in aquaculture (Zapata, 2013). There are several other reports suggesting isolation of LAB or probiotic LAB from fish samples; however, the composition changes in each fish samples due to different environmental conditions. In most of the case, the composition remains same that is same LAB were found in a number of different fish samples.
Lactic acid bacteria are known for their beneficial functions including metabolic characteristics and production of biological compounds. A number of LAB isolated from fish showed anti-obesity effects. Bajpai et al. (2016) isolated Lactobacillus sakei 1l1 from fish showed antidiabetic and antiviral activity. Honda et al., 2012a, Honda et al., 2012b reported the antidiabetic activity of two lactic acid bacteria strains Lactobacillus rhamnosus GG and Lactobacillus delbrueckii susbsp. bulgaricus LB3 in an animal model. However, very less study has been conducted on other LAB species. There are several reports showing the inhibitory effect of α-Glucosidase by different strains of lactic acid bacteria (Ramchandran and Shah, 2008, Michlmayr and Kneifel, 2014, Panwar et al., 2014). Several studies reported the α-glucosidase inhibitory activity of yogurt isolated LAB strains and it was found that strains of Lactobacillus casei, acidophilus, and Bifidobacterium showed approximately 80% of α-glucosidase inhibitory activity (Ramchandran and Shah, 2008).
4. Conclusion
In conclusion our results suggest the antibacterial, and α-glucosidase inhibitory activity of Leuconostoc mesenteroides KS-TN11 from Nile Tilapia, O. niloticus combined with its possible anti-obesity effect. Thus, KS-TN11’s pharmaceutical properties may be explored for therapeutic purposes. However, further studies are required to validate the data and solidify its role.
Acknowledgments
Acknowledgement
The authors would like to express their sincere appreciation to the Deanship of Scientific Research at the King Saud University, Riyadh, Saudi Arabia for funding this Research Group project no RGP-289.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Bilal Ahmad Paray, Email: bparay@ksu.edu.sa.
Irfan A. Rather, Email: rather@ynu.ac.kr.
References
- Ashraf R., Shah N.P. Selective and differential enumerations of Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium spp. in yoghurt - a review. Int. J. Food. Microbiol. 2011;149:194–208. doi: 10.1016/j.ijfoodmicro.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Bajpai V.K., Han J.H., Nam G.J., Majumder R., Park C., Lim J., Paek W.K., Rather I.A., Park Y.H. Characterization and pharmacological potential of Lactobacillus sakei 1l1 isolated form fresh water fish Zacco koreanus. DARU J. Pharm. Sci. 2016;24:8. doi: 10.1186/s40199-016-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar J.L., Venderll D., de Blas I., Ruiz-Zarzuela I., Muzquiz J.L., Girones O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture. 2008;278:188–219. [Google Scholar]
- Bielecka M., Biedrzycka E., Majkowska A., Biedrzycka E. Method of Lactobacillus acidophilus viable cell enumeration in the presence of thermophilic lactic acid bacteria and bifidobacteria. Food Biotech. 2000;17:399–404. [Google Scholar]
- Cho Y.H., Hong S.M., Kim C.H. Isolation and characterization of lactic acid bacteria from Kimchi, Korean traditional fermented food to apply into fermented dairy products. Korean J. Food Sci. Anim. Resour. 2013;33:75–82. [Google Scholar]
- Choi I., Kim Y., Park Y., Seog H., Choi H. Anti-obesity activities of fermented soygerm isoflavones by Bifidobacterium breve. Biofactors. 2007;29:105–112. doi: 10.1002/biof.552029201. [DOI] [PubMed] [Google Scholar]
- Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T., Williams, S.T., 1994. Bergey’s Manual of Determinative Bacteriology, ninth ed., William and Wilkins, MD, pp. 559–564.
- Honda K., Moto M., Uchida N., He F., Hashizume N. Effects of Lactobacillus rhamnosus GG on the development of diabetes in mice with genetic type 2 diabetes. J. Jpn. Soc. Clin. Nutr. 2011;33:39–45. [Google Scholar]
- Honda K., Moto M., Uchida N., He F., Hashizume N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012;51(2):96–101. doi: 10.3164/jcbn.11-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Moto M., Uchida N., He F., Hashizume N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012;51:96–101. doi: 10.3164/jcbn.11-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jini R., Swapna H.C., Rai A.K., Vrinda R., Halami P.M., Sachindra N.M., Bhaskar N. Isolation and characterization of potential lactic acid bacteria (LAB) from freshwater fish processing wastes for application in fermentative utilization of fish processing waste. Braz. J. Microbiol. 2011;42:1516–1525. doi: 10.1590/S1517-838220110004000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T., Watanabe Y., Makino S., Kano H., Tsuji N.M. Improvement of intestinal immune cell function by lactic acid bacteria for dairy products. Microorganisms. 2017;5:1. doi: 10.3390/microorganisms5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O., Weiss N. Bergey’s Manual of Systematic Bacteriology. Williams and Wilkins; Baltimore: 1986. pp. 1209–1234. [Google Scholar]
- Kim J.S., You H.J., Kang H.Y., Ji G.E. Enhancement of the tyrosinase inhibitory activity of Mori Cortex radicis extract by biotransformation using Leuconostoc paramesenteroides PR. Biosci. Biotechnol. Biochem. 2012;76:1425–1430. doi: 10.1271/bbb.111002. [DOI] [PubMed] [Google Scholar]
- Kim N.H., Moon P.D., Kim S.J., Choi I.Y., An H.J., Myung N.Y., Jeong H.J., Um J.Y., Hong S.H., Kim H.M. Lipid profile lowering effect of Soypro fermented with lactic acid bacteria isolated from Kimchi in high-fat diet-induced obese rats. Biofactors. 2008;33:49–60. doi: 10.1002/biof.5520330105. [DOI] [PubMed] [Google Scholar]
- Kober M.M., Bowe W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Womens Derm. 2015;1:85–89. doi: 10.1016/j.ijwd.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Ishii M., Sekimizu K. An in vivo invertebrate evaluation system for identifying substances that suppress sucrose-induced postprandial hyperglycemia. Sci. Rep. 2016;6:26354. doi: 10.1038/srep26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlmayr H., Kneifel W. β-Glucosidase activities of lactic acid bacteria: mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014;352:1–10. doi: 10.1111/1574-6968.12348. [DOI] [PubMed] [Google Scholar]
- Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolke R.H. sixth ed. ASM; Washington: 1995. Manual Clinical Microbiology. [Google Scholar]
- Nair P.S., Surendran P.K. Biochemical characterization of lactic acid bacteria isolated from fish and prawn. J. Culture Collections. 2005;4:48–52. [Google Scholar]
- Panwar H., Calderwood D., Grant I.R., Grover S., Green B.D. Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur. J. Nutr. 2014;53:1465–1474. doi: 10.1007/s00394-013-0649-9. [DOI] [PubMed] [Google Scholar]
- Ramchandran L., Shah N.P. Proteolytic profiles and angiotensin-I converting enzyme and alpha-glucosidase inhibitory activities of selected lactic acid bacteria. J. Food Sci. 2008;3:75–81. doi: 10.1111/j.1750-3841.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Rather I.A., Seo B.J., Kumar V.J.R., Choi U.H., Choi K.H., Lim J.H., Park Y.H. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML007 and its application as a food preservative. Lett. Appl. Microbiol. 2013;57:69–76. doi: 10.1111/lam.12077. [DOI] [PubMed] [Google Scholar]
- Rather I.A., Seo B.J., Kumar V.J.R., Choi U.H., Choi K.H., Lim J.H., Park Y.H. Biopreservative potential of Lactobacillus plantarum YML007 and efficacy as a replacement for chemical preservatives in animal feed. Food Sci. Biotechnol. 2014;23:195–200. [Google Scholar]
- Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shin S.Y., Bajpai V.K., Kim H.R., Kang S.C. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int. J. Food Microbiol. 2007;113:233–236. doi: 10.1016/j.ijfoodmicro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Tabuchi M., Morita H., He F., Hosoda M., Yamada N., Ishida T. Effect of administration of Lactobacillus GG on postprandial blood glucose levels in rats. Milk Sci. 2004;54:249–253. [Google Scholar]
- Tominaga S., Sugahara T., Nishimoto S., Yamawaki M., Nakashima Y., Kishida T., Akiyama K., Maruyama M., Yamauchi S. The effect of secoisolariciresinol on 3T3-L1 adipocytes and the relationship between molecular structure and activity. Biosci. Biotechnol. Biochem. 2009;73:35–39. doi: 10.1271/bbb.80393. [DOI] [PubMed] [Google Scholar]
- Tsai Y.T., Cheng P.C., Pan T.M. Immunomodulating activity of Lactobacillus paracasei subsp. paracasei NTU 101 in enterohemorrhagic Escherichia coli O157:H7-infected mice. J. Agric. Food Chem. 2010;58:11265–11272. doi: 10.1021/jf103011z. [DOI] [PubMed] [Google Scholar]
- Tsai Y.T., Cheng P.C., Pan T.M. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl. Microbiol. Biotechnol. 2014;98:1–10. doi: 10.1007/s00253-013-5346-3. [DOI] [PubMed] [Google Scholar]
- Yuan T., Wan C., Liu K., Seeram N.P. New maplexin FI and phenolic glycosides from red maple (Acer rubrum) bark. Tetrahedron. 2012;68:959–964. [Google Scholar]
- Yun S.I., Park H.O., Kang J.H. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J. Appl. Microbiol. 2009;107:1681–1686. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- Zapata A.A. Antimicrobial activities of lactic acid bacteria strains isolated from Nile Tilapia intestine (Oreochromis niloticus) J. Biol. Life Sci. 2013;4:164–171. [Google Scholar]