Abstract
Background:
The Vibralung Acoustical Percussor is a new airway clearance therapy (ACT) utilizing intrapulmonary sound waves in addition to positive expiratory pressure (PEP). We evaluated the safety of the Vibralung and collected preliminary data on its ability to mediate sputum expectoration in individuals with cystic fibrosis (CF).
Methods:
Over two separate studies, 10 and 11 mild to moderate CF patients were recruited for study I and II, respectively. Study I: Vibralung was used for 20 min with either no sound (NS: PEP only) or sound (S: PEP and sound) on randomized visits. Pulmonary function, diffusion capacity of the lungs for carbon monoxide and nitric oxide (DLCO/DLNO), symptoms, and peripheral oxygen saturation (SpO2) were measured at baseline and at 1 and 4 h post treatment. Expectorated sputum was collected over 4 h post treatment. Study II: over 5 days of in-hospital therapy, the Vibralung or vibratory vest therapy (Vest) were used for two therapy sessions per day, with sputum collected for 20 min following each therapy and pulmonary function accessed pre and post each 5-day period (days 1–5 or 7–11) in a randomized crossover design.
Results:
Vibralung usage resulted in no change from baseline to 4 h post in pulmonary function, SpO2 or symptoms (p > 0.05). At 4 h post therapy, the DLCO- and DLNO-derived measure of alveolar–capillary unit function (DM/VC) showed improvement (DM/VC = 12.5 ± 5.5 versus 7.3 ± 18.8% change, S versus NS) with no difference between S and NS (p = 0.74). Sputum expectoration was similar between S and NS conditions (wet sputum = 10.5 ± 4.6 versus 9.9 ± 3.2 g, S versus NS, p = 0.25). There were no differences in the improvement in pulmonary function between Vibralung and Vest during either 5-day period during the hospital stay.
Conclusions:
Vibralung was well tolerated and caused no detrimental changes in pulmonary function metrics. The Vibralung appears to be a safe ACT in individuals with CF.
Keywords: airway clearance therapy, cystic fibrosis, diffusing capacity of the lungs for carbon monoxide and nitric oxide, high-frequency chest wall oscillation/compression, oscillatory positive expiratory pressure, pulmonary function, sputum expectoration/clearance
Introduction
Cystic fibrosis (CF) is an inherited autosomal recessive disease where gene mutation of cystic fibrosis transmembrane conductance regulator chloride channel impairs the secretory abilities of various endocrine organs and tissues.1,2 In the lungs, the impairments in salt regulation cause a drastic depletion of the airway surface liquid (ASL). With the ASL depleted, the mucus compresses the cilia such that they cannot properly beat to facilitate mucus clearance. The stagnant thick mucus that is not cleared can obstruct the distal airways and submucosal glands and becomes a breeding ground for bacteria and other pathogens.3 A chronic cyclic state of infection and inflammation causes damage to the airways, a progressive decline in lung function, and for many, eventual failure if there is no lung transplant.2,3 For this reason, removal of mucus and airway secretions, termed airway clearance therapy (ACT) is a crucial piece of the treatment and management of CF.4
There are a variety of airway clearance methods and devices available for ACT. To be effective, the method used must increase mucus clearance. It is crucial to move stagnant mucus to improve gas exchange, minimize colonization of infections, and support the overall goal to attenuate decline in pulmonary function. Currently, ACT methods include postural drainage and percussion therapy (PD and P) performed by a respiratory therapist or family member, high-frequency chest wall oscillation/compression (HFCWO/HFCWC, wearing a vibratory vest), positive expiratory pressure (PEP) masks or oscillatory PEP devices such as the Acapella® and Flutter®, and breathing techniques (active cycle of breathing and autogenic drainage). All methods are generally followed by cough to facilitate sputum expectoration. Although an important part of CF management is to maintain health, prevent deterioration, and treat pulmonary exacerbations, the extent of adherence for ACT has been reported to be as low as 54% and as high as 96.5%.5–10
Previous research has compared ACT methods against one another, and systematic reviews have concluded that efficacy for preserving pulmonary function, and facilitating mucus clearance is comparable between most devices.11–13 With no method demonstrating superiority, it is important to note this does not mean that all therapies are equally effective for all patients. The key is for the individual and their CF care team to choose an ACT method that is most effective, but probably more importantly, that the individual will have the highest adherence rate using it.
Recently, the Vibralung Acoustical Percussor (Vibralung, Westmed, Inc., Tucson, AZ) device became a new ACT option that is cleared by the US Food and Drug Administration (FDA) and commercially available. The Vibralung is a handheld device a patient breathes through as it applies sound waves, at various frequencies, over a 10-min treatment protocol, directly to the intrapulmonary airways. The Vibralung device is hypothesized to internally vibrate the airways by the phenomenon of sympathetic resonance, akin to vibrating windows in a home if a stereo music system is played at high volumes. It has been postulated that the increase in sputum expectoration is derived from the sound waves effectively vibrating the boundary between mucus and the airway surface to which it adheres. This technique allows direct airway vibration that is accomplished without the need to vibrate the entire body. In addition, the Vibralung device applies a wide range of progressively increasing frequencies throughout the treatment session, rather than a single frequency. This sequential frequency-stepping technique is used to accommodate the acoustic theory that, the smaller the tube or cylinder (airway segment), the higher the frequency applied must be to vibrate it at its resonant frequency. As such, most Vibralung settings progress from a set of low frequency tone pulses (for large diameter/longer airways) to increasingly higher sets of tone pulses (for smaller diameter/shorter airways) in order to effectively vibrate as many different sized airway segments as possible.14 Additionally, the Vibralung incorporates PEP to slightly dilate airways during exhalation, to mitigate airway collapse and promote effective mucus expectoration that is currently being used as an effective ACT on its own.15–17
Therefore, the purpose of this research was to determine the safety of the Vibralung Acoustical Percussor and collect preliminary data on its efficacy for mediating sputum expectoration in individuals with CF in two studies: (a) to compare the use of sound (S) versus no sound (NS) (to determine if the intrapulmonary sound waves were harmful to the lungs); and (b) to compare Vibralung versus vibratory vest therapy (Vest) in hospitalized individuals with CF.
Methods
Study population
Individuals with CF with mild to moderate lung disease [forced expiratory volume in 1 s (FEV1) > 40%] between the ages of 10 and 55 years, clinically diagnosed with a positive chloride sweat test and who had not had a pulmonary hemorrhage within the past 6 months or pneumothorax within the past year, and were not taking any experimental drugs, were recruited for participation in these studies. In study I, the 10 CF patients recruited were clinically stable (no pulmonary exacerbations or hospitalizations within the previous month or taking any antibiotics for a pulmonary exacerbation). In contrast, for study II, the 11 CF patients recruited were those who had been admitted to the hospital for a pulmonary exacerbation, who were expected to be admitted for a minimum of 2 weeks, and who could be recruited within the first 48 h of their admission. The protocol was reviewed and approved by the University of Arizona Institutional Review Board. All participants provided written informed consent prior to study, and all aspects of the study were performed according to the Declaration of Helsinki.
Study device
The Vibralung Acoustic Percussor device couples sound waves emitted by the speaker directly to the tracheobronchial tract at frequencies between 5 and 1200 Hz that encompass many of the natural resonant frequencies of the human tracheobronchial tract.14 The device is capable of generating various sound frequencies and patterns which have been assigned descriptive names by the manufacturer: Random Noise, Low, Medium and High. The Random Noise mode causes the speaker to emit a continuous ‘hash’ of random, simultaneous frequencies between 5 and 1200 Hz, such that the resulting sound is perceived by humans as ‘static’. For this study, a single usage would last 10 min and entail 1 min of Random Noise, followed by 7 min of sequential frequency-stepping pulses (perceived by humans as a series of musical ‘beeps’) over the range of 69.3–662.2 Hz, and then ended with 2 min of Random Noise.
Study participants were instructed to keep the original nozzle-shaped mouthpiece in their mouth, to couple the sound waves to their airways. Patients wore a nose clip during the treatment period to obviate nose breathing and direct all sound waves into the tracheobronchial tract through the mouthpiece. Patients would inhale around the mouthpiece, but exhale through the mouthpiece, where the exhaled air would escape through two small holes in the housing. These holes were of sufficient size to allow exhalation while also producing PEP of approximately 4–5 cm H2O.
The Vibralung device utilized in our study was the single-piece commercial handheld device, available at that time (years 2010–2011), which contained the mouthpiece attached to a small acoustical loud speaker that produces audible pulsatile tones generated by an electronic controller that is part of the device handle and where the battery was also housed. After this study, the two-piece commercial device, consisting of (a) a separate hand-held unit with speaker housing and mouthpiece, and (b) a separate electronic controller and battery unit, was cleared for marketing by the FDA in 2014 and is currently in clinical use in 10 CF centers across the US.
Study design
The data collected encompassed two studies.
Study I
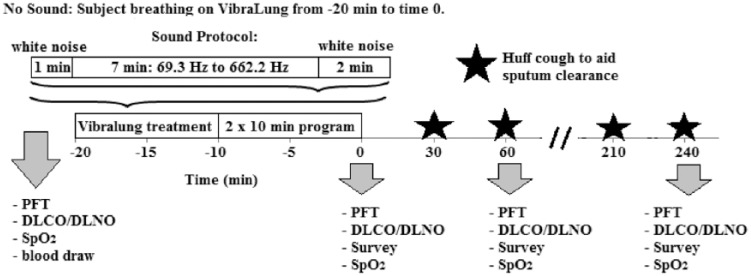
Participants completed two randomized treatment visits (Figure 1). On one visit, the patients used the Vibralung device with S through two of its programmed 10-min treatments as described above for a 20-min total treatment. For the other visit, the patients used the Vibralung device with NS, such that they were breathing on the device incorporating only PEP for 20 min. For both visits, patients arrived fasted and refrained from taking albuterol the morning of their treatment visit. Upon arrival, patients were equipped with a 12-lead electrocardiogram and had a single blood draw to determine hemoglobin concentration. Indices of safety for this study included assessment of cardiovascular function (cardiac output and heart rate), pulmonary function, diffusion capacity of the lungs for carbon monoxide and nitric oxide (DLCO/DLNO) as primary outcomes, and peripheral oxygen saturation (SpO2) and symptoms as secondary outcomes. These measures were performed at baseline, 1 h, and 4 h post treatment. Patients were instructed to perform the huff cough maneuver every 30 min to aid in sputum clearance and the total sputum produced over the 4 h was then measured (wet, pellet, and dry weight). Patients completed a Likert scale survey that asked about pain, comfort, ease of breathing before, during, and after treatment. These metrics plus the perceived change in mucus clearance and the patient’s impression on ease of use and likelihood to use the device were also assessed at 1 and 4 h post treatment.
Figure 1.
Phase I schematic.
The horizontal axis represents time (min) relative to the Vibralung treatment. Two randomized visits were performed where the participants breathed on the Vibralung device: one with sound and one without sound. Assessments were performed at the time points as marked by the grey arrows. Huff cough was performed every 30 min indicated by the black star.
PFT, pulmonary function; DLCO/DLNO, diffusion capacity of the lungs for carbon monoxide and nitric oxide; SpO2, peripheral oxygen saturation; Survey, Likert survey to assess symptoms.
Study II
Patients were recruited within the first 48 h of admission to the hospital. Based on the order that the patient was recruited in, s/he was assigned the condition that had been randomly assigned to each patient number from 1 to 30 using a random number generator to assign conditions A–C to each of the 30 recruitment slots, such that there would be 10 patients in each condition with successful enrollment of the desired 30 total participants. A detailed timeline of the schedule of ACT and pre, post and daily measurements for each condition is provided in Table 1. A Vibralung treatment consisted of using the 10-min program three times in succession for a total treatment time of 30 min. This kept the treatment time the same between the two airway clearance modalities (Vibralung and Vest). The Vest treatments were 30 min, using the frequency and pressure settings prescribed by the respiratory therapist per physician-approved protocol specific for each patient. For the health and safety of the participants, the Vibralung treatment arm utilized the Vibralung at two of the four daily treatment times, while still having the patient utilize the Vest at three of their treatment times, which was the current prescribed minimum for individuals with CF at our center. This ensured that the patient always received three conventional airway clearance treatments daily, since the safety and efficacy of the Vibralung had not yet been proven in humans and we did not wish to inadvertently diminish the standard of care during the study. The Vest was chosen as the comparator because it was the most common airway clearance technique used by individuals with CF in our center when they were at home. Additionally, postural drainage and percussion by a respiratory therapist was not allowed as an airway clearance treatment modality to keep with only nonmanual methods.
Table 1.
Schedule of airway clearance therapy treatment based on condition and daily measurement timing.
| Treatment time | Condition A: (days 1–5 Vibralung) | Condition B: (days 7–11 Vibralung) | Condition C: (standard therapy: Vest) |
|---|---|---|---|
| Pre: admission days 1–5 | Full PFT and survey | Full PFT and survey | Full PFT and survey |
| 8 a.m. | Vibralung | Vest | Vest |
| Assessments | SpO2/20 min, sputum | SpO2/20 min, sputum | SpO2/20 min, sputum |
| 12 p.m. | Vest | Vest | Vest |
| 3 p.m. | Vibralung | Vest | Vest |
| Assessments | SpO2/20 min, sputum | SpO2/20 min, sputum | SpO2/20 min, sputum |
| Second 3 p.m. ACT | Vest | None | None |
| 8 p.m. | Vest | Vest | Vest |
| Post: admission days 1–5 | Full PFT and survey | Full PFT and survey | Full PFT and survey |
| Day 6: washout | Standard of care | Standard of care | Standard of care |
| Pre: admission days 7–11 | Full PFT and survey | Full PFT and survey | Full PFT and survey |
| 8 a.m. | Vest | Vibralung | Vest |
| Assessments | SpO2/20 min, sputum | SpO2/20 min, sputum | SpO2/20 min, sputum |
| 12 p.m. | Vest | Vest | Vest |
| 3 p.m. | Vest | Vibralung | Vest |
| Assessments | SpO2/20 min, sputum | SpO2/20 min, sputum | SpO2/20 min, sputum |
| Second 3 p.m. ACT | None | Vest | None |
| 8 p.m. | Vest | Vest | Vest |
| Post: admission day 12 | Full PFT and survey | Full PFT and survey | Full PFT and survey |
A full PFT and a survey of pain, comfort, ease of use and changes in breathing and sputum clearance when using the Vibralung or Vest was performed every 5 days to cover the pre- and post-time periods for crossover design of Vibralung or Vest use. ACT: a single ACT treatment (Vest or Vibralung) was 30 min in duration. Condition C was the control condition, where for the entire 12-day duration of their stay/participation in this study, patients utilized the Vest for all their daily airway clearance treatments. Assessments: sputum was collected for 20 min post ACT treatment and oxygen saturation was assessed at the end of sputum collection. For Vibralung 3 p.m. treatment in Condition A, the assessment was performed after the Vibralung before the Vest treatment was started.
ACT, airway clearance therapy; PFT, pulmonary function; SpO2, peripheral oxygen saturation; survey, Likert survey to assess symptoms; Vest, vibratory vest therapy.
The primary endpoint for study II was again confirming safety of the device. For an initial evaluation of effectiveness of sputum clearance mean weight of the sputum expectorated following the morning and afternoon treatments over a 5-day treatment period (days 1–5 or days 7–11) was compared between Vibralung and Vest usage participants for that 5-day period.
Experimental procedures
Pulmonary function testing
Spirometry was performed according to American Thoracic Society guidelines using the Medical Graphics CPXD (Minneapolis, MN, US) for study I and the portable CareFusion VMAX Encore PFT System (Franklin Lakes, NJ, US) for study II. Predicted values for all pulmonary function measures were based on predicted equations from NHANES III.18
Quantification of sputum sample weight
Wet weight (sputum and saliva) was determined as the total sample weight excluding the empty container weight. The sample was then centrifuged at 27,000 RPM for 15 min at 4°C and the supernatant (saliva) was removed and the pellet weight determined. Finally, the samples were dried using a vacuum and room air, to determine the dry weight.
Measurement of diffusing capacity of the lungs for carbon monoxide and nitric oxide, alveolar–capillary membrane conductance, and pulmonary capillary blood volume and cardiac output
Briefly, DLCO and DLNO were assessed using the rebreathe technique where patients were rapidly switched from breathing room air to breath 8–10 breaths at 32 breaths per min from a 5 l anesthesia rebreathe bag filled with 1575 ml of the gas mixture containing 9% helium, 0.3% carbon monoxide (C18O), 40 parts per million nitric oxide (NO), 0.7% acetylene, and 35% O2 as previously described.16–20 The patients were instructed to almost completely empty the bag, and the disappearance of the gases with each breath was sampled using a mass spectrometer (Perkin Elmer, St. Louis, MO, USA) and NO analyzer. The rate of disappearance of acetylene from the exhaled gas mixture during rebreathing was used to assess cardiac output. Since acetylene does not bind to hemoglobin, the rate-limiting step in its disappearance is the flow of a new volume of blood through the lungs. Because all the blood in the pulmonary circulation per min is equal to the volume of blood in the systemic circulation per min, the measure of the disappearance of acetylene provides a measure of cardiac output.19 The rate of disappearance of the gases with each breath can be calculated from the slope of the exponential disappearance for each gas with respect to helium, using custom software.20
The two resistances determining DLCO are the conductance of carbon monoxide (CO) across the alveolar–capillary membrane and plasma barrier, DM, and the erythrocyte that includes rate of CO uptake by the blood and of binding to Hb (ӨCO) and the pulmonary–capillary blood volume (VC). This model of diffusion capacity of the lungs was described by Roughton and Forster:21
| (1) |
In contrast to DLCO, DLNO has been considered a relatively direct measure of membrane conductance (DMNO), as the diffusion resistance of the blood is trivial,22–25 but not infinite, and for our purposes of comparing change in response to a stimulus, it gives reliable results for evaluating changes in DLCO and its components in one maneuver rather than multiple maneuvers at different oxygen tensions.26 Using this assumption, the DMNO value is used to calculate the DM for carbon monoxide (DMCO) by adjusting for differences in diffusion constants based on molecular weight and solubility between the two gases as described previously using an alpha ratio of 2.2.22,27 Pulmonary–capillary blood volume (VC) is then calculated from the DLCO measured by subtracting the resistance to diffusion associated with alveolar–capillary barrier (DMCO) and correcting for differences in the rate of uptake and binding to hemoglobin (Hb; 1/θ) due to differences in Hb concentrations and the alveolar pressure of oxygen, as described previously using the Roughton and Forester 2.5 ӨCO equation.22,27,28
Statistical analysis
The SPSS statistical software package (v.20; SPSS, Inc., Chicago, IL) and GraphPad Prism 7.03 (La Jolla, CA, USA) were used for all statistical analyses. Due to the small sample size and non-normal distribution, for study I data, the Wilcoxon signed-rank test was used to compare within-patient response to the Vibralung with and without sound, and for study II data, the Wilcoxon Mann–Whitney U test was used for comparisons between patients using the Vest versus those using the Vibralung during the first 5 days or the last 5 days. Due to outliers, skewness and non-normal distribution in some of the variables’ summary values are present as median and interquartile range (IQR).
Results
To determine the safety of the Vibralung Acoustical Percussor and its ability to mediate sputum clearance, data were collected over two studies. In study I, the Vibralung was used for a single treatment, either with S or with NS (only PEP) to assess the device’s safety over a 4 h post-treatment follow-up period and to determine the ability of the Vibralung’s utilization of sound waves to improve sputum clearance. In study II, the safety and the ability of the Vibralung to facilitate mucus clearance was compared with traditional Vest in individuals with CF who had been admitted to the hospital for a pulmonary exacerbation. Although the current condition of study II patients had necessitated a hospital admission, only age was significantly lower than that of the study I patients (Table 2).
Table 2.
Study I and II population demographics and baseline pulmonary function.
| Study I | Study II | |||
|---|---|---|---|---|
| n | 10 | 11 | ||
| Sex (male) | 7 (70%) | 5 (45%) | ||
| Age (years) | 30 | (25–34) | 20* | (17–29) |
| Height (cm) | 170 | (159–176) | 163 | (160–168) |
| Weight (kg) | 63 | (54–79) | 59 | (50–65) |
| BMI (kg/m2) | 24 | (19–26) | 22 | (20–26) |
| VO2PEAK (ml/min/kg) | 19 | (10–24) | ||
| VO2PEAK (% predicted) | 59 | (30–61) | ||
| Pulmonary function | ||||
| FVC (l) | 4.1 | (2.8–4.8) | 3.1 | (2.7–3.4) |
| FVC (% predicted) | 89 | (75–98) | 76 | (65–88) |
| FEV1 (l/s) | 2.6 | (1.4–3.3) | 2.1 | (1.6–2.4) |
| FEV1 (% predicted) | 76 | (46–89) | 57 | (47–69) |
| FEV1/FVC (%) | 63 | (54–74) | 64 | (58–74) |
| FEF25–75 (l/s) | 1.3 | (0.5–2.7) | 1.2 | (0.7–1.6) |
| FEF25–75 (% predicted) | 33 | (14–67) | 27 | (18–46) |
Values are median, and the interquartile range is in brackets.
p < 0.05 versus study I Mann–Whitney U test.
BMI, body mass index; FVC, forced vital capacity; FEF25–75, forced expiratory volume at 25–75% of the FVC; FEV1, forced expiratory volume in 1 s of the FVC; VO2PEAK, maximal oxygen consumption.
Study I
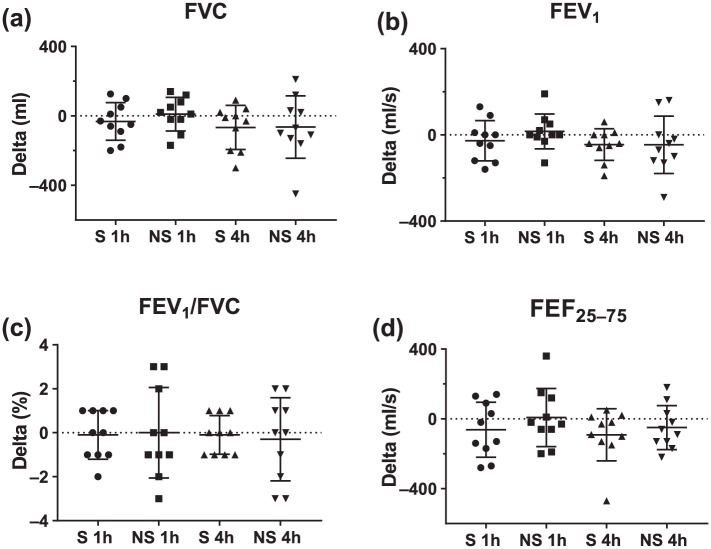
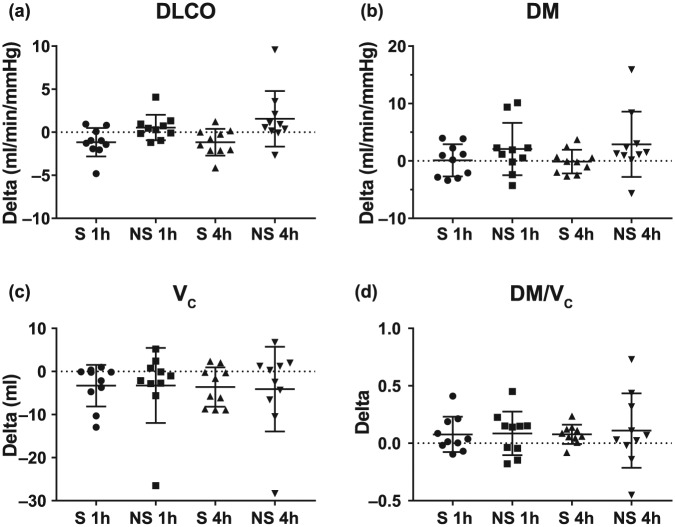
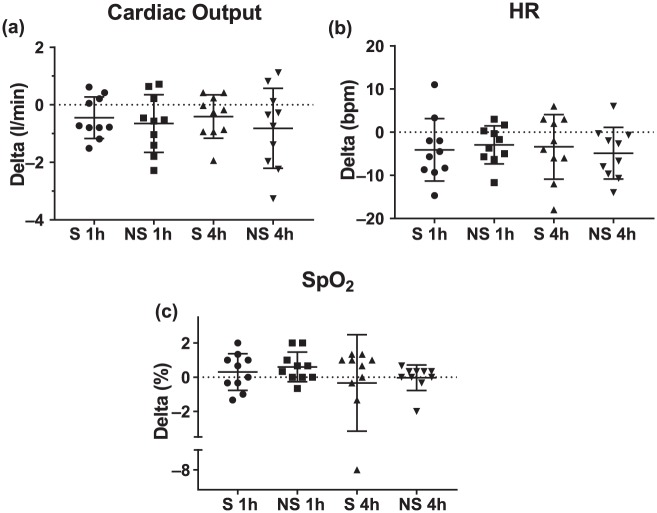
Across the 10 participants in study I, there was no clinically significant decline in any of the pulmonary-function-test-measured indices of safety after treatment with the Vibralung device with both S and NS with the change from baseline provided in the box plots in Figure 2 and average absolute values for the three time points provided in Table 3. There was no real change in DLCO and alveolar–capillary membrane conductance (DM), as well as no change or an improvement in DM/VC, a measure of diffusion at a functional unit (Figure 3 and Table 3). On the NS visit, there was one outlier who demonstrated a large increase in the diffusion parameters. Since a few days after the NS visit, this individual was admitted for an exacerbation; this large increase was likely the result of having no medications the morning of the visit in her more exacerbated state, which reduced her baseline lung diffusion, such that performing ACT to move some of the mucus around was more beneficial for this patient. During both visits where the participants remained seated and relaxed there was no change in cardiac output, but this long period of sedentary activity did result in a reduction in heart rate of less than 5 beats per min on average and a related drop in VC which averaged around 4 ml for both visits (Figure 4(a,b) and Figure 3(c), Table 3). Finally, there was no significant drop in SpO2 noted, except in one patient who desaturated by 8%, but demonstrated no major decline in pulmonary function and diffusion metrics over the post period (Figure 4c, Table 3).
Figure 2.
Change in pulmonary function following sound- or no-sound-Vibralung use.
Absolute change (delta) from baseline to 1 or 4 h post-Vibralung treatment for the sound (S: PEP and sound, 1 h post = circles; 4 h post = triangles) and no sound (NS: PEP only, 1 h post = squares; 4 h post = inverted triangle) visits for basic spirometry metrics. Scatter plots provide the individual data, as well as summarize the median (middle band) and the first and third quartiles (whiskers) across all patients: (a) forced vital capacity (FVC); (b) forced expiratory volume in 1 s of the FVC (FEV1); (c) ratio of FEV1 over FVC (FEV1/FVC); (d) forced expiratory volume at 25–75% of the FVC (FEF25–75).
PEP, positive expiratory pressure.
Table 3.
Phase I safety data.
| Baseline | 1 h post | 4 h post | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Sound | No sound | Sound | No sound | Sound | p value | No sound | ||
| FVC (l) | 4.1 (2.8–4.8) | 4.1 (2.8–4.8) | 4.0 (2.8–4.7) | 4.0 (2.9–4.8) | 4.0 (2.6–4.7) | 0.25 | 4.0 (2.5–4.7) | 0.38 |
| FVC (% pred) | 90 (74–98) | 88 (76–100) | 89 (76–99) | 88 (71–98) | 90 (71–98) | 0.22 | 90 (67–97) | 0.46 |
| FEV1 (l/s) | 2.6 (1.4–3.2) | 2.6 (1.4–3.4) | 2.6 (1.4–3.3) | 2.7 (1.4–3.3) | 2.5 (1.3–3.1) | 0.13 | 2.7 (1.3–3.3) | 0.43 |
| FEV1 (%) | 72 (45–89) | 77 (47–91) | 68 (47–89) | 77 (47–94) | 69 (43–89) | 0.11 | 74 (40–92) | 0.40 |
| FEV1/FVC (%) | 61 (53–73) | 64 (55–75) | 60 (54–74) | 64 (56–75) | 61 (53–73) | 0.71 | 64 (56–75) | 0.59 |
| FEF25–75 (l/s) | 1.1 (0.5–2.6) | 1.4 (0.6–2.7) | 1.1 (0.5–2.7) | 1.5 (0.5–2.6) | 1.1 (0.5–2.5) | 0.053 | 1.4 (0.5–2.7) | 0.26 |
| FEF25–75 (%) | 30 (13–65) | 36 (15–69) | 28 (15–66) | 38 (14–65) | 27 (15–61) | 0.09 | 36 (13–67) | 0.48 |
| SpO2 (%) | 97 (96–98) | 98 (97–98) | 97 (97–98) | 98 (97–99) | 98 (96–99) | 0.41 | 98 (97–98) | 0.59 |
| Cardiac output (l/min) | 4.1 (2.6–5.6) | 3.9 (2.7–5.4) | 3.7 (2.5–4.8) | 3.6 (2.9–4.2) | 3.6 (2.5–4.7) | 0.19 | 3.4 (2.8–3.8) | 0.13 |
| HR (bpm) | 89 (83–104) | 95 (81–115) | 90 (79–96) | 89 (77–117) | 88 (78–99) | 0.24 | 89 (79–113) | 0.02 |
| Stroke volume (ml) | 43 (29–59) | 38 (28–66) | 36 (31–54) | 36 (27–52) | 39 (28–58) | 0.38 | 32 (31–45) | 0.16 |
| DLCO (l/min/mmHg) | 15.8 (12.4–18.3) | 14.9 (12.1–16.9) | 14.1 (11.8–16.6) | 15.0 (12.2–19.7) | 14.7 (11.8–16.3) | 0.06 | 16.4 (12.7–19.7) | 0.047 |
| DM (l/min/mmHg) | 25.5 (17.9–28.4) | 24.1 (18.2–28.2) | 23.2 (18.1–26.4) | 26.0 (20–30) | 23.9 (18.4–26.5) | 0.19 | 25.9 (19.1–36.2) | 0.047 |
| VC (ml) | 32.9 (27.9–42.5) | 30.3 (24.6–42.6) | 33.6 (25.4–34.1) | 27.7 (23.9–41.7) | 32.3 (26.6–35.7) | 0.07 | 28.2 (24.4–33.5) | 0.38 |
| DM/VC | 0.72 (0.57–0.76) | 0.68 (0.54–0.88) | 0.75 (0.67–0.82) | 0.82 (0.63–0.92) | 0.76 (0.68–0.82) | 0.04 | 0.72 (0.68–1.05) | 0.28 |
Values are median (interquartile range). p values represent Wilcoxon signed ranks baseline versus 4 h post for each treatment.
SpO2, peripheral oxygen saturation; DLCO, diffusion capacity of the lungs for carbon monoxide; DM, alveolar–capillary membrane conductance; VC, pulmonary–capillary blood volume; DM/VC, functional unit of diffusion; FVC, forced vital capacity; FEV1, forced expiratory volume at 1 s of the FVC; FEF25–75, forced expiratory volume at 25–75% of the FVC; HR, heart rate; bpm, beats per min.
Figure 3.
Change in diffusion capacity of the lung for carbon monoxide and its components following sound or no sound Vibralung use.
Absolute change (delta) from baseline to 1 h or 4 h post-Vibralung treatment for the sound (S: PEP and sound, 1 h post = circles; 4 h post = triangles) and no sound (NS: PEP only, 1 h post = squares; 4 h post = inverted triangle) visits for lung diffusion capacity for carbon monoxide and its components metrics. Scatter plots provide the individual data as well as summarize the median (middle band) and the first and third quartiles (whiskers) across all patients: (a) diffusion capacity of the lung for carbon monoxide (DLCO); (b) alveolar–capillary membrane conductance (DM); (c) pulmonary–capillary membrane conductance (VC); (d) ratio of DM/VC, measure of a function unit of diffusion.
PEP, positive expiratory pressure.
Figure 4.
Change in cardiac function following sound or no sound Vibralung use.
Absolute change (delta) from baseline to 1 or 4 h post Vibralung treatment for the sound (S: PEP and sound, 1 h post = circles; 4 h post = triangles) and no sound (NS: PEP only, 1 h post = squares and 4 h post = inverted triangle) visits for basic cardiac function metrics. Scatter plots provide the individual data as well as summarize the median (middle band) and the first and third quartiles (whiskers) across all patients: (a) cardiac output; (b) heart rate (HR); (c) peripheral oxygen saturation (SpO2).
PEP, positive expiratory pressure; bpm, beats per min.
From the user feedback gathered through a questionnaire, the most common comments were that: (a) the mouthpiece was uncomfortable, tiring and made lips sore; and (b) having to wear the nose clip during the treatment caused some mild soreness. Overall, the Vibralung was found to be easy to use. Patients appreciated its compactness, and 50% of patients stated that using the Vibralung would help improve their compliance. From a Likert 5-point scaling system (1–5), patients rated on average no pain (1), but a slight discomfort (2.1), found the device easy to use (1) and did feel there was a noticeable change in their breathing (2.3) or in their ease of clearing mucus (2.6).
The Vibralung with addition of sound did not appear to further enhance sputum clearance in addition to PEP alone within the 4 h time period as mean sputum weights were not different between the two treatments overall (Table 4). However, reviewing the weights of the specific fractions within each individual participant showed that the use of sound did tend to facilitate slightly larger pellet and dry weights for some patients, and these weights are more representative of the viscous mucus that airway clearance therapy is aiming to mobilize (Supplement Figure 6).
Table 4.
Sputum weights with sound and no sound.
| Sound | No sound | p value | |
|---|---|---|---|
| Wet weight (g) | 10.5 (3.9–14.4) | 10.0 (1.6–13.0) | 0.25 |
| Pellet weight (g) | 5.9 (3.4–6.9) | 4.4 (1.3–7.8) | 0.25 |
| Dry weight (g) | 0.58 (0.05–1.02) | 0.67 (0.42–1.03) | 0.57 |
Weights: wet = total sample: sputum + saliva; pellet = wet weight with saliva removed; dry = after samples dried with vacuum and room air. Values are median (interquartile range). p values represent no sound versus sound.
Study II
Out of the 11 patients that were recruited, 2 completed condition A, but only 1 completed the crossover as the other two were discharged after 5 days; 5 patients were in condition B and 4 patients completed condition C. As such, for days 1–5, the Vest group included patients from both conditions B and C who were using the Vest during this 5-day period and for days 7–11 the Vest group included patients from conditions A (had used the Vibralung days 1–5) and C (had used the Vest for days 1–5) (Table 1 for condition reference).
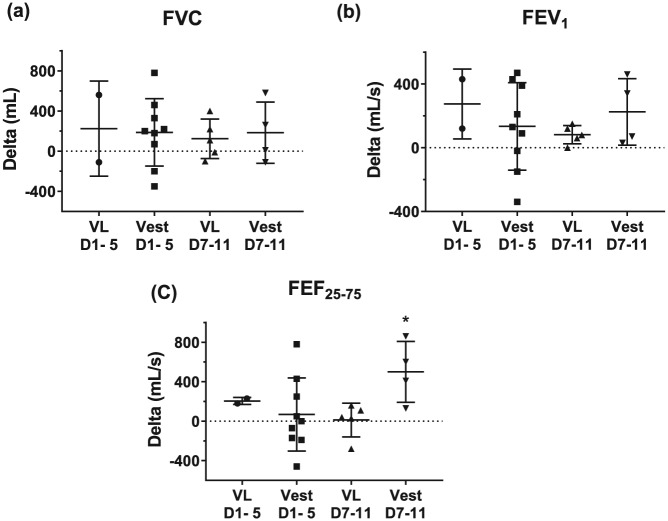
When using the Vibralung or the Vest, all but two patients demonstrated improvements in their pulmonary function over the 12 days of the study (Supplement Figure 7); the only difference between treatments was seen over days 7–11 where patients using the Vest had a greater increase in FEF25–75 compared with those patients using the Vibralung (Figure 5).
Figure 5.
Change in pulmonary function over 5-day period with Vibralung or Vest use.
Absolute change (delta) from day 1 to 5 or day 7 to 11 (days 1–5 or days 7–11) when using Vibralung (Vibralung: days 1–5 = circles; days 7–11 = triangles) or Vest (days 1–5 = squares; days 7–12 = inverted triangles) as primary ACT treatment for basic spirometry metrics. Scatter plots provide the individual data as well as summarize the median (middle band) and the first and third quartiles (whiskers) across all patients: (a) forced vital capacity (FVC); (b) forced expiratory volume in 1 s of the FVC (FEV1); (c) forced expiratory volume at 25–75% of the FVC (FEF25–75). *p < 0.05 versus Vibralung days 7–12.
ACT, airway clearance therapy; VL, Vibralung; Vest, vibratory vest therapy.
As expected, patients produced more sputum (mean sputum over a 5-day period using the Vest or Vibralung), across all fractions, over the first 5 days of their admission compared with the final 5 days of the study, but there was no difference in sputum fraction weights between Vibralung and Vest usage over either 5-day period (Table 5). The ease of sputum expectoration was variable amongst patients, with some unable to clear much of anything, while others were able to clear a considerable amount of sputum and the Vibralung appeared to be as effective as the Vest for some patients (Supplement Figure 8).
Table 5.
Sputum weights with Vest and Vibralung days 1–5 and days 7–11.
| All | Vest | Vibralung | p value | |
|---|---|---|---|---|
| Wet weight (g) | ||||
| Days 1–5 | 3.6 (1.8–6.1) | 3.6 (1.7–5.2) | 6.0 (1.77,10.29) | 0.73 |
| Days 7–11 | 2.4 (1.3–3.1)* | 2.7 (1.6–4.2) | 1.5 (0.8–2.7) | 0.3 |
| Pellet weight (g) | ||||
| Days 1–5 | 2.1 (0.9–3.8) | 2.1 (1.3–3.6) | 4.1 (0.3–8.0) | 0.91 |
| Days 7–11 | 1.1 (0.4–1.6)* | 1.0 (0.3–2.5) | 1.2 (0.6–1.7) | 0.84 |
| Dry weight (g) | ||||
| Days 1–5 | 0.37 (0.30–0.68) | 0.37 (0.32–0.61) | 0.77 (0.04–1.5) | 0.91 |
| Days 7–11 | 0.36 (0.15–0.55) | 0.36 (0.26–0.52) | 0.23 (0.07–0.57) | 0.69 |
Days 1–5: Vest: n = 9 and Vibralung: n = 2; days 7–11: n = 5 for both Vest and Vibralung. Weights: wet = total sample: sputum + saliva; pellet = wet weight with saliva removed; dry = after samples dried with vacuum and room air. Values are median (IQR). *Days 1–5 versus days 7–11, Wilcoxon signed ranks. p values represent Vest versus Vibralung, Mann–Whitney U.
Vest, vibratory vest therapy.
Discussion
This study demonstrates that the use of the Vibralung device was well tolerated in individuals with CF. The application of sound directly to the tracheobronchial tract did not result in dramatic decrements in lung function nor demonstrable damage to the alveolar–capillary membrane in individuals with CF. There were small decreases in expiratory flowrates throughout the 4-h study period, but this is likely due to: (a) within-day variability with repeated pulmonary function testing; and (b) the time that had elapsed since last bronchodilator use. Evaluation of the repeatability of FEV1 measurements in CF adults demonstrated that the within-day variability between repeated measurements when there was no true change in clinical status was 0.106 l or 3.3% of predicted,29 and any changes we measured from baseline to 1 h or 4 h post treatment were within this variability. Also Horvick and colleagues have previously demonstrated that both short- and long-acting beta-agonist daily use significantly increases pulmonary function across the day, but with albuterol, there is a fall back to baseline overnight, which can be minimized with use of a longer-acting beta-agonist.30,31 As such, we feel it is safe to conclude the Vibralung does not induce any detrimental changes in pulmonary function metrics that would equate to decline in clinical status. Since there was no difference in sputum production between S and NS PEP only, the added benefit of sound provided by the Vibralung was not demonstrated with this study.
In phase II of the study, we observed that the CF patients using the Vibralung for ACT during a hospital admission did not demonstrate any hindrance to improvement in pulmonary function. The observed variability in individual sputum weights between Vest and Vibralung between and within individuals reaffirms that not all therapies are equally effective for all patients.
For the majority of individuals with CF in the USA, the Vest is the primary ACT device used, but it is not ideal. For those who have never worn a vest, the feeling of being shaken is not always tolerable, especially when the individual has gastrointestinal issues or a feeding or access port, as in these settings, it can be uncomfortable to the point that treatment is skipped all together. With vest treatments done three to four times daily, the large and bulky nature of the Vest devices make them difficult to travel with. Finally, although more convenient in the sense that it allows independence in performing one’s ACT, Vest devices can be costly, where at one time Vests were retailing at $15,000–20,000,32 and now can be found new or used between $4000–8000. As such, assistance navigating the health insurance system to find coverage is needed or else the Vest is not economically viable for all patients. This high cost is why PEP breathing and oscillatory positive expiratory pressure (OPEP) devices are the primary ACT used by individuals with CF in Canada.11 The Vibralung is more expensive than the PEP or OPEP devices, but at a lower retail cost of $3000, than most Vest options. Additionally, the Vibralung can also allow for simultaneous delivery of aerosol medications, a common technique used by individuals with CF while performing a vest treatment to save time. As such, the Vibralung, now commercially available, is another ACT method available for individuals with CF and one that can be considered by patients and their care teams as an ACT option.
Limitations
The sample size used in this study was relatively small for both phases of study. Further, in study I, the patients were relatively healthy, suggesting that there may be more beneficial effects of the Vibralung in the short term that were not observed in the present study, due to the relatively healthy lungs of these patients. Because this is the first time the Vibralung has been studied in humans, and with concern for the care of the patients, the second study was designed such that certain Vest treatments were substituted with Vibralung treatments. Now that the Vibralung is FDA approved, future studies should compare solely Vibralung treatments with solely Vest (or other ACT device) treatments. Additionally, we chose to compare the Vibralung to the Vest because that was the most frequently used ACT by individuals with CF in our center when they are at home and an alternative oscillating wave technology, but comparison of the efficacy of the Vibralung to other PEP-only devices would also be important.
Conclusion
This study demonstrates that the Vibralung, an acoustic ACT device that couples sound waves directly to the tracheobronchial airways for the purpose of loosening and mobilizing mucus, is well tolerated in individuals with CF with or without a pulmonary exacerbation and does not induce lung damage that could be detected by conventional pulmonary function tests. In two small studies, we observed: (a) no enhancement of sputum production between S- and NS-PEP only after a single use of the Vibralung; and (b) similar sputum expectoration levels with Vibralung therapy when compared with Vest treatment in individuals with CF who were hospitalized with a pulmonary exacerbation. The Vibralung appears to be a safe treatment in individuals with CF. Additional long-term clinical studies, in both hospitalized and homecare individuals with CF using the current commercially available and FDA-approved Vibralung should be undertaken to fully understand the potential benefits and efficacy of acoustical ACT in comparison with other available modalities.
Supplemental Material
Supplemental material, Suplemental_Figures for Influence of the Vibralung Acoustical Percussor on pulmonary function and sputum expectoration in individuals with cystic fibrosis by Courtney M. Wheatley, Sarah E. Baker, Cori M. Daines, Hanna Phan, Marina G. Martinez, Wayne J. Morgan and Eric M. Snyder in Therapeutic Advances in Respiratory Disease
Acknowledgments
We are sincerely grateful to the CF patients who willingly donated their time and effort to be involved in this study. We would also like to thank Mary Morgan who helped facilitate patient recruitment. Mary Morgan, Eric Wong and Steven Karpen also assisted with data collection for this study.
The authors contributed to this research by way of the following: CW: performed literature search, helped design the study, performed data collection, analysis and interpretation, manuscript preparation and revised the manuscript. SB: performed data collection and revised the manuscript. CD: helped design the study, recruit participants and revised the manuscript. HP: helped recruit participants and revised the manuscript. MM: performed data collection and revised the manuscript. WM: helped design the study, recruit participants and revised the manuscript. ES: designed the study designed the study, analyzed and interpreted the data, and revised the manuscript. All authors read and approved the final manuscript.
These data were presented by Dr Wheatley at the North American Cystic Fibrosis Foundation Annual Meeting 2013, at the PT and RT workshop: updates on airway clearance and inhalation therapies: ‘Influence of the Vibralung device on pulmonary function and sputum expectoration in patients with cystic fibrosis’, Salt Lake City, UT, 17 October 2013.
Footnotes
Authors’ Note: Courtney M. Wheatley is now affiliated to Mayo Clinic AZ and Mayo Clinic Rochester, Sarah E. Baker to Mayo Rochester and Eric M. Snyder affiliated to UMN.
Funding: This study was performed at the University of Arizona, College of Pharmacy in Tucson, AZ.
The study was funded by WestMed Inc., Tucson, AZ, USA.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental Material: Supplementary material for this article is available online.
ORCID iD: CM Wheatley  https://orcid.org/0000-0002-2854-462X
https://orcid.org/0000-0002-2854-462X
Contributor Information
Courtney M. Wheatley, Department of Cardiovascular Diseases, Mayo Clinic, 13400 East Shea Boulevard, Scottsdale, AZ 85259, USA Department of Pharmacy Practice and Science, University of Arizona, Tucson, AZ, USA.
Sarah E. Baker, Department of Anesthesiology, Mayo Clinic, Rochester, MN, USA Department of Pharmacy Practice and Science, University of Arizona, Tucson, AZ, USA
Cori M. Daines, Department of Pediatrics—Pediatric Pulmonology and Sleep, University of Arizona, Tucson, AZ, USA Banner—University Medical Center, Tucson, AZ, USA
Hanna Phan, Department of Pharmacy Practice and Science, University of Arizona, Tucson, AZ, USA Department of Pediatrics—Pediatric Pulmonology and Sleep, University of Arizona, Tucson, AZ, USA.
Marina G. Martinez, Arizona Clinical Research Center, University of Arizona, Tucson, AZ, USA
Wayne J. Morgan, Department of Pediatrics—Pediatric Pulmonology and Sleep, University of Arizona, Tucson, AZ, USA Banner—University Medical Center, Tucson, AZ, USA
Eric M. Snyder, Department of Kinesiology, University of Minnesota, Minneapolis, MN, USA; and Department of Pharmacy Practice and Science, University of Arizona, Tucson, AZ, USA
References
- 1. Quinton PM. Cystic fibrosis: a disease in electrolyte transport. FASEB J 1990; 4: 2709–2717. [DOI] [PubMed] [Google Scholar]
- 2. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005; 352: 1992–2001. [DOI] [PubMed] [Google Scholar]
- 3. Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 2007; 58: 157–170. [DOI] [PubMed] [Google Scholar]
- 4. Flume PA, Robinson KA, O’Sullivan BP, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care 2009; 54: 522–537. [PubMed] [Google Scholar]
- 5. Shepherd SL, Hovell MF, Harwood IR, et al. A comparative study of the psychosocial assets of adults with cystic fibrosis and their healthy peers. Chest 1990; 97: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 6. Abbott J, Dodd M, Bilton D, et al. Treatment compliance in adults with cystic fibrosis. Thorax 1994; 49: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conway SP, Pond MN, Hamnett T, et al. Compliance with treatment in adult patients with cystic fibrosis. Thorax 1996; 51: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konstan MW, Butler SM, Schidlow DV, et al. Patterns of medical practice in cystic fibrosis: part II. Use of therapies. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Pediatr Pulmonol 1999; 28: 248–254. [DOI] [PubMed] [Google Scholar]
- 9. White D, Stiller K, Haensel N. Adherence of adult cystic fibrosis patients with airway clearance and exercise regimens. J Cyst Fibros 2007; 6: 163–170. [DOI] [PubMed] [Google Scholar]
- 10. Flores JS, Teixeira FA, Rovedder PM, et al. Adherence to airway clearance therapies by adult cystic fibrosis patients. Respir Care 2013; 58: 279–285. [DOI] [PubMed] [Google Scholar]
- 11. Health Quality Ontario. Airway clearance devices for cystic fibrosis: an evidence-based analysis. Ont Health Technol Assess Ser 2009; 9: 1–50. [PMC free article] [PubMed] [Google Scholar]
- 12. Langenderfer B. Alternatives to percussion and postural drainage. A review of mucus clearance therapies: percussion and postural drainage, autogenic drainage, positive expiratory pressure, flutter valve, intrapulmonary percussive ventilation, and high-frequency chest compression with the ThAIRapy Vest. J Cardiopulm Rehabil 1998; 18: 283–289. [DOI] [PubMed] [Google Scholar]
- 13. Lester MK, Flume PA. Airway-clearance therapy guidelines and implementation. Respiratory care 2009; 54: 733–750; discussion 751–733. [DOI] [PubMed] [Google Scholar]
- 14. McPeck M. Vibralung acoustical percussor: a new paradigm in airway clearance therapy. Respir Ther 2014; 9: 45–47. [Google Scholar]
- 15. Konstan MW, Stern RC, Doershuk CF. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. J Pediatr 1994; 124: 689–693. [DOI] [PubMed] [Google Scholar]
- 16. Homnick DN, Anderson K, Marks JH. Comparison of the flutter device to standard chest physiotherapy in hospitalized patients with cystic fibrosis: a pilot study. Chest 1998; 114: 993–997. [DOI] [PubMed] [Google Scholar]
- 17. Mortensen J, Falk M, Groth S, et al. The effects of postural drainage and positive expiratory pressure physiotherapy on tracheobronchial clearance in cystic fibrosis. Chest 1991; 100: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 18. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 19. Hsia CC, Herazo LF, Ramanathan M, et al. Cardiac output during exercise measured by acetylene rebreathing, thermodilution, and Fick techniques. J Appl Physiol 1995; 78: 1612–1616. [DOI] [PubMed] [Google Scholar]
- 20. Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Physiol 2005; 99: 1985–1991. [DOI] [PubMed] [Google Scholar]
- 21. Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 1957; 11: 290–302. [DOI] [PubMed] [Google Scholar]
- 22. Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest 2001; 120: 1850–1856. [DOI] [PubMed] [Google Scholar]
- 23. Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest 2002; 122: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 24. Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 1957; 11: 290–302. [DOI] [PubMed] [Google Scholar]
- 25. Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med 2005; 118: 205–211. [DOI] [PubMed] [Google Scholar]
- 26. Coffman KE, Chase SC, Taylor BJ, et al. The blood transfer conductance for nitric oxide: infinite vs. finite thetaNO. Respir Physiol Neurobiol 2017; 241: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wheatley CM, Foxx-Lupo WT, Cassuto NA, et al. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. J Cyst Fibros 2011; 10: 45–53. [DOI] [PubMed] [Google Scholar]
- 28. Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 1957; 11: 290–302. [DOI] [PubMed] [Google Scholar]
- 29. Stanbrook MB, Corey M, Tullis DE. The repeatability of forced expiratory volume measurements in adults with cystic fibrosis. Chest 2004; 125: 150–155. [DOI] [PubMed] [Google Scholar]
- 30. Hordvik NL, Sammut PH, Judy CG, et al. The effects of albuterol on the lung function of hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med 1996; 154: 156–160. [DOI] [PubMed] [Google Scholar]
- 31. Hordvik NL, Sammut PH, Judy CG, et al. Effects of standard and high doses of salmeterol on lung function of hospitalized patients with cystic fibrosis. Pediatr Pulmonol 1999; 27: 43–53. [DOI] [PubMed] [Google Scholar]
- 32. Marks JH. Airway clearance devices in cystic fibrosis. Paediatr Respir Rev 2007; 8: 17–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Suplemental_Figures for Influence of the Vibralung Acoustical Percussor on pulmonary function and sputum expectoration in individuals with cystic fibrosis by Courtney M. Wheatley, Sarah E. Baker, Cori M. Daines, Hanna Phan, Marina G. Martinez, Wayne J. Morgan and Eric M. Snyder in Therapeutic Advances in Respiratory Disease