Abstract
The properties of lipid bilayers in sucrose solutions have been intensely scrutinized over recent decades because of the importance of sugars in the field of biopreservation. However, a consensus has not yet been formed on the mechanisms of sugar-lipid interaction. Here, we present a study on the effect of sucrose on 1,2-dipalmitoyl-sn-glycero-3-phosphocholine bilayers that combines calorimetry, spectral fluorimetry, and optical microscopy. Intriguingly, our results show a significant decrease in the transition enthalpy but only a minor shift in the transition temperature. Our observations can be quantitatively accounted for by a thermodynamic model that assumes partial delayed melting induced by sucrose adsorption at the membrane interface.
Introduction
There is strong evidence that lipid membrane structures are well stabilized by small sugars (1, 2). Disaccharides, and also other sugars, play a key role in preserving the structure and the functionality of biological membranes during periods of environmental stress (3). Besides their significant role in cellular regulation, carbohydrates also have a broad range of applications in biophysics and industrial research, particularly in the field of biopreservation and cryopreservation. Some sugars, such as sucrose and trehalose, are very efficient cryoprotectors (1, 4, 5). They have been shown to readily reduce the liquid-gel transition temperature Tm in highly dehydrated lipid bilayers and to increase the survivability of membranes undergoing freezing/thawing processes (6). Although this mechanism was initially associated with the ability of disaccharides to insert between adjacent lipid headgroups during dehydration and to hydrogen bond to them, an alternative model has been proposed that explains the observed effects in terms of sugar changes on the hydration repulsion (7).
Moreover, it has been proven that sugars play a role in the properties of hydrated bilayers. Döbereiner et al. (8) observed the strong influence of glucose on the spontaneous curvature of liposomes. Genova et al. (9) showed by fluctuation analysis that high concentrations of sucrose reduce the bending modulus kb of stearoyl-oleoyl phosphatidylcholine giant vesicles by up to 25%, whereas Vitkova (10) found a 60% reduction using micropipette aspiration. Nagle (11) also showed by x-ray scattering that the bending rigidity of 1,2-dioleoyl-sn-glycero-3-phosphocholine bilayers is reduced, although it should be noted that recently the opposed effect has been reported (12).
Characteristic chain-melting temperatures of phospholipid dispersions are known to increase as the activity of water decreases in the presence of increased solutes (13). Strauss et al. (13) found that the addition of more than 10% sucrose to hydrated multilamellar vesicles of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) elevated the melting temperature by several degrees; they suggested a hydrogen-bonded sucrose network as the cause. Crowe and Crowe (14) found that several mono- and disaccharides raise and broaden the main transition of large DPPC multilamellar vesicles (MLVs). However, the addition of sugars to unilamellar vesicles created multiple thermodynamic populations. High concentrations of trehalose, sucrose, and fructose created a low-temperature shoulder on the DPPC endotherm, indicating a second population with a lower than that of pure hydrated DPPC.
Despite the observed influence of sugars on dry, semidry, and hydrated bilayers, relatively few studies have been conducted to understand the main effect of disaccharides on the lipid bilayer phase transition, and the mechanisms of interaction are yet to be understood. Although there is some agreement that high concentrations of sugar increase the transition temperature of the bilayer melting, the effect on the enthalpic contribution is quite discordant, reporting in certain cases no effect on the enthalpy of the transition and, in other studies, a significant decrease in energy. This discordance, likely due to differences in sample preparation methods, which lead to differing bilayer exposures to the sugars, calls for a more consistent approach.
In this work, we expose well-hydrated bilayers of DPPC to increasing concentrations of sucrose, up to 1.50 M. We probe the effect of sugar on the membrane phase behavior using a combination of differential scanning calorimetry (DSC) and Laurdan emission spectra to obtain structural and thermodynamical information. We also visualize changes in the phase behavior and kinetics of the transition by fluorescence microscopy of giant unilamellar vesicles (GUVs). Based on our experimental observations, we propose a thermodynamic model that quantitatively accounts for the effects of the interaction of sucrose with the lipid bilayer.
Materials and Methods
Materials
Chloroform solutions of DPPC (C40H80NO8P, Mw = 734.039, 10 mg/mL) and DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) (Mw = 677.933, 10 mg/mL) were purchased from Avanti Polar Lipid (Birmingham, AL). DiI stain (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, C50H97ClN2O4, Mw = 933.8793) was provided by Thermo Fisher Scientific (Waltham, MA) as a powder and dissolved in chloroform at 10 mg/mL final concentration. Sucrose (C12H22O11 Mw = 342.3) and Laurdan (6-dodecanoyl-N,N-dimethyl-2-naphthylamine) were purchased from Sigma-Aldrich (Saint-Quentin, France). All chemicals had high purity and were used without further purification. The osmolarities of the sucrose solutions were measured with a cryoscopy osmometer Osmomat 030 (Gonotec, Berlin, Germany).
Liposomal preparation
2.5 mg of DPPC in chloroform was transferred to a glass vial, and organic solvent was evaporated using first an argon stream for 20 min, followed by 8 h of vacuum pumping. For fluorescence measurements, the lipids were stained with 1% mol Laurdan in chloroform before evaporation. The lipid film was then hydrated with aqueous solution (buffer or sucrose solution) at 70°C to reach the desired concentration and gently vortexed. The resulting MLV suspensions were sonicated for 15 min to disperse larger aggregates. Liposomal solutions remained stable over a period of days, as they were routinely checked with dynamic light scattering (Malvern Zetasizer Nano, Royston, United Kingdom).
Fluorimeter
3 mL of liposomal suspension stained with Laurdan of total concentration 3 mg/mL was placed in a quartz silica cuvette with a 1 mm path length. Acquisition of Laurdan emission spectra was performed with a Jobin Horiba FluoroMax equipped with a Peltier unit to control temperature. The excitation wavelength was set at 350 nm with a bandpass of 1 nm, and the emission was also recorded with slit of 1 nm. The solution was equilibrated at a given temperature for 10 min before each acquisition. For each sample, we performed two cycles of heating and cooling. Each spectrum acquisition was repeated three times on a new sample.
Generalized polarization (GP) was calculated using the standard expression provided by Parasassi (15):
| (1) |
where and are the values of the emitted intensity recorded at 440 and 490 nm, respectively.
DSC
The calorimetry measurements were performed using high sensitivity DSC (μDSC Setaram). The measurement cell was filled with the sonicated sample (MLVs at different concentrations of sucrose), whereas the reference cell was filled with the same sucrose solution. The heating rate was fixed at 0.5 K × min–1, and the cooling rate was fixed at 0.3 K × min–1. The system was equilibrated ∼20 min before each heating or cooling ramp. The analysis of DSC data was performed using OriginPro 9.0 (OriginLab, Northampton, MA).
Results and Discussion
Laurdan emission spectra
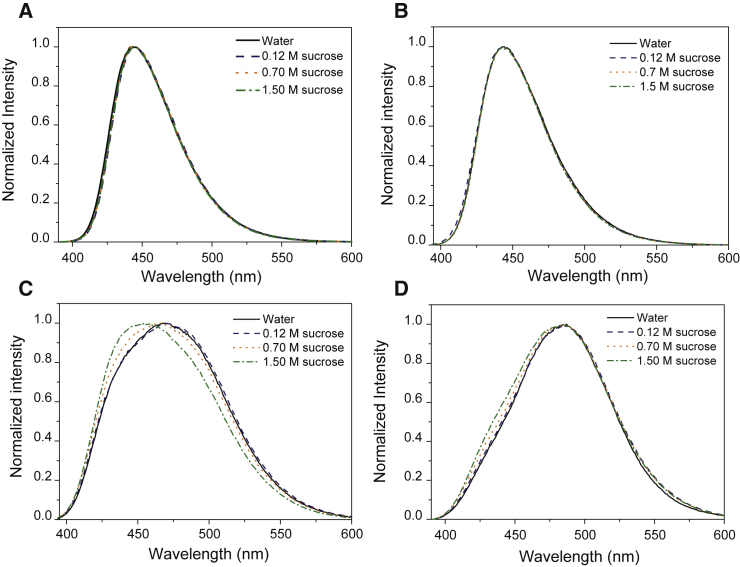
Fig. 1 illustrates the emission spectra of Laurdan for DPPC MLVs at different concentrations of sucrose at 20°C (Fig. 1 A) and 60°C (Fig. 1 B). Sucrose concentrations were chosen up to 1.5 M, thus covering well the relevant range for cryoprotection, i.e., 0.15–0.50 M (16, 17). As shown also in Fig. S1, the spectra exhibit a continuous shift to longer wavelengths with increasing temperature while lipids undergo the gel-to-fluid transition. At 20°C, the maximal intensity is centered at 440 nm, corresponding to the signal associated with the gel phase (Fig. 1 A) (15). The figure further shows that the emission spectrum does not evolve with the sucrose concentration, remaining identical to that of DPPC in water, indicating that below , the presence of sugar does not significantly modify the structure or order of the bilayer.
Figure 1.
Comparison of emission curves of Laurdan for DPPC MLVs in pure water and 0.12, 0.70, and 1.50 M sucrose at (A) 20°C, (B) 30°C, (C) 42°C, and (D) 60°C. To see this figure in color, go online.
As temperature increases above the transition temperature , we observe a decrease of total intensity together with a broadening of the emission spectrum, corresponding to an increasing red emission centered at 490 nm (Fig. 1 B). Such a decrease of intensity has been reported elsewhere (18). It is noteworthy that increasing concentrations of sucrose result in the persistence of a 440 nm shoulder in the emission spectra. This additional contribution is found to be dependent on the sucrose concentration and remains at any temperature above . A Laurdan emission centered at 490 nm is attributed to a more relaxed Laurdan excited state, which would be favored by a more hydrated environment (19, 20). Conversely, a contribution at 440 nm reflects a stiffer and/or less hydrated system surrounding the Laurdan naphthalene moiety. The evolution of GP with temperature and sucrose concentration appears to quantify the observed spectral changes, as shown in Fig. 2.
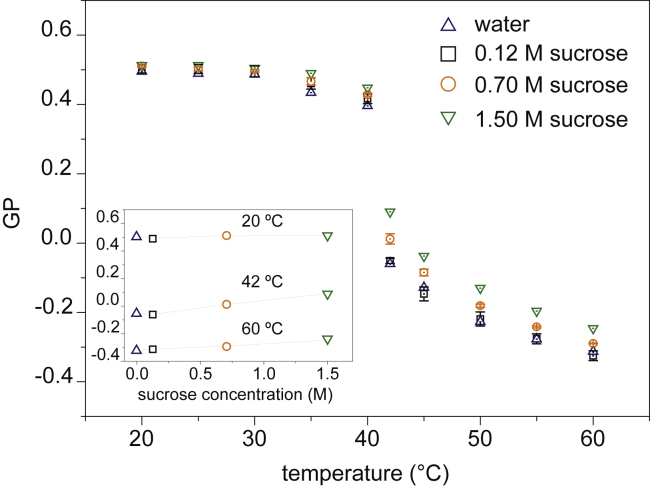
Figure 2.
Variation of general polarization (GP) over temperature for multilamellar liposomes of DPPC formed in water and at different sucrose concentrations. A rise in GP values proportional to sucrose concentration is observed. Inset: the dependence of GP on sucrose concentration for 20°C, 42°C, and 60°C is shown. When not visible, error bars are smaller than symbol sizes. To see this figure in color, go online.
The values for DPPC in water are comparable to the values reported by Bagatolli et al. (21), as the GP decreases from 0.51 0.02 for temperatures below to −0.33 0.01 for the fluid phase above . The value for extracted by interpolating the GP curves to determine the temperature at which GP = 0 is 41.5 0.3°C, which is in good agreement with our DSC measurement of 41.3°C and with previously reported values (22). The GP transition from gel phase to liquid crystalline phase is sharp for any concentration of sucrose. A higher transition temperature is found by the same method for larger concentrations of sugar, as displayed in Table 1. The values of GP at temperatures below are almost independent of sucrose content, although for temperatures near the transition (35 and 40°C), a slight increase of GP with an increasing amount of sugar can be observed; see the inset in Fig. 2.
Table 1.
Summary of DSC Results for DPPC Liposomes in Sucrose Solution
| Sucrose Concentration |
Tm |
|
H |
Laurdan Tm |
|---|---|---|---|---|
| [M] | [°C] | [°C] | [kJ/mol] | [°C] |
| 0 | 41.8 0.2 | 0.40 0.01 | 38.5 0.7 | 40.9 0.6 |
| 0.39 | 41.9 0.2 | 0.44 0.01 | 33.6 1.9 | 40.9 0.4 |
| 0.7 | 42.1 0.1 | 0.48 0.01 | 27.9 1.6 | 42.0 0.5 |
| 1.5 | 42.7 0.2 | 0.55 0.02 | 23.9 0.7 | 43.5 0.4 |
The inset also shows that above in the fluid phase, GP values increase significantly with sucrose concentration, with GP increasing from −0.38 to −0.22 at 60°C for 1.50 M sucrose. Interestingly, the maximal variation of GP with sugar concentration can be observed at , whereas for higher temperatures, the sensitivity of GP with respect to sugar content gradually decreases (Fig. 2, inset). The variation owed to the presence of sucrose appears to follow a linear dependence on sugar concentration in bulk for any analyzed temperature.
Laurdan is insoluble in water; therefore, any information from the emission spectra and GP arises entirely from the probe in the membrane. In lipid bilayers, the fluorescent moiety of Laurdan is located at the level of the glycerol backbone, and the emission shift upon change in temperature is due to a dipolar relaxation process (23). For MLVs formed in water, the red shift and decrease in GP generally associated with the phase transition are caused by a change in lipid packing, as the more disordered membrane allows for deeper penetration of the polar solvent molecules in the interfacial region.
Variations of emission spectra and GP, such as those shown in this study, have been reported before in cases of high ionic strength or in the presence of cations in the buffer for dimyristoylphosphatidylglycerol liposomes (24, 25). In these studies, the effect was due to changes in lipid packing upon electrostatic attraction caused by ionic charges. Despite sucrose being highly polar, our results show that when it is present in high concentration, the Laurdan emission spectra display a small decrease in the polarity of the solvent surrounding the probe naphtalene moiety (18). Conversely, increased GP values in the presence of sugar are linked to a lower mobility for water or a smaller number of water molecules around the Laurdan naphthalene moiety.
Two possible mechanisms can be invoked to explain such an effect: 1) tighter lipid packing of the membrane, as observed for example in liquid-ordered phases in the presence of cholesterol, and 2) depletion of water molecules at the lipid headgroup region, which reduces the emission shift of Laurdan.
Simulations and previous experimental studies showed that the adsorption of sugar to the membrane leads to a smaller number of molecules around the lipid headgroup (5, 26, 27, 28). These results are consistent with our GP data, which show a progressive reduction of water molecules surrounding the Laurdan naphthalene moiety, resulting in higher GP values.
DSC
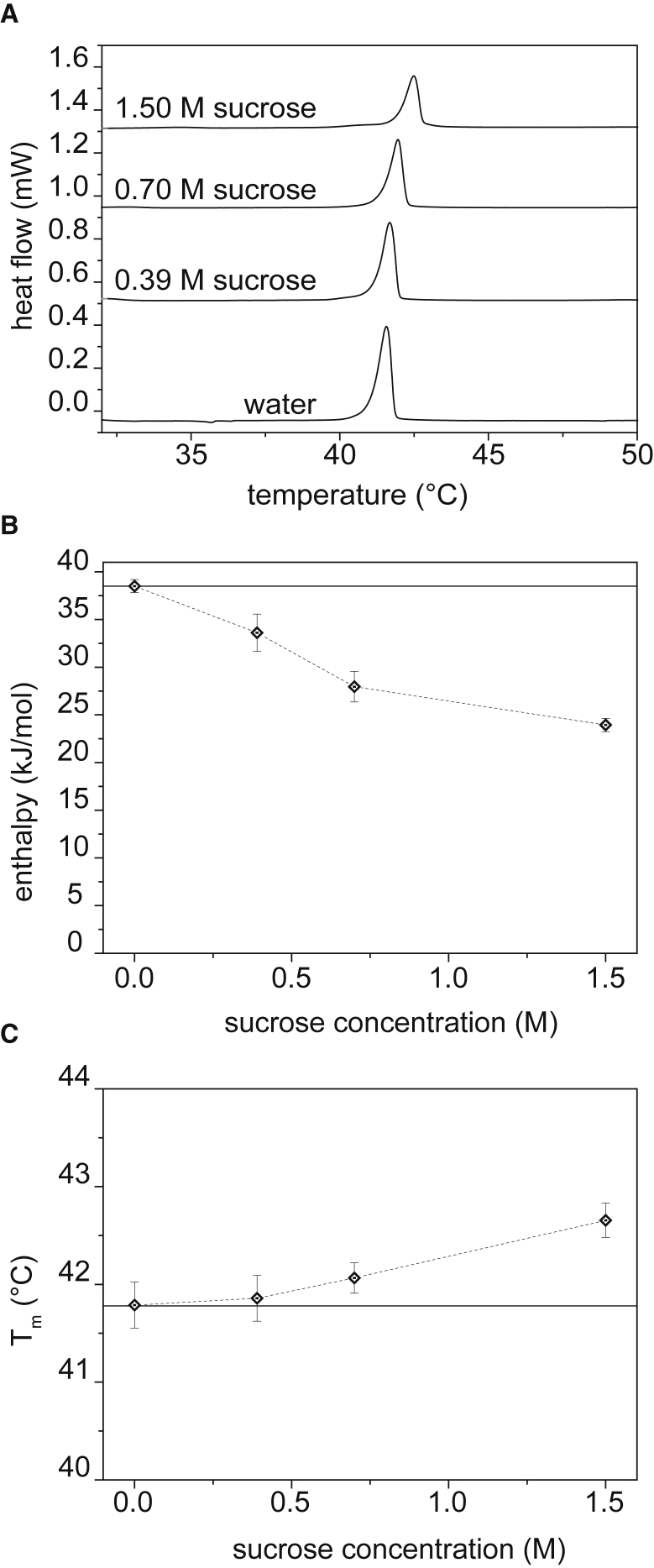
Fig. 3 reports DSC results on DPPC MLVs hydrated with water, and 0.39, 0.70, and 1.50 M sucrose. Fig. 3 A shows the raw data; integration of the area under the peak around gives the energy associated with the transition from the gel to liquid crystalline phases, which is related to the local packing of the membrane upon melting (29).
Figure 3.
Summary of DSC results for DPPC MLVs at different concentrations of sucrose. (A) A DSC calorimetric signal is shown. (B) Decreases in enthalpy at different sucrose concentrations are shown. (C) Variation of Tm with sucrose concentration is shown. In both (B) and (C), the full straight line represents the initial value in pure water, and the dashed lines connect the experimental points.
The intensity of the peak decreases with increasing amounts of sugar, and the center of the peak shifts to slightly higher temperatures with the sucrose concentration. However, the curves remain sharp, indicating that the transition is still highly cooperative. Broadening of the peaks is usually associated with disruption of lipid packing and cooperativity (30), as reported by Mannock et al. (31) for cholesterol. Here, such large broadening is not present, suggesting no significant disruption of the number of lipids participating in the transition cooperatively.
The enthalpy , transition temperature , and midheight width of the peaks are summarized in Table 1. For DPPC membranes formed in pure water, the enthalpy of the transition is 38.5 kJ/mol with temperature at 41.8°C, which is in good agreement with previously reported values (32, 33). A significant drop in enthalpy in the presence of sucrose is observed, with a final value of 23.9 kJ/mol at 1.50 M sucrose. A drop in sucrose concentration of DPPC enthalpy of transition has been observed previously by Chowdhry and Chen (32, 33). Our values are in good agreement with the variation of 10.9 kJ/mol for 1 M sucrose measured by Chowdhry. Chen, however, reported a drop of 11.6 kJ/mol earlier at 0.2 M sucrose, which we did not observe. Surprisingly, our results show a nonlinear trend for the enthalpy change with sucrose concentration (Fig. 3 B). The enthalpy dropping rate slows down at 1.50 M, suggesting a saturation of the sucrose effect on the membrane transition. This saturation could be linked to a maximal adsorption of sucrose at the surface, as it was measured with electron spin echo envelope modulation by Konov et al. (34), who reported Langmuir adsorption isotherms of sucrose on DPPC bilayers.
The transition temperature for DPPC increases only slightly with sucrose concentration, with a final value of 42.7°C for 1.50 M of sucrose. These changes in , albeit small, are in good agreement with the increase of 0.6°C at 1 M reported by Chowdhry, measured via calorimetry (32). Stümpel et al. (35) also observed a shift of ∼1°C in the of DMPC MLVs in the presence of 1.17 M sucrose. Similar effects have been reported on DMPC vesicles (36). Other studies instead reported little or no changes in , contrary to our results (33). As a matter of fact, many studies report variations in the of lipid bilayers upon interaction with macromolecules located either at the surface or in the hydrophobic region of the membrane (37, 38). An increase in the transition temperature is generally linked to a tighter packing of the lipids, with different possible causes. In this study, we argue that the observed shift of to higher temperatures is due to a dehydration of the lipid bilayer. Indeed, sugar molecules, by accumulating on the membrane surface, substitute water molecules from the first hydration layer, increasing the transition temperature. This is in agreement with our observation of the Laurdan emission spectra, which indicate a decrease in the number of water molecules at the water-membrane interface.
In poorly hydrated (20% water content) DPPC vesicles, Gabrielle-Delmont (39) reported an enthalpy drop and a small shift comparable to our observations. Such low hydration conditions, however, lead to a high temperature peak in the DSC curves, which is a contribution we do not see for our experimental conditions in which minimal water content is ∼60% (or 1.50 M) sucrose. The effect of substitution of water molecules by sucrose cannot therefore be reduced to simple bilayer dehydration. Finally, we observe a slight increase of , which is consistent with the values reported by Chen (33).
Thermodynamic model
We now attempt to rationalize our experimental observations on the interaction of sucrose with a DPPC bilayer by means of a thermodynamic model.
In discussing the interaction between dry bilayers and sugars, a frequent model is the water replacement hypothesis, which postulates direct hydrogen bonding between sugars and phospholipids (1). By creating H bonds with lipid headgroups, sugars keep lipids separated in the liquid phase upon dehydration. In some cases, the hypothesis considers the sugars to be able to penetrate the interfacial region of the membrane and therefore keep the lipids apart. Although our data do support the removal of water molecules from the membrane, the water replacement hypothesis cannot explain both the increase in and the decrease of enthalpy.
Another proposed model is the hydration forces explanation (40, 41), which assumes no direct interaction between sugars and lipids. Sugars and nonspecific volumetric and osmotic effects are the causes of change in the bilayer phase transition. This model has been able to quantitively predict changes in the transition temperature (7, 42); however, the variations of enthalpy reported here and in previous studies have not been explained.
A thermodynamic model that would fully explain the observed phenomena should include the following: 1) a mild increase in the transition temperature, 2) a significant drop in enthalpy, and 3) a decrease in the cooperativity of the transition. As we will see below, the last point is required to reconcile the apparent contraction of the first two without invoking some unknown reason for an almost perfect balance of the enthalpic and entropic variations.
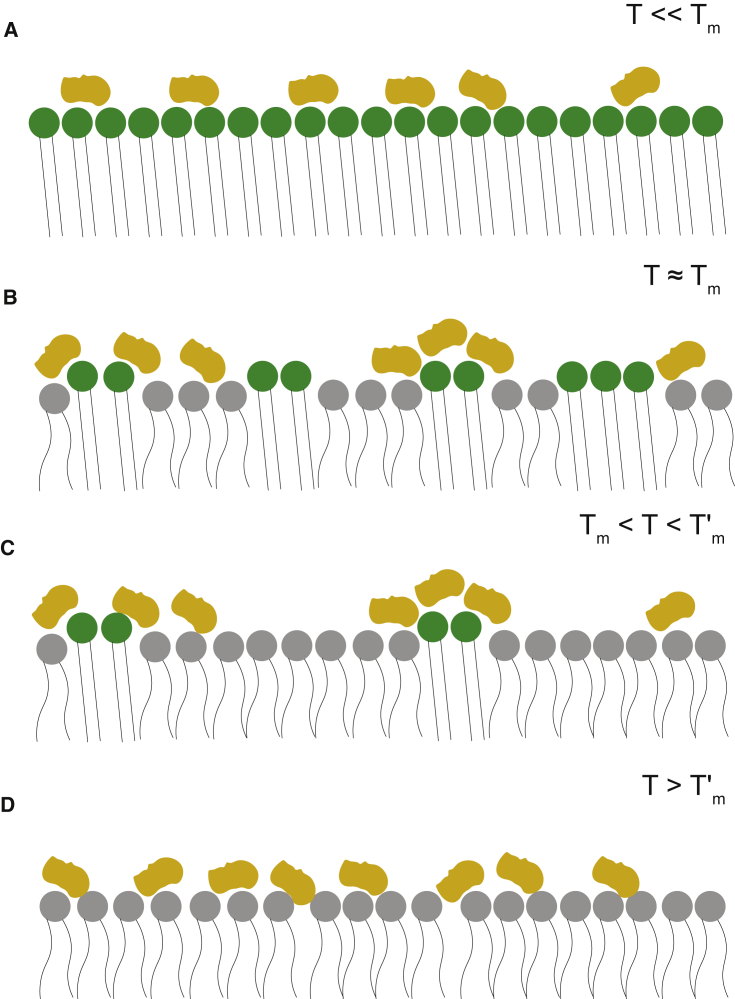
We propose a model in which the adsorption of sucrose at the membrane surface locally dehydrates the lipid bilayer, resulting in the formation of clusters with intrinsically different transition temperatures; see Fig. 4. As change of state occurs, only a fraction of the lipids participate, resulting in a lower enthalpic contribution and a higher effective melting temperature, coupled with a broader peak.
Figure 4.
Schematic representation of the interaction of sucrose with the lipid bilayer at different temperatures. (A) Below , the lipid bilayer is in the phase, with sucrose molecules excluded from the interfacial region of the lipid headgroup. (B) At temperatures near , the membrane undergoes a gel-to-fluid transition in which clusters of lipid melt into the fluid phase. The lipids that interact less with sucrose melt at , whereas lipids dehydrated by interaction with saccharides will have higher effective transition temperatures . (C) In the intermediate region between and , the lipids interacting with sucrose are still in the gel phase. (D) Above , the membrane is completely in the fluid phase, with sucrose molecules adsorbed on the membrane and penetrating the interfacial region. To see this figure in color, go online.
To interpret our data, we use a formalism previously introduced to reproduce the calorimetry of pure and binary lipid systems (43, 44, 45). This description is akin to an Ising spin model, where the spin represents the internal state (phase) of the lipid. Based on (44), the configurational energy difference of the reference gel state for a pure lipid bilayer is given by:
| (2) |
where is the number of lipids in the liquid phase, is the enthalpy of melting per molecule at the transition, is the corresponding entropy contribution, J is the cooperativity parameter, and is the number of gel-liquid lipid pairs in contact. A penalty J is counted every time a nearest-neighbor pair of lipids is found with different internal states, i.e., a mismatched pair.
We assume that sucrose molecules in contact with lipid molecules slightly modify the enthalpy and entropy of melting while decreasing the cooperativity parameter (the mismatch energy). A full derivation of our model can be found in the Supporting Materials and Methods. Following this approach, the configurational energy difference is defined as follows:
| (3) |
where represents, as in Eq. 2, the internal mismatch energy between neighboring lipids in different states. For the case without sucrose, is the mismatch energy when neighboring lipids in different states are both in contact with sucrose, and is the mismatch energy when only one lipid is in contact with sucrose. is the total number of gel-fluid lipid pairs.
We now introduce the probability σ for a lipid to be in contact with a sucrose molecule, assuming that σ is constant and controlled by the concentration of sucrose in solution. The number of lipids in the gel and liquid states thus depends on the site coverage σ and the probabilities for a lipid to be in a specific state. The number of lipids is determined by
| (4) |
for free lipids and
| (5) |
for sucrose-bound lipids. To estimate the interaction terms, we use a mean-field approximation involving the probabilities and the average coordination number z of lipid molecules in each plane leaflet:
| (6) |
The average enthalpy difference can be expressed using the above mean-field approximation as follows:
| (7) |
where is the sum of all the cooperativity terms listed in Eq. 3. As pointed out in (44), the respective enthalpy and entropy contributions to are arbitrary. We assume here that is purely of enthalpic origin. Fortunately, it turns out that depends only marginally on this assumption.
Adding to the configurational energy (3) the mean-field configurational entropy given by
| (8) |
and minimizing with respect to p and leads to the following set of self-consistent equations:
| (9) |
As explained in Supporting Materials and Methods, we performed a numerical calculation using our model for a coordination number with the following parameter values: K, K, with J corresponding to 200 cal/mol, corresponding to 100 cal/mol, and . As a result, the enthalpy (7) increases sharply around the nominal melting transition temperature. We retain the change in enthalpy across a K temperature interval centered around 315 K as representative of the experimental heat adsorbed at the transition. The inflection point of with respect to temperature gives us the apparent melting temperature. No attempt was made to reproduce the shape of the measured excess-specific heat curve, given the mean-field character of our treatment.
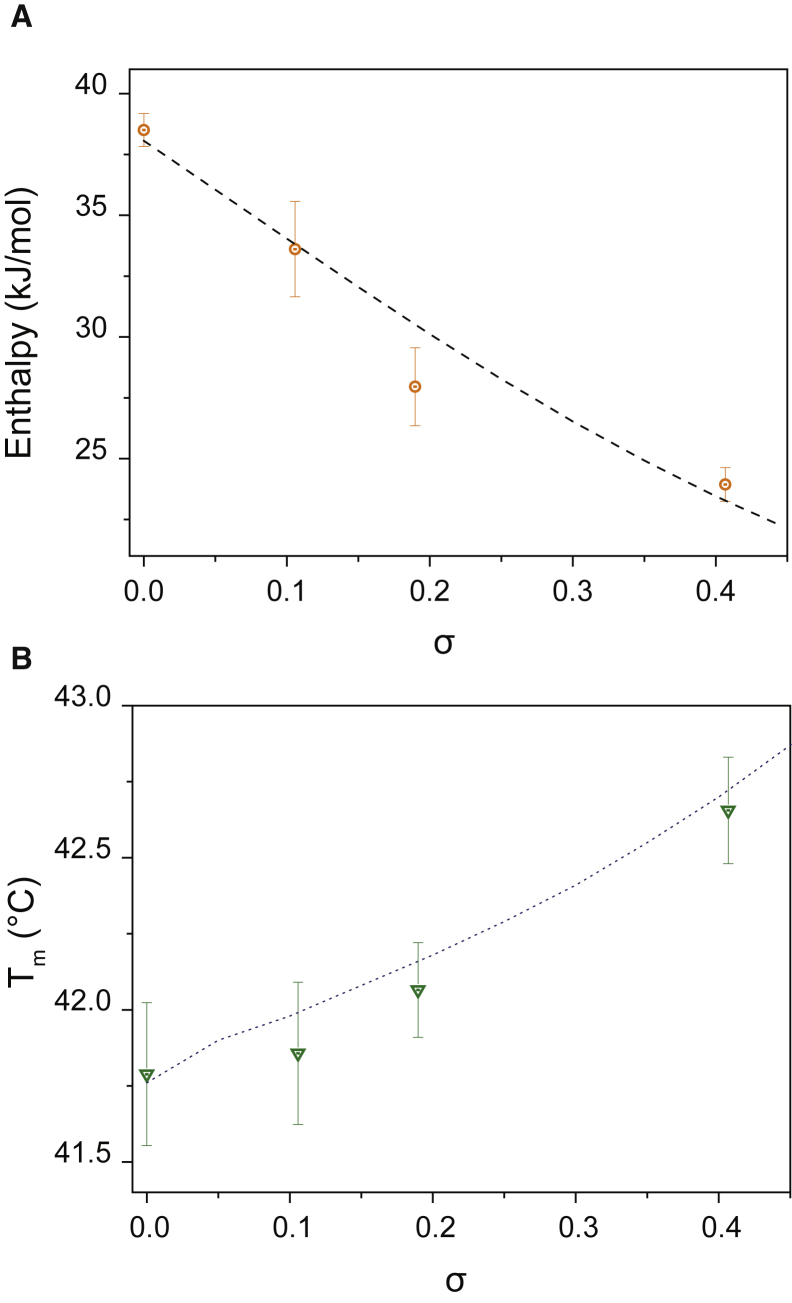
Our coverage parameter σ was adjusted to the experimental conditions by comparing the theoretical value of σ required to obtain the enthalpy reduction measured for given mass concentrations of sucrose, as shown in Fig. S2, with an approximately linear variation being found between σ and the sucrose concentration. The experimental changes in enthalpy (Fig. 5 A) and the shift in transition temperatures (Fig. 5 B) are found to be well described by this theoretical model, thus supporting its main assumptions.
Figure 5.
(A) Experimental values of for the DPPC gel-to-liquid transition at different concentrations of sucrose (circles), and theoretical predictions from the thermodynamics model (dotted line). (B) Experimental values of for the DPPC gel-to-liquid transition are shown at different concentration of sucrose (triangles), and theoretical predictions from the thermodynamics model are also shown (dotted line). To see this figure in color, go online.
Conclusions
In this study, we have shown that sucrose can alter the gel-liquid phase transition of DPPC lipid bilayers, slightly increasing while lowering the enthalpy of the transition by a significant amount. Our experimental results bring together complementary techniques to probe a consistent set of samples, thus reducing the previous dispersion of conclusions likely because of the different degrees of exposure of the bilayers to the sugars as a consequence of different preparation methods. We not only clarify previous observations concerning the effects of sugars in well-hydrated lipid bilayers of DPPC but also raise puzzling questions about the mechanisms of sugar-bilayer interactions. Our spectrometric results based on GP measurements of Laurdan do point to a dehydration of the membrane interfacial region. However, if the main action of the sugar was to dehydrate the bilayers, then a much larger shift of to larger values would be expected. We propose here an explanation for this conundrum by a mechanism of partially delayed transition. More specifically, we assume that lipids in contact with sugars have their transition shifted to higher temperatures, whereas the others behave in an unperturbed manner. As our model quantitatively predicts based on these assumptions, this suppresses the measurable heat release around the calorimetric maximum, shifting the value of the maximum by a few degrees.
The excellent agreement between our model and the thermodynamic and spectrometric data reported in this article suggests that further investigations of the bilayer properties must be conducted. In particular, it would be important to inspect bilayer behavior at the optical length scales with lipid platforms such as GUVs. Although for technical reasons we were not able to perform such experiments for DPPC GUVs, we did perform preliminary experiments for DMPC GUVs in solutions with different sucrose concentrations; see Supporting Materials and Methods. As our results with DMPC show, the sugar reduces the width of the metastable region above , where out-of-equilibrium domains are observed. Such reduction is compatible with a sugar-induced decrease of the mismatch energy between lipids in the gel and liquid states, which is a key ingredient of our model. Also, the interaction between the sugars and the lipids might induce different kinetics for sugar-free and sugar-bound lipids that could be probed not only by a systematic study of the influence of the cooling and heating rates on the transition but also by changing the nature of the sugars.
Author Contributions
M.I.M., M.S., and M.K. performed the experiments. F.T. developed the theoretical model. F.T., A.P.S., and C.M.M. designed and managed the project. M.I.M., F.T., A.P.S., and C.M.M. contributed to the writing of the article.
Acknowledgments
The ISO9001 Characterization Platform of Institut Charles Sadron is gratefully acknowledged for access to μDSC.
M.I.M., M.K., F.T., A.P.S., and C.M.M. acknowledge funding from the European FP7-MSCA International Training Network SNAL 608184 (Smart Nano-Objects for Alteration of Lipid Bilayers) for support for this work.
Editor: Arne Gericke.
Footnotes
Supporting Materials and Methods and six figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30440-5.
Supporting Citations
References (46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59) appear in the Supporting Material.
Supporting Material
References
- 1.Crowe L.M., Reid D.S., Crowe J.H. Is trehalose special for preserving dry biomaterials? Biophys. J. 1996;71:2087–2093. doi: 10.1016/S0006-3495(96)79407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowe J.H., Crowe L.M., Tablin F. The trehalose myth revisited: introduction to a symposium on stabilization of cells in the dry state. Cryobiology. 2001;43:89–105. doi: 10.1006/cryo.2001.2353. [DOI] [PubMed] [Google Scholar]
- 3.Yancey P.H., Clark M.E., Somero G.N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 4.Crowe J.H., Crowe L.M., Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzardo M.C., Amalfa F., Disalvo E.A. Effect of trehalose and sucrose on the hydration and dipole potential of lipid bilayers. Biophys. J. 2000;78:2452–2458. doi: 10.1016/s0006-3495(00)76789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrigan P.R., Madden T.D., Cullis P.R. Protection of liposomes during dehydration or freezing. Chem. Phys. Lipids. 1990;52:139–149. doi: 10.1016/0009-3084(90)90157-m. [DOI] [PubMed] [Google Scholar]
- 7.Lenné T., Garvey C.J., Bryant G. Effects of sugars on lipid bilayers during dehydration--SAXS/WAXS measurements and quantitative model. J. Phys. Chem. B. 2009;113:2486–2491. doi: 10.1021/jp808670t. [DOI] [PubMed] [Google Scholar]
- 8.Döbereiner H.G., Selchow O., Lipowsky R. Spontaneous curvature of fluid vesicles induced by trans-bilayer sugar asymmetry. Eur. Biophys. J. 1999;28:174–178. [Google Scholar]
- 9.Genova J., Zheliaskova A., Mitov M.D. The influence of sucrose on the elasticity of SOPC lipid membrane studied by the analysis of thermally induced shape fluctuations. Colloids Surf. A Physicochem. Eng. Asp. 2006;282–283:420–422. [Google Scholar]
- 10.Vitkova V., Genova J., Bivas I. Sugars in the aqueous phase change the mechanical properties of lipid mono- and bilayers. Mol. Cryst. Liq. Cryst. (Phila. Pa.) 2006;449:95–106. [Google Scholar]
- 11.Nagle J.F., Jablin M.S., Akabori K. What are the true values of the bending modulus of simple lipid bilayers? Chem. Phys. Lipids. 2015;185:3–10. doi: 10.1016/j.chemphyslip.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagle J.F., Jablin M.S., Tristram-Nagle S. Sugar does not affect the bending and tilt moduli of simple lipid bilayers. Chem. Phys. Lipids. 2016;196:76–80. doi: 10.1016/j.chemphyslip.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Strauss G., Schurtenberger P., Hauser H. The interaction of saccharides with lipid bilayer vesicles: stabilization during freeze-thawing and freeze-drying. Biochim. Biophys. Acta. 1986;858:169–180. doi: 10.1016/0005-2736(86)90303-2. [DOI] [PubMed] [Google Scholar]
- 14.Crowe L.M., Crowe J.H. Solution effects on the thermotropic phase transition of unilamellar liposomes. Biochim. Biophys. Acta. 1991;1064:267–274. doi: 10.1016/0005-2736(91)90311-u. [DOI] [PubMed] [Google Scholar]
- 15.Parasassi T., Krasnowska E.K., Gratton E. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998;8:365–373. [Google Scholar]
- 16.Rodrigues J.P., Paraguassú-Braga F.H., Porto L.C. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56:144–151. doi: 10.1016/j.cryobiol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Anchordoguy T.J., Rudolph A.S., Crowe J.H. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987;24:324–331. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- 18.Parasassi T., Di Stefano M., Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys. J. 1994;66:120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vequi-Suplicy C.C., Benatti C.R., Lamy M.T. Laurdan in fluid bilayers: position and structural sensitivity. J. Fluoresc. 2006;16:431–439. doi: 10.1007/s10895-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 20.Bagatolli L.A., Gratton E., Fidelio G.D. Water dynamics in glycosphingolipid aggregates studied by Laurdan fluorescence. Biophys. J. 1998;75:331–341. doi: 10.1016/S0006-3495(98)77517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagatolli L.A., Maggio B., Fidelio G.D. Laurdan properties in glycosphingolipid-phospholipid mixtures: a comparative fluorescence and calorimetric study. Biochim. Biophys. Acta. 1997;1325:80–90. doi: 10.1016/s0005-2736(96)00246-5. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975;14:152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- 23.Vequi-Suplicy C.C., Coutinho K., Lamy M.T. New insights on the fluorescent emission spectra of Prodan and Laurdan. J. Fluoresc. 2015;25:621–629. doi: 10.1007/s10895-015-1545-x. [DOI] [PubMed] [Google Scholar]
- 24.Lúcio A.D., Vequi-Suplicy C.C., Lamy M.T. Laurdan spectrum decomposition as a tool for the analysis of surface bilayer structure and polarity: a study with DMPG, peptides and cholesterol. J. Fluoresc. 2010;20:473–482. doi: 10.1007/s10895-009-0569-5. [DOI] [PubMed] [Google Scholar]
- 25.Riske K.A., Barroso R.P., Lamy M.T. Lipid bilayer pre-transition as the beginning of the melting process. Biochim. Biophys. Acta. 2009;1788:954–963. doi: 10.1016/j.bbamem.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Sum A.K., Faller R., de Pablo J.J. Molecular simulation study of phospholipid bilayers and insights of the interactions with disaccharides. Biophys. J. 2003;85:2830–2844. doi: 10.1016/s0006-3495(03)74706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy A., Dutta R., Sarkar N. A comparative study of the influence of sugars sucrose, trehalose, and maltose on the hydration and diffusion of DMPC lipid bilayer at complete hydration: investigation of structural and spectroscopic aspect of lipid-sugar interaction. Langmuir. 2016;32:5124–5134. doi: 10.1021/acs.langmuir.6b01115. [DOI] [PubMed] [Google Scholar]
- 28.Pereira C.S., Lins R.D., Hünenberger P.H. Interaction of the disaccharide trehalose with a phospholipid bilayer: a molecular dynamics study. Biophys. J. 2004;86:2273–2285. doi: 10.1016/S0006-3495(04)74285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demetzos C. Differential scanning calorimetry (DSC): a tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008;18:159–173. doi: 10.1080/08982100802310261. [DOI] [PubMed] [Google Scholar]
- 30.Marsh D., Watts A., Knowles P.F. Cooperativity of the phase transition in single- and multibilayer lipid vesicles. Biochim. Biophys. Acta. 1977;465:500–514. doi: 10.1016/0005-2736(77)90268-1. [DOI] [PubMed] [Google Scholar]
- 31.Mannock D.A., Lewis R.N., McElhaney R.N. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys. J. 2006;91:3327–3340. doi: 10.1529/biophysj.106.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhry B.Z., Lipka G., Sturtevant J.M. Thermodynamics of phospholipid-sucrose interactions. Biophys. J. 1984;46:419–422. doi: 10.1016/S0006-3495(84)84038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C.H., Berns D.S., Berns A.S. Thermodynamics of carbohydrate-lipid interactions. Biophys. J. 1981;36:359–367. doi: 10.1016/S0006-3495(81)84737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konov K.B., Leonov D.V., Dzuba S.A. Membrane-sugar interactions probed by pulsed electron paramagnetic resonance of spin labels. J. Phys. Chem. B. 2015;119:10261–10266. doi: 10.1021/acs.jpcb.5b06864. [DOI] [PubMed] [Google Scholar]
- 35.Stümpel J., Vaz W.L., Hallmann D. An X-ray diffraction and differential scanning calorimetric study on the effect of sucrose on the properties of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1985;821:165–168. doi: 10.1016/0005-2736(85)90167-1. [DOI] [PubMed] [Google Scholar]
- 36.Fabrie C.H., de Kruijff B., de Gier J. Protection by sugars against phase transition-induced leak in hydrated dimyristoylphosphatidylcholine liposomes. Biochim Biophys Acta. 1990;1024:380–384. doi: 10.1016/0005-2736(90)90368-x. [DOI] [PubMed] [Google Scholar]
- 37.Ali S., Minchey S., Mayhew E. A differential scanning calorimetry study of phosphocholines mixed with paclitaxel and its bromoacylated taxanes. Biophys. J. 2000;78:246–256. doi: 10.1016/S0006-3495(00)76588-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bothun G.D. Hydrophobic silver nanoparticles trapped in lipid bilayers: size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnology. 2008;6:13. doi: 10.1186/1477-3155-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabielle-Madelmont C., Perron R. Calorimetric studies on phospholipidwater systems. J. Colloid Interface Sci. 1983;95:471–482. [Google Scholar]
- 40.Koster K.L., Lei Y.P., Bryant G. Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys. J. 2000;78:1932–1946. doi: 10.1016/S0006-3495(00)76741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfe J., Bryant G. Freezing, drying, and/or vitrification of membrane- solute-water systems. Cryobiology. 1999;39:103–129. doi: 10.1006/cryo.1999.2195. [DOI] [PubMed] [Google Scholar]
- 42.Lenné T., Garvey C.J., Bryant G. Kinetics of the lamellar gel-fluid transition in phosphatidylcholine membranes in the presence of sugars. Chem. Phys. Lipids. 2010;163:236–242. doi: 10.1016/j.chemphyslip.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Doniach S. Thermodynamic fluctuations in phospholipid bilayers. J. Chem. Phys. 1978;68:4912–4916. [Google Scholar]
- 44.Heimburg T., Biltonen R.L. A Monte Carlo simulation study of protein-induced heat capacity changes and lipid-induced protein clustering. Biophys. J. 1996;70:84–96. doi: 10.1016/S0006-3495(96)79551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heimburg T. Wiley-VCH; Weinheim, Germany: 2007. Thermal Biophysics of Membranes. [Google Scholar]
- 46.Bagatolli L.A., Gratton E. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 1999;77:2090–2101. doi: 10.1016/S0006-3495(99)77050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirst L.S., Ossowski A., Selinger R.L. Morphology transition in lipid vesicles due to in-plane order and topological defects. Proc. Natl. Acad. Sci. USA. 2013;110:3242–3247. doi: 10.1073/pnas.1213994110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klymchenko A.S., Kreder R. Fluorescent probes for lipid rafts: from model membranes to living cells. Chem. Biol. 2014;21:97–113. doi: 10.1016/j.chembiol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Baumgart T., Hunt G., Feigenson G.W. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim. Biophys. Acta. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen D., Santore M.M. Large effect of membrane tension on the fluid-solid phase transitions of two-component phosphatidylcholine vesicles. Proc. Natl. Acad. Sci. USA. 2014;111:179–184. doi: 10.1073/pnas.1314993111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metso A.J., Zhao H., Kinnunen P.K. Observation of the main phase transition of dinervonoylphosphocholine giant liposomes by fluorescence microscopy. Biochim. Biophys. Acta. 2005;1713:83–91. doi: 10.1016/j.bbamem.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Jørgensen K., Mouritsen O.G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys. J. 1995;69:942–954. doi: 10.1016/S0006-3495(95)79968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crowe J.H., Crowe L.M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 54.Angelova M.I., Dimitrov D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986;81:303–311. [Google Scholar]
- 55.Moiset G., López C.A., Marrink S.J. Disaccharides impact the lateral organization of lipid membranes. J. Am. Chem. Soc. 2014;136:16167–16175. doi: 10.1021/ja505476c. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein R.E., Leibler S. Structural phase transitions of interacting membranes. Phys. Rev. A Gen. Phys. 1989;40:1025–1035. doi: 10.1103/physreva.40.1025. [DOI] [PubMed] [Google Scholar]
- 57.Jerala R., Almeida P.F., Biltonen R.L. Simulation of the gel-fluid transition in a membrane composed of lipids with two connected acyl chains: application of a dimer-move step. Biophys. J. 1996;71:609–615. doi: 10.1016/S0006-3495(96)79261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivanova V.P., Heimburg T. Histogram method to obtain heat capacities in lipid monolayers, curved bilayers, and membranes containing peptides. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2001;63:041914. doi: 10.1103/PhysRevE.63.041914. [DOI] [PubMed] [Google Scholar]
- 59.Fisher M.E. The theory of equilibrium critical phenomena. Rep. Prog. Phys. 1967;30:615. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.