Abstract
Background
Three-dimensional printing (3DP) has become popular for development of anatomic models, preoperative planning, and production of tailored implants. A novel laparoscopic, transgastric procedure for distal esophageal mucosectomy was developed. During this procedure a space holder had to be introduced into the distal esophagus for exposure during suturing. The production process and evaluation of a 3DP space holder are described herein.
Material and methods
Computer-aided design software was used to develop models printed from polylactic acid. The prototype was adapted after testing in a cadaveric model. Subsequently the device was evaluated in a non-survival porcine model. A mucosal purse-string suture was placed as orally as possible in the esophagus, in the intervention group with and in the control group without use of the tool (n=8 each). The distance of the stitches from the Z-line was measured. The variability of stitches indicated the suture quality.
Results
The median maximum distance from Z-line to purse-string suture was larger in the intervention group (5.0 [3.3-6.4] versus 2.4 [2.0-4.1] cm;P=0.013). The time taken to place the sutures was shorter in the control group (P<0.001). Stitch variance tended to be greater in the intervention group (2.3 [0.9-2.5] versus 0.7 [0.2-0.4] cm;P=0.051). The time required for design and production of a tailored tool was below 24 h.
Conclusions
3DP in experimental surgery enables rapid production, permits repeated adaptation until a tailored tool is obtained, and ensures independence from industrial partners. With the aid of the space holder more orally located esophageal lesions came within reach.
Keywords: 3D printing, device development, surgical instruments, experimental surgery, esophageal surgery
Introduction
There is rising interest in the use of three-dimensional printing (3DP) in medicine and particularly in surgical disciplines.1–4 When 3DP was introduced more than 30 years ago, the technique was expensive and exclusive. Nowadays, however, 3DP has become affordable and printers are commercially available. It has become possible to produce an individual product in a short amount of time. As a consequence, 3DP has gained importance in different fields of surgery for preoperative planning and better intraoperative orientation.5–8 The 3D models are based on high-quality radiological images of complex anatomical structures. Furthermore, 3DP is used to create prototypes of individual implants. In preclinical experiments, surgical instruments such as trocars for laparoscopy, retractors, and implants such as ureteral stents have been printed and tested in porcine models and humans.9,10
However, there is scant literature on the production of prototypes for experimental surgery using 3DP. A novel laparoscopic surgical procedure aimed at resecting the distal esophageal mucosa with the aid of a circular surgical stapler. The procedure, intended for use in patients with dysplastic Barrett’s esophagus,11 involved placement of two submucosal purse-string sutures in the distal esophagus. The instruments and the stapler were introduced transabdominally and transgastrically. As the distal esophagus is narrow and the exposure of the mucosal esophageal aspect critical, there was a need for a customized space holder to be inserted in order to expose the esophageal mucosa. The goal was to ensure sufficient exposure of the esophageal mucosa and achieve an adequate height to reach Barrett mucosa extensions. We describe herein the production process of a 3DP tool and the evaluation of the printed space holder in a porcine model.
Material and methods
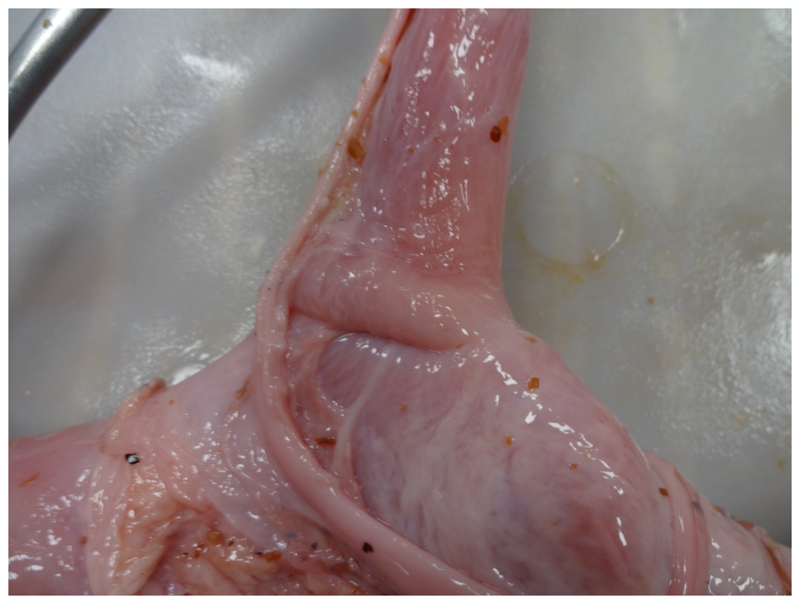
The idea of the esophageal space holder derived from existing anal retractors that expose the anal canal and enable the surgeon to place a purse-string suture in the mucosa of the anal canal. Such tools are used for stapled hemorrhoidopexy.12 The prototype of the esophageal space holder was developed and evaluated in a multi-step process. Instrument requirements were determined by anatomical measurements in an ex vivo porcine esophageal specimen (Fig. 1) and the size of laparoscopic suturing instruments. The space holder needed to advance along the distal esophagus without tension and ensure good exposure of the mucosa. The aperture of the space holder had to be large enough to permit placement of sutures with laparoscopic instruments. This includes a surgical needle holder and the needle of a polypropylene monofilament suture (Surgipro II, Covidien™, Dublin, Ireland) with 19 mm diameter. After 3DP (Fig. 2), prototypes (Fig. 3) were tested in ex vivo porcine esophageal specimens in a box trainer and adjustments were made according to the need of the surgeon (Table 1).
Fig. 1.
Gastroesophageal junction in porcine cadaver model used for definition of the dimensions of the space holder.
Fig. 2.
3D printing of the designed retractor using Ultimaker 2.
Fig. 3.
Different prototypes of the retractor (A-C) and the final version (D).
Table 1.
Dimensions of the first and final versions of the esophageal space holder
| Characteristic | First space holder | Final space holder |
|---|---|---|
| Length (mm) | 50 | 50 |
| Apical diameter (mm) | 10 | 17 |
| Basal diameter (mm) | 26 | 19 |
| Wall thickness (mm) | Not defined | 6 mm |
| Aperture diameter | One third of circumference | One third of circumference |
| Aperture length (mm) | 35 | 45 |
The final space holder was evaluated in whole-body freshly euthanized German Landrace pigs that had previously been used for experimental pancreatic resection. Ethical approval was obtained from the local animal care committee in Karlsruhe, Germany (reference number: 35-9185.81/G-63/15). The animals had been sacrificed by an intravenous injection of 30 mmol KCl under general anesthesia before testing the space holder. A single surgeon (DCS) performed all procedures in this experiment. He has extensive experience in laparoscopic surgery and holds a certificate in advanced laparoscopic surgery from the Swiss Association for Laparoscopic and Thoracoscopic Surgery (SALTS).
Development of the prototype
The prototype was created using the computer-aided design (CAD) software Autodesk® Inventor (Version 2016, Autodesk, San Rafael, CA, USA) and Autodesk® Meshmixer (Version 10.10.170, Autodesk). The 3D model was printed with the Ultimaker 2 (Ultimaker B.V., Geldermalsen, Netherlands) using polylactic acid (PLA) (Ultimaker). The 3D printer works by fused filament fabrication, distributing and adding melted PLA two-dimensionally to construct the model layer by layer (Fig. 2). Time needed for development of the space holder and production costs were evaluated. The prototypes were tested in a cadaveric stomach model (purchased from a butcher) in a box trainer until the final model was dimensioned.
Evaluation in the animal model
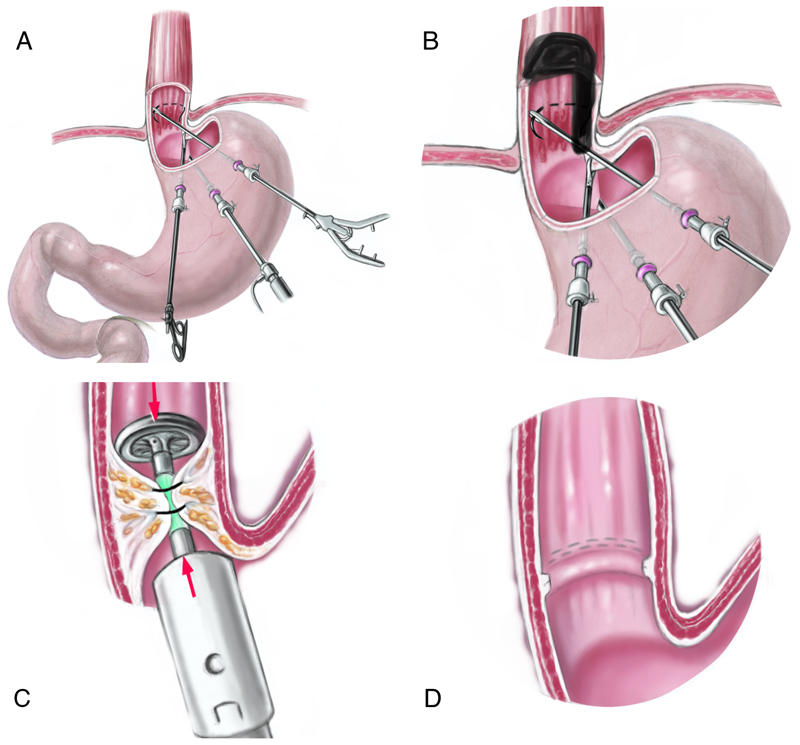
The final version of the prototype esophageal space holder was evaluated in freshly sacrificed German Landrace pigs. The full technique of laparoscopic transgastric stapler-assisted mucosectomy (SAM) is depicted in Figure 4 and has been reported in detail elsewhere.11 In brief, after laparoscopic placement of three transabdominal intragastric trocars and establishment of 12-mmHg pneumogastrium, two submucosal purse-string sutures using monofilament suture material are placed in the distal esophagus. A circular stapler is then introduced via a gastrostomy to perform a circular mucosectomy. In the current experiment, the procedure was performed up to the placement of the more proximal of the two purse-string sutures in the esophagus. The surgeon placed the suture as far proximal from the Z-line as possible. In the control group (n=8) the suture was placed without the esophageal retractor (Fig. 5a). In the intervention group (n=8) the suture was placed with the aid of the printed space holder (Fig. 5b). After insertion of the space holder into the stomach it was gently positioned in the distal esophagus by means of laparoscopic graspers (Video 1). After placement of the purse-string suture, the ends of the suture were secured with clips. A median laparotomy was performed and the esophagogastric junction was resected. In the control group a purse-string suture was placed as far orally as possible in the distal esophagus without the aid of a space holder (Video 2).
Fig. 4.
Stapler-assisted mucosectomy: With the aid of three transabdominal-transgastric trocars, a purse-string suture is placed in the distal esophagus without (A) or with the retractor (B). The anvil of a circular stapler is introduced into the distal esophagus and the purse-string suture is knotted (C). After firing of the stapler a circular mucosectomy specimen is obtained while staples approximate the mucosa borders (D).
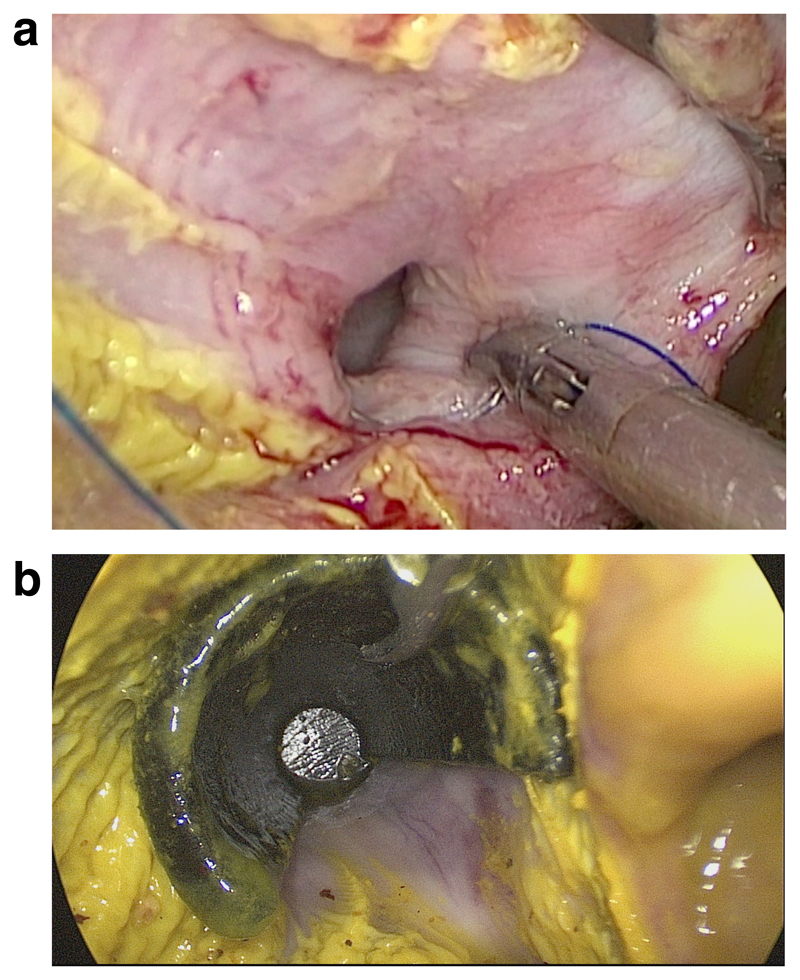
Fig. 5.
Exposure of the esophageal epithelium from an intragastric perspective: (a) without and (b) with 3D-printed space holder.
Outcome measures
The outcome was evaluated in the macroscopic surgical specimen of the esophagogastric junction. The primary outcome measure was the distance of the sutures above the Z-line, measured at 6 and 12 o’clock in lithotomy position (Fig. 6). The secondary endpoints were intraoperative complications (injury to the esophagus, esophageal perforation, transmural stitches), the time needed to place the suture, including insertion of the retractor in the intervention group, and suture quality. The difference in length between the longest and shortest stitch was measured as surrogate marker for suture regularity, and the height difference between the 6 o’clock and the 12 o’clock stitch as surrogate marker for suture skewness.
Fig. 6.
Assessment of the quality of the purse-string suture, including stitch variability in tissue explant. The z-line is marked in red.
Statistics
For this pilot study on a novel surgical tool there are no literature data on which a sample size calculation might be based. The reproducibility of results in a pilot study is, however, usually determined after the procedure has been carried out in 6-8 animals.13,14
Statistical analysis was performed using SPSS® Statistics Version 22 (IBM, Armonk, New York). Given the low sample size, non-parametric data distribution was assumed. Continuous data were expressed as median and interquartile range. Proportions between groups were compared using a Mann–Whitney test, while categorical variables were compared using a two-sided Fisher’s exact test. A two-sided p-value ≤ 0.05 was considered to show a statistically significant difference.
Results
Development of the prototype
The initial model design process took 4 h for a resident who was familiar with the CAD software. The time needed to print each prototype was 5 (4.3 – 5.9) hours. Four prototypes were printed, with various adjustments of the dimensions (Table 1). In the first prototype the chosen material density was too low. Therefore the space holder was too brittle and fragile and could not be pushed into the esophagus (Fig. 3a). The apical inner diameter of the second prototype of the space holder was too small and thus it was impossible to maneuver the needle of the purse-string suture, which had a diameter of 19 mm. Furthermore, the outer diameter of the lower end of the space holder was too large for it to be advanced into the esophagus (Fig. 3b). Therefore, prototypes with a thinner wall and a smaller cap were produced. The third prototype had an excessively large lateral aperture, impairing good exposure of the esophageal mucosa (Fig. 3c). The final prototype had a larger apical inner diameter and a smaller distal outer diameter. The needle could comfortably be maneuvered within the whole aperture of the space holder and the tool could be advanced as far as necessary into the esophagus (Fig. 3d). The purchase price of the printer used was € 2,500 and the annual licensing fee for the software was € 2,000. The production costs were € 5 per disposable tool.
Evaluation in the animal model
The weight and sex of the animals in the two groups did not differ significantly (Table 2). Insertion of the retractor into the esophagus was feasible in all eight pigs. The retractor kept the esophagus open, and while turning the tool with the open third of the circumference, stiches could be placed as intended (Fig. 5). The median distance from Z-line to purse-string suture, measured at 6 and 12 o’clock in lithotomy position in each animal, was greater in the intervention group (P=0.013 and P=0.017). In neither group were any of the expected complications (injury to the esophagus, esophageal perforation, transmural stitches) observed (P=1.0). The time taken up by suture placement was shorter in the control group (P<0.001). In the intervention group, the overall procedure time included the time needed to introduce the space holder into the esophagus. Variability of stitch height in relation to the Z-line tended to be greater in the intervention group (P=0.051), but there was no difference in suture quality in terms of variance in stitch length (Table 2).
Table 2.
Evaluated parameters of the esophageal space holder
| Objectives | With retractor (n=8) | Control (n=8) | p |
|---|---|---|---|
| Weight of animal (kg) | 42.3 (40.2 – 44.9) | 39.5 (36.2 – 42.9) | 0.122 |
| Sex of animals (m:f) | 5 : 3 | 4 : 4 | 1.0 |
| Height above Z-line at 6 o’clock° (cm) | 5.0 (3.3 – 6.4) | 2.4 (2.0 – 4.1) | 0.013 |
| Height above Z-line at 12 o’clock° (cm) | 3.8 (2.1 – 5.3) | 2.3 (1.3 – 3.8) | 0.017 |
| Time needed for procedure (min) | 20.2 (12.8 – 23.3) | 5.9 (4.3 – 6.9) | <0.001 |
| Variability of stitch height* (cm) | 2.3 (0.9 – 2.5) | 0.7 (0.2 – 1.4) | 0.051 |
| Stitch distance$ (cm) | 0.4 (0.3 – 1.2) | 0.4 (0.3 – 1.2) | 1.00 |
in lithotomy position
Difference between stitch at 3 o’clock and at 9 o’clock
Difference between longest and shortest luminal stitch
Discussion
The need for a customized surgical tool arose during the development of SAM as a novel surgical technique for resection of Barrett’s epithelium (BE).11 In SAM a purse-string suture marks the proximal resection border and must be placed orally to the BE. The space holder developed in this experiment brought a significantly higher proportion of the distal esophagus within reach compared with intraesophageal sutures without the aid of the space holder. The ability to reach more orally sited lesions is of crucial importance, enabling the use of SAM in long-segment BE.
The process from initial idea to 3D-printed esophageal space holder required less than 24 h of working time. After initial assessment in an organ model the tailored tool could be repeatedly adapted in a multi-step process until it fulfilled the purpose. Since the printing and prototyping process was carried out in-house, the necessary adjustments could be made quickly.
In terms of material properties, the final space holder showed no deformation during the experiments, including positioning with the laparoscopic grasper, despite its relatively thin wall. Although this stability is desired, a more flexible and pliable material for the space holder might facilitate intra-esophageal suturing.
An anticipated disadvantage of the space holder was the increased time it took to perform the purse string suture. Furthermore, the ability to control the stitches was impaired, as shown by a tendency towards greater variability of stitch height. This might be explained by the task of intraesophageal suturing being more difficult when the space holder is used. The rigid space holder limits the freedom of movement. However, for the success of SAM it is crucial that the purse-string suture be placed exactly proximal to the oral border of the BE in order to ensure complete resection.
The requirement for 3DP prototyping is the availability of a 3D printer and dedicated software. Furthermore, user know-how in CAD software is a prerequisite. The most time-consuming step in development of a 3D model is processing the requirements in the software. In the current experiment the model was designed in the CAD software based on a simple drawing and anatomic measurements by a resident who was familiar with the software and had worked with it for more than 100 hours. The design of the model took less than half of a working day; however, more time might be needed if the designer has less experience with the CAD software. Once the model has been designed in the software, the production process from idea to first prototype can be completed in less than 24 h, depending on the size and complexity of the printed model.17–19 In this study the printing time for one model was 5 hours. After preliminary testing of the prototype, adaptation could be rapidly performed in the software. The rapid production time and the low threshold for adaptations of the prototypes are important advantages of self-produced 3DP models relative to prototype development with external industrial partners. In the later stages of development of SAM and when it comes to clinical introduction, however, the space holder should be commercially produced and certified for clinical use.
Although 3DP is considered cost effective by many, one has to bear in mind the initial investments in software and hardware.15,20,21 Several research groups have stated that the cost to the final 3D print represents a limitation.7,22,23 A range of investment between € 13,000 and € 40,000 has been reported for software and hardware. However, the hardware has become considerably cheaper in recent years. In our experiment the printer cost € 2,500 and the yearly licensing fee for the software was € 2,000. The cost per disposable space holder was very low, at € 5. Given that the printer used was a basic version of a model aimed at the general public and the software was shared among several groups in our department, the actual costs of 3DP prototypes are low and may be reduced even further with a wider range of open-access software.5,18 Compared to a prototype development in partnership with an industrial company, 3DP is inexpensive.
Secrecy is of crucial importance during the development of a surgical innovation. A lack of confidentiality may impede retention of the intellectual property and later patenting of a novel surgical method.25 Therefore, it is potentially hazardous to seek industrial collaboration at an early stage of development. Dependency on industry is avoided by rapid self-production of prototypes. In the present study no patent was requested. Nevertheless, at a later stage of development intellectual property may be secured before industrial cooperation is sought.
Conclusion
The present study describes the use of 3DP for a self-produced prototype used in experimental esophageal surgery. 3DP enabled production of a space holder for intraesophageal suturing, fulfilling the aim of higher intraesophageal suture placement, and therefore permitting higher intraesophageal resection. The advantages of 3DP in experimental surgery are its speed, the possibility of multiple adaptations, its cost effectiveness and the freedom from dependence on industrial partners. The development of novel surgical methods involving a medical device may be facilitated by the use of 3DP.
Supplementary Material
Intraesophageal suturing using the 3D-printed space holder
Intraesophageal suturing without space holder
Funding
The study was funded by institutional means. Regarding the presented device, no patent agreement has been made.
Footnotes
Conflicts of interest: Daniel C. Steinemann is supported by the Swiss National Science Foundation (Grant P300PB-161099/1) and the Margarete and Walter Lichtenstein Foundation, Basel, Switzerland (DMS2321). All other authors have nothing to disclose.
Authors’ contributions: DCS: study design, performing the experiments, statistical analysis, writing the manuscript; PCM, MA: study design, performing the experiments, writing the manuscript; FN, HGK, BPM: interpretation of data, critical revision of the manuscript; GRL: study design, interpretation of data, critical revision of the manuscript.
References
- 1.Choi JW, Kim N. Clinical application of three-dimensional printing technology in craniofacial plastic surgery. Arch Plast Surg. 2015;42(3):267–277. doi: 10.5999/aps.2015.42.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltorai AE, Nguyen E, Daniels AH. Three-Dimensional Printing in Orthopedic Surgery. Orthopedics. 2015;38(11):684–687. doi: 10.3928/01477447-20151016-05. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Yang X, Li D. The Application of Three-Dimensional Surface Imaging System in Plastic and Reconstructive Surgery. Ann Plast Surg. 2016;77(Suppl 1):S76–83. doi: 10.1097/SAP.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 4.Malik HH, Darwood AR, Shaunak S, et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199(2):512–522. doi: 10.1016/j.jss.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Ayoub AF, Rehab M, O'Neil M, et al. A novel approach for planning orthognathic surgery: the integration of dental casts into three-dimensional printed mandibular models. Int J Oral Maxillofac Surg. 2014;43(4):454–459. doi: 10.1016/j.ijom.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs S, Grunert R, Mohr FW, Falk V. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interact Cardiovasc Thorac Surg. 2008;7(1):6–9. doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 7.Kurenov SN, Ionita C, Sammons D, Demmy TL. Three-dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J Thorac Cardiovasc Surg. 2015;149(4):973–979 e971. doi: 10.1016/j.jtcvs.2014.12.059. [DOI] [PubMed] [Google Scholar]
- 8.Xiang N, Fang C, Fan Y, et al. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int J Clin Exp Med. 2015;8(10):18873–18878. [PMC free article] [PubMed] [Google Scholar]
- 9.del Junco M, Okhunov Z, Yoon R, et al. Development and initial porcine and cadaver experience with three-dimensional printing of endoscopic and laparoscopic equipment. J Endourol. 2015;29(1):58–62. doi: 10.1089/end.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rankin TM, Giovinco NA, Cucher DJ, Watts G, Hurwitz B, Armstrong DG. Three-dimensional printing surgical instruments: are we there yet? J Surg Res. 2014;189(2):193–197. doi: 10.1016/j.jss.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinemann DC, Zerz A, Muller PC, et al. Laparoscopic transgastric circumferential stapler-assisted vs. endoscopic esophageal mucosectomy in a porcine model. Endoscopy. 2017 doi: 10.1055/s-0043-103407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozdag AD, Nazli O, Tansug T, Derici H, Deniz V. Special anoscope for easy purse-string suture application in stapled hemorrhoidopexy. World J Surg. 2007;31(3):538–541. doi: 10.1007/s00268-006-0416-7. [DOI] [PubMed] [Google Scholar]
- 13.Bordeianou L, Sylla P, Kinnier CV, Rattner D. Perineal sigmoidopexy utilizing transanal endoscopic microsurgery (TEM) to treat full thickness rectal prolapse: a feasibility trial in porcine and human cadaver models. Surg Endosc. 2015;29(3):686–691. doi: 10.1007/s00464-014-3722-4. [DOI] [PubMed] [Google Scholar]
- 14.Zdichavsky M, Krautwald M, Meile T, et al. Single-port live donor nephrectomy using a novel Curved Radius r2 Surgical System in an in vivo model. Minim Invasive Ther Allied Technol. 2015;24(2):63–67. doi: 10.3109/13645706.2014.975134. [DOI] [PubMed] [Google Scholar]
- 15.Dean D, Min KJ, Bond A. Computer aided design of large-format prefabricated cranial plates. J Craniofac Surg. 2003;14(6):819–832. doi: 10.1097/00001665-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Tandon A, Byrne N, Nieves Velasco Forte Mde L, et al. Use of a semi-automated cardiac segmentation tool improves reproducibility and speed of segmentation of contaminated right heart magnetic resonance angiography. Int J Cardiovasc Imaging. 2016;32(8):1273–1279. doi: 10.1007/s10554-016-0906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birnbaum K, Schkommodau E, Decker N, Prescher A, Klapper U, Radermacher K. Computer-assisted orthopedic surgery with individual templates and comparison to conventional operation method. Spine (Phila Pa 1976) 2001;26(4):365–370. doi: 10.1097/00007632-200102150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Gerstle TL, Ibrahim AM, Kim PS, Lee BT, Lin SJ. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg. 2014;133(2):446–451. doi: 10.1097/01.prs.0000436844.92623.d3. [DOI] [PubMed] [Google Scholar]
- 19.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 20.Condino S, Carbone M, Ferrari V, et al. How to build patient-specific synthetic abdominal anatomies. An innovative approach from physical toward hybrid surgical simulators. Int J Med Robot. 2011;7(2):202–213. doi: 10.1002/rcs.390. [DOI] [PubMed] [Google Scholar]
- 21.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101(5):1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillis JA, Morris SF. Three-dimensional printing of perforator vascular anatomy. Plast Reconstr Surg. 2014;133(1):80e–82e. doi: 10.1097/01.prs.0000436523.79293.64. [DOI] [PubMed] [Google Scholar]
- 23.Kim BJ, Hong KS, Park KJ, Park DH, Chung YG, Kang SH. Customized cranioplasty implants using three-dimensional printers and polymethyl-methacrylate casting. J Korean Neurosurg Soc. 2012;52(6):541–546. doi: 10.3340/jkns.2012.52.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stitely ML, Paterson H. Using Three-Dimensional Printing to Fabricate a Tubing Connector for Dilation and Evacuation. Obstet Gynecol. 2016;127(2):317–319. doi: 10.1097/AOG.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 25.Vecht JA, Athanasiou T, Ashrafian H, Mayer E, Darzi A, von Segesser LK. Surgeons produce innovative ideas which are frequently lost in the labyrinth of patents. Eur J Cardiothorac Surg. 2009;35(3):480–488. doi: 10.1016/j.ejcts.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Meseguer-Olmo L, Vicente-Ortega V, Alcaraz-Banos M, et al. In-vivo behavior of Si-hydroxyapatite/polycaprolactone/DMB scaffolds fabricated by 3D printing. J Biomed Mater Res A. 2013;101(7):2038–2048. doi: 10.1002/jbm.a.34511. [DOI] [PubMed] [Google Scholar]
- 27.Rozema FR, Bos RR, Boering G, van Asten JA, Nijenhuis AJ, Pennings AJ. The effects of different steam-sterilization programs on material properties of poly(L-lactide) J Appl Biomater. 1991;2(1):23–28. doi: 10.1002/jab.770020104. [DOI] [PubMed] [Google Scholar]
- 28.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraesophageal suturing using the 3D-printed space holder
Intraesophageal suturing without space holder